Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Squid Waste and Lyophilization
2.2. Moisture Determination and Conventional Lipid Extraction
2.3. Lipid Extraction with Ethanol/Acetone
2.4. Lipid Extract Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Moisture Values of Starting and Lyophilized Waste
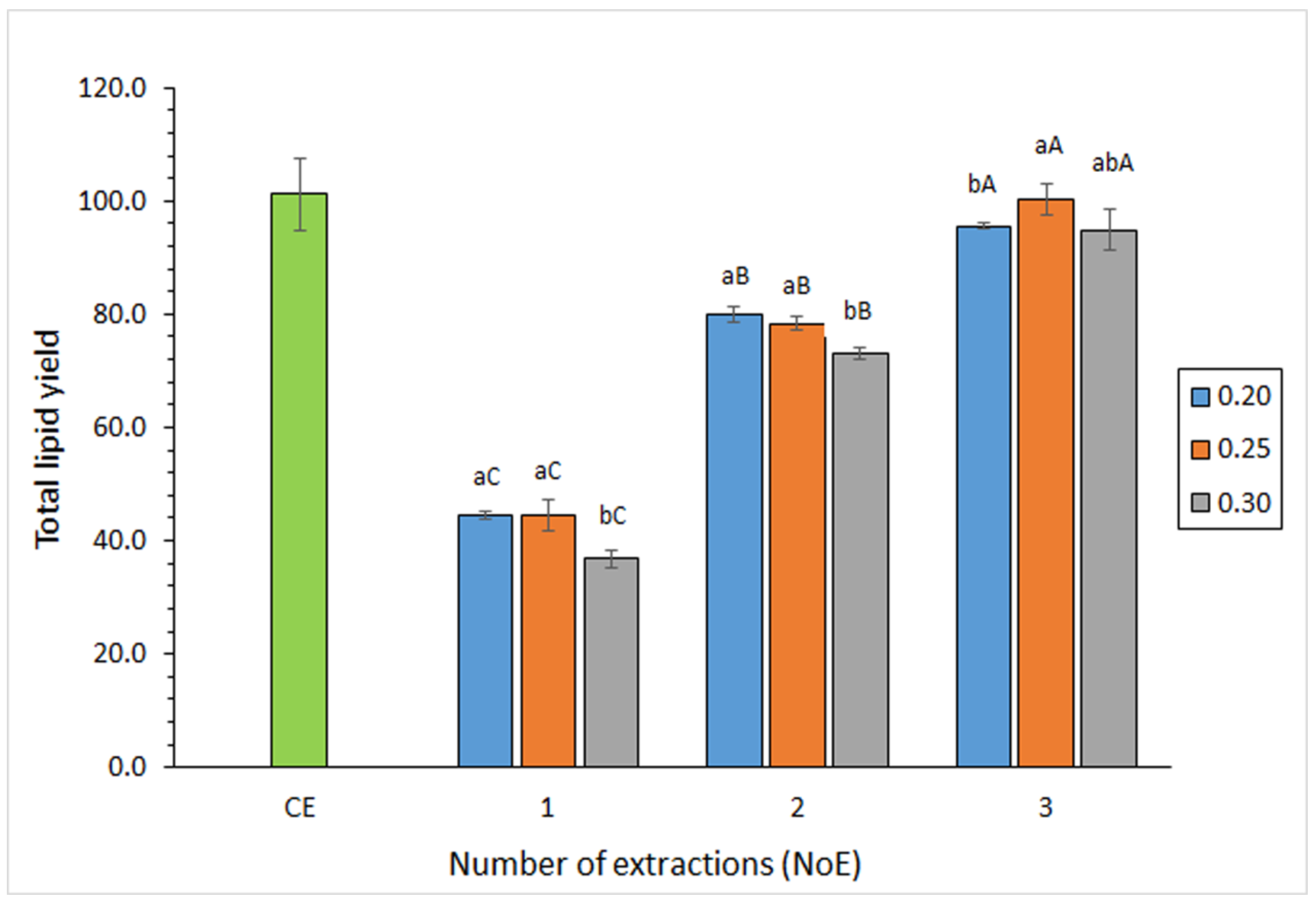
3.2. Effect of the Extraction Conditions on the TL Yield
3.3. Effect of the Extraction Conditions on the PL Yield
3.4. Effect of the Extracting Conditions on the Tocopherol Content
3.5. Effect of the Extracting Conditions on the FA Composition
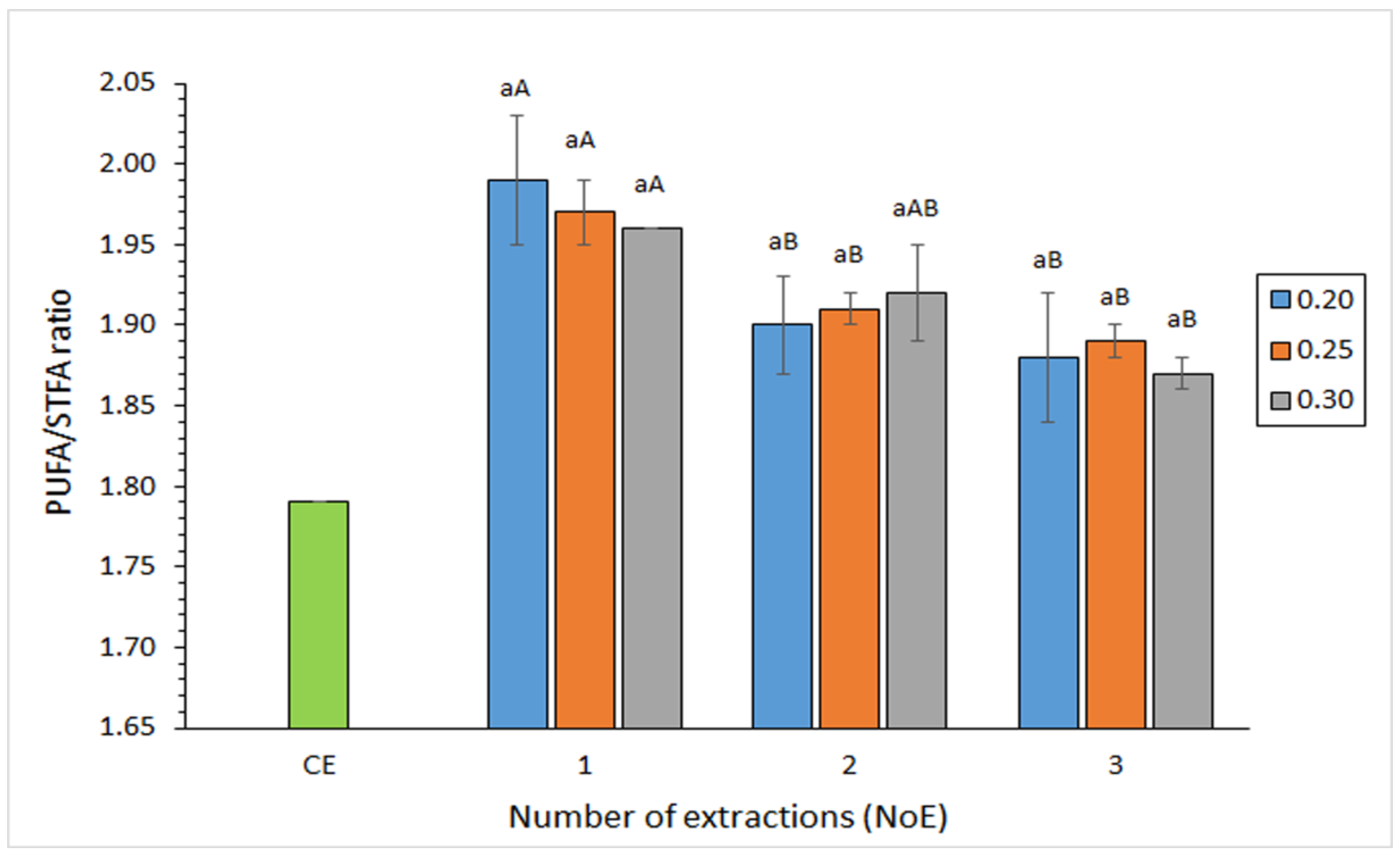
3.6. Effect of the Extraction Conditions on the FA Ratios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Tilami, S.K.; Sampels, S. Nutritional Value of Fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. 2018, 26, 242–253. [Google Scholar]
- Swanson, S.; Block, R.; Mousa, S. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.M.; Lee, B.H.; Yu, X. Current trends and future perspectives on omega-3 fatty acids. Res. J. Biol. 2017, 5, 11–20. [Google Scholar]
- Takahashi, K.; Inoue, Y. Marine by-product phospholipids as booster of medicinal compounds. Adv. Food Nutr. Res. 2012, 65, 31–46. [Google Scholar] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A. Antioxidants. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; pp. 309–326. [Google Scholar]
