LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. By-Products’ Selection and Extraction
2.3. Incorporation of the Lemon Extract into LDPE and PLA
2.4. Oxygen and Water Permeability Tests of Active Films
2.5. Migration Assay of Active Films
2.5.1. HPLC–DAD Analysis
2.5.2. Total Phenolic Content
2.5.3. Radical Scavenging Activity through DPPH Radical Assay
2.6. Selection of High-Fat Content Foods
2.7. Sample Preparation and Packaging
2.8. Lipid Oxidation Assays
2.8.1. TBARS Assay
2.8.2. Fat Extraction
2.8.3. p-Anisidine Value Determination
2.8.4. Peroxide Value Determination
2.9. Microbiological Analysis
2.10. Statistical Analysis
3. Results
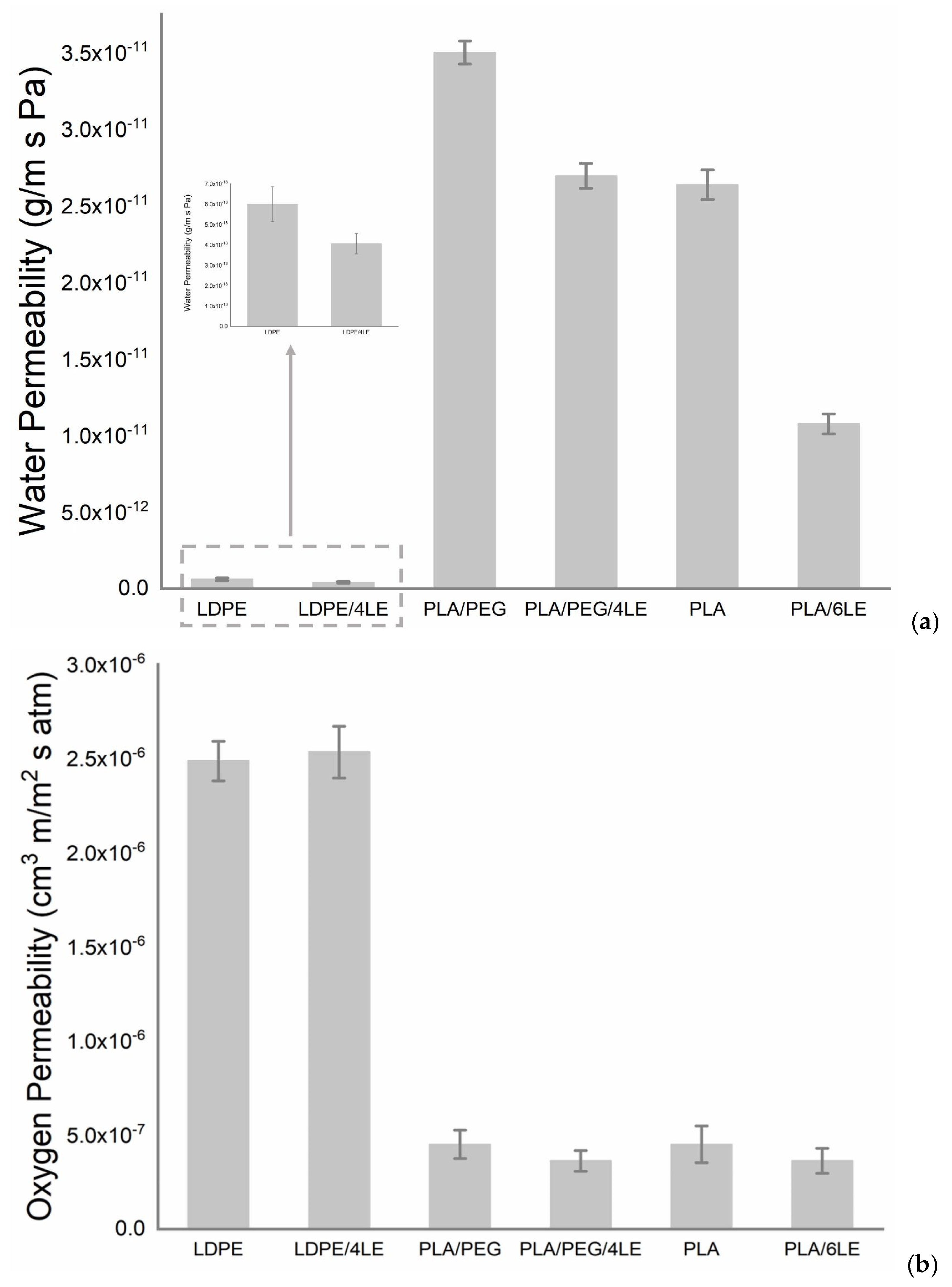
3.1. Oxygen and Water Permeability Tests of Active Films
3.2. Migration of Polyphenols and Antioxidant Capacity of the Active Films
3.3. Selection of Model Foods
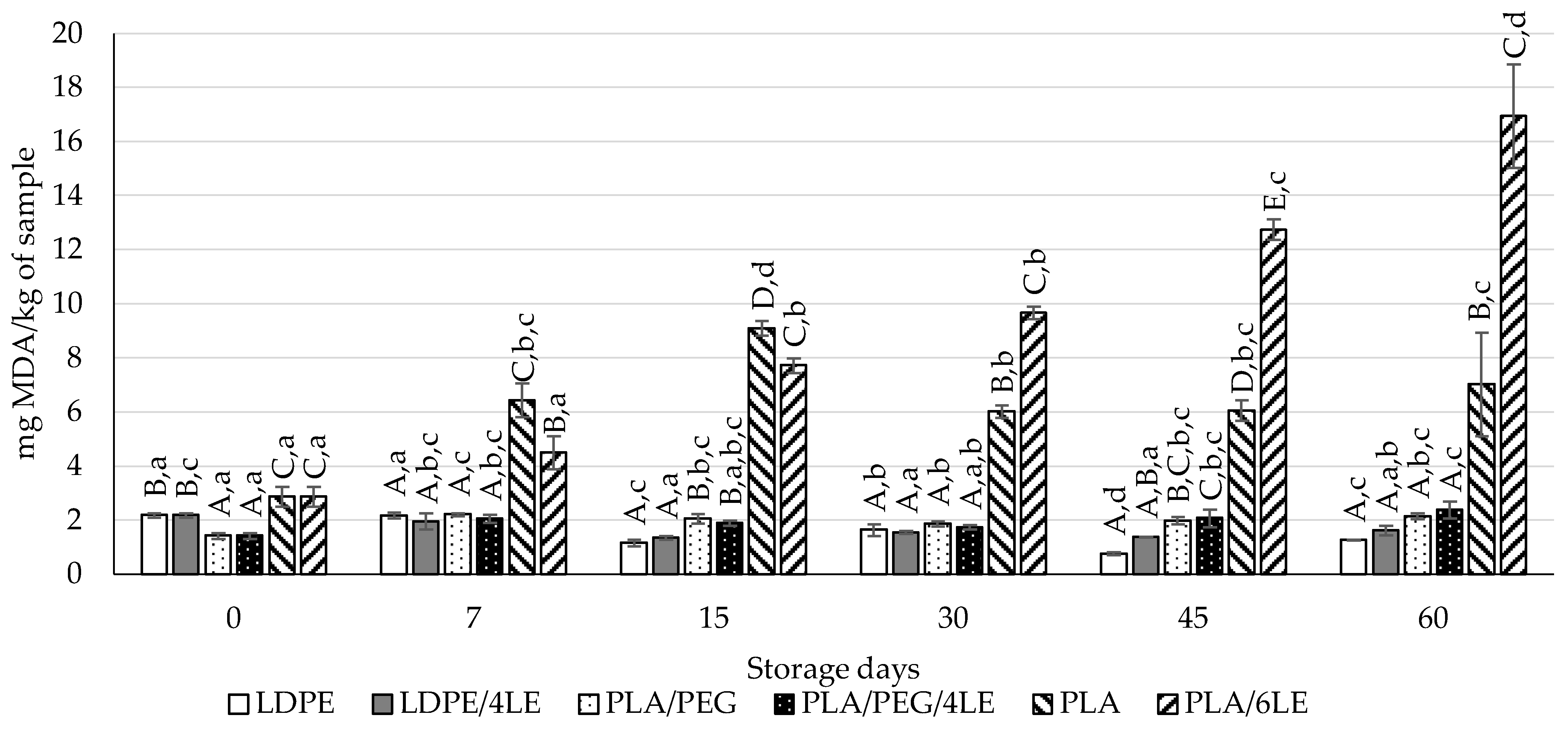
3.4. Lipid Oxidation Evaluation of Almond