- Kim, Y.N. Vitamins. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Francis and Taylor Group: Boca Raton, FL, USA, 2010; pp. 327–350. [Google Scholar]
- FAO. El Estado Mundial de la Pesca y la Acuicultura; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2020; pp. 2–9. [Google Scholar]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2223. [Google Scholar] [CrossRef]
- Özyurt, G.; Özkütük, S. Advances in discard and by-product processing. In Innovative Technologies in Seafood Processing; Özoğull, Y., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 323–350. [Google Scholar]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduiña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef]
- Rustad, T.; Storro, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Terra Crexi, V.; Legemann Monte, M.; Almeida de Souza Soares, L.; Almeida Pinto, L.A. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem. 2010, 119, 945–950. [Google Scholar] [CrossRef]
- Okada, T.; Morrisey, M.T. Recovery and characterization of sardine oil extracted by pH adjustment. J. Agric. Food Chem. 2007, 55, 1808–1813. [Google Scholar] [CrossRef]
- Pudtikajorn, K.; Benjakul, S. Simple wet rendering method for extraction of prime quality oil from skipjack tuna eyeballs. Eur. J. Lipid Sci. Technol. 2020, 122, 2000077. [Google Scholar] [CrossRef]
- Linder, M.; Fanni, J.; Parmentier, M. Proteolytic extraction of salmon oil and PUFA concentration by lipases. Marine Biotechnol. 2005, 15, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Pando, M.E.; Rodríguez, A.; Galdames, A.; Berríos, M.M.; Rivera, M.; Romero, N.; Valenzuela, M.A.; Ortiz, J.; Aubourg, S.P. Maximization of the docosahexaenoic and eicosapentaenoic acids content in concentrates obtained from a by-product of rainbow trout (Oncorhynchus mykiss) processing. Eur. Food Res. Technol. 2018, 5, 937–948. [Google Scholar] [CrossRef]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Li, C.J.; Xin, M.R.; Sun, Z.L. Selection of extraction solvents for edible oils from microalgae and improvement of the oxidative stability. J. Biosci. Bioeng. 2021, 132, 365–371. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agricultural Organisation of the United Nations. Fishery Division. Species Fact Sheets. Loligo gahi (Orbigny, 1835). 2021. Available online: www.fao.org/fishery/species/2713/en (accessed on 1 January 2023).
- Aubourg, S.P.; Trigo, M.; González, M.J.; Lois, S.; Medina, I. Comparative study of bioactive lipid extraction from squid (Doryteuthis gahi) by-products by green solvents. Foods 2022, 11, 2188. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of low-toxicity solvent employment for total lipid and tocopherol compound extraction from Patagonian squid by-products. Foods 2023, 12, 504. [Google Scholar] [CrossRef]
- Jiménez Callejón, M.J.; Robles Medina, A.; González Moreno, P.A.; Cerdán, L.E.; Orta Guillén, S.; Molina Grima, E. Simultaneous extraction and fractionation of lipids from microalga Nannochloropsis sp. for the production of EPA-rich polar lipid concentrates. J. Applied Phycol. 2020, 32, 1117–1128. [Google Scholar] [CrossRef]
- AOAC. Official Methods for analysis of the Association of Analytical Chemistry, 15th ed.; Association of Official Chemists, Inc.: Arlington, VA, USA, 1990; pp. 931–937. [Google Scholar]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Herbes, S.E.; Allen, C.P. Lipid quantification of freshwater invertebrates: Method modification for microquantitation. Can. J. Fish. Aquat. Sci. 1983, 40, 1315–1317. [Google Scholar] [CrossRef]
- Raheja, R.; Kaur, C.; Singh, A.; Bhatia, A. New colorimetric method for the quantitative determination of phospholipids without acid digestion. J. Lipid Res. 1973, 14, 695–697. [Google Scholar] [CrossRef]
- Cabrini, L.; Landi, L.; Stefanelli, C.; Barzanti, V.; Sechi, A. Extraction of lipid and lipophilic antioxidants from fish tissues: A comparison among different methods. Comp. Biochem. Physiol. Biochem. Molec. Biol. 1992, 101, 383–386. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Prego, R.; Fett, R.; Aubourg, S.P. The chemical composition of different edible locations (central and edge muscles) of flat fish (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2018, 53, 271–281. [Google Scholar] [CrossRef]
- Chantachum, S.; Benjakul, S.; Sriwirat, N. Separation and quality of fish oil from precooked and non-precooked tuna heads. Food Chem. 2000, 69, 289–294. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Bunga, S.; Stewart, I.; Tian, H.; Carne, A.; Bekhit, A.E.-D.A. Simple and efficient one-pot extraction method for phospholipidomic profiling of total oil and lecithin by phosphorus-31 nuclear magnetic resonance measurements. J. Agric. Food Chem. 2020, 68, 14286–14296. [Google Scholar] [CrossRef]
- Rodríguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of healthy-lipid content and oxidative stability during oil extraction from squid (Illex argentinus) viscera by green processing. Mar. Drugs 2021, 19, 616. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Jin, W.; Gu, Q.; Zhou, X.; Xi, Y.; Tu, R.; Han, S.F.; Xie, G.J.; Gao, S.H.; Wang, Q. Subcritical n-hexane/isopropanol extraction of lipid from wet microalgal pastes of Scenedesmus obliquus. World J. Microb. Biotechnol. 2018, 34, 39. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; Sarkinnen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects–a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef]
- Grey, A.; Bolland, M. Clinical trial evidence and use of fish oil supplements. JAMA Intern. Med. 2014, 174, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.P.; Müller, M.; Rainger, G.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef]
- Głowacz-Rozynska, A.; Tynek, M.; Malinowska-Panczyk, E.; Martysiak-Zurowska, D.; Pawłowicz, R.; Kołodziejsk, I. Comparison of oil yield and quality obtained by different extraction procedures from salmon (Salmo salar) processing byproducts. Eur. J. Lipid Sci. Technol. 2016, 118, 1759–1767. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Trigo, M.; Prego, R.; Cobelo-García, A.; Medina, I. Nutritional and healthy value of chemical constituents obtained from Patagonian squid (Doryteuthis gahi) by-products captured at different seasons. Foods 2021, 10, 2144. [Google Scholar] [CrossRef]
- Piclet, G. Le poisson aliment. Composition-Intérêt nutritionnel. Cah. Nutr. Diét. 1987, XXII, 317–335. [Google Scholar]
- Sieiro, M.P.; Aubourg, S.P.; Rocha, F. Seasonal study of the lipid composition in different tissues of the common octopus (Octopus vulgaris). Eur. J. Lipid Sci. Technol. 2006, 108, 479–487. [Google Scholar] [CrossRef]
- Uauy, R.; Valenzuela, A. Marine oils: The health benefits of n-3 fatty acids. Nutrition 2000, 16, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Reddy, C.R.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Šimat, V.; Vlahović, J.; Soldo, B.; Mekinić, I.G.; Čagalj, M.; Hamed, I.; Skroza, D. Production and characterization of crude oils from seafood processing by-products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
| Extraction Condition Number | Experimental Conditions | Process Variables | ||
|---|---|---|---|---|
| Waste Weight (WW) (g) | Solvent Volume (SV) (mL) | WW/SV Ratio (g·mL−1) | Numer of Extractions (NoE) | |
| EC-1 | 3 | 15 | 0.20 | 1 |
| EC-2 | 3 | 12 | 0.25 | 1 |
| EC-3 | 3 | 10 | 0.30 | 1 |
| EC-4 | 3 | 15 | 0.20 | 2 |
| EC-5 | 3 | 12 | 0.25 | 2 |
| EC-6 | 3 | 10 | 0.30 | 2 |
| EC-7 | 3 | 15 | 0.20 | 3 |
| EC-8 | 3 | 12 | 0.25 | 3 |
| EC-9 | 3 | 10 | 0.30 | 3 |
| Extraction Condition | Lipid Parameter | |||
|---|---|---|---|---|
| WW/SV (g·mL−1) | NoE | PLs | α-Tocopherol | γ-Tocopherol |
| 0.20 | 1 | 343.0 aA (6.6) | 624.8 bA (44.6) | 10.4 abA (1.5) |
| 0.25 | 1 | 343.9 aA (7.0) | 699.0 aA (11.8) | 15.4 aA (3.0) |
| 0.30 | 1 | 334.6 aA (4.8) | 643.4 abA (23.9) | 9.4 bAB (0.5) |
| 0.20 | 2 | 329.2 bA (9.0) | 687.5 aA (23.5) | 10.9 aA (0.2) |
| 0.25 | 2 | 322.7 bB (2.2) | 593.4 abB (8.5) | 7.8 bB (0.3) |
| 0.30 | 2 | 341.3 aA (13.4) | 575.7 bB (24.0) | 7.8 bB (0.2) |
| 0.20 | 3 | 328.5 aA (4.7) | 667.6 aA (19.7) | 10.3 aA (1.3) |
| 0.25 | 3 | 315.5 bB (6.9) | 596.1 aB (70.1) | 8.1 aB (0.7) |
| 0.30 | 3 | 316.3 abA (19.8) | 647.9 aA (21.1) | 8.9 aA (0.1) |
| Conventional extraction | 323.2 (3.2) | 617.4 (82.4) | 8.1 (2.4) | |
| Extraction Condition | FA Parameter | |||
|---|---|---|---|---|
| WW/SV (g·mL−1) | NoE | EPA | DHA | ω3/ω6 |
| 0.20 | 1 | 17.73 aA (0.39) | 34.67 aA (0.33) | 12.24 bA (0.36) |
| 0.25 | 1 | 17.52 aA (0.08) | 34.93 aA (0.14) | 12.91 aA (0.00) |
| 0.30 | 1 | 17.40 aA (0.20) | 34.76 aA (0.08) | 12.38 bA (0.39) |
| 0.20 | 2 | 17.50 aA (0.04) | 34.29 bA (0.07) | 12.90 aA (0.74) |
| 0.25 | 2 | 17.48 aA (0.21) | 34.52 abB (0.14) | 12.62 aB (0.02) |
| 0.30 | 2 | 17.36 aA (0.08) | 34.56 aA (0.14) | 12.67 aA (0.53) |
| 0.20 | 3 | 17.38 aA (0.17) | 34.09 aA (0.18) | 12.64 aA (0.34) |
| 0.25 | 3 | 17.39 aA (0.11) | 34.25 aC (0.07) | 12.26 aC (0.25) |
| 0.30 | 3 | 17.38 aA (0.21) | 34.33 aB (0.02) | 13.00 aA (0.52) |
| Conventional extraction | 17.82 (0.00) | 33.13 (0.00) | 12.38 (0.00) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubourg, S.P.; Rodríguez, A.; Trigo, M.; Medina, I. Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction. Foods 2023, 12, 2649. https://doi.org/10.3390/foods12142649
Aubourg SP, Rodríguez A, Trigo M, Medina I. Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction. Foods. 2023; 12(14):2649. https://doi.org/10.3390/foods12142649
Chicago/Turabian StyleAubourg, Santiago P., Alicia Rodríguez, Marcos Trigo, and Isabel Medina. 2023. "Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction" Foods 12, no. 14: 2649. https://doi.org/10.3390/foods12142649
APA StyleAubourg, S. P., Rodríguez, A., Trigo, M., & Medina, I. (2023). Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction. Foods, 12(14), 2649. https://doi.org/10.3390/foods12142649







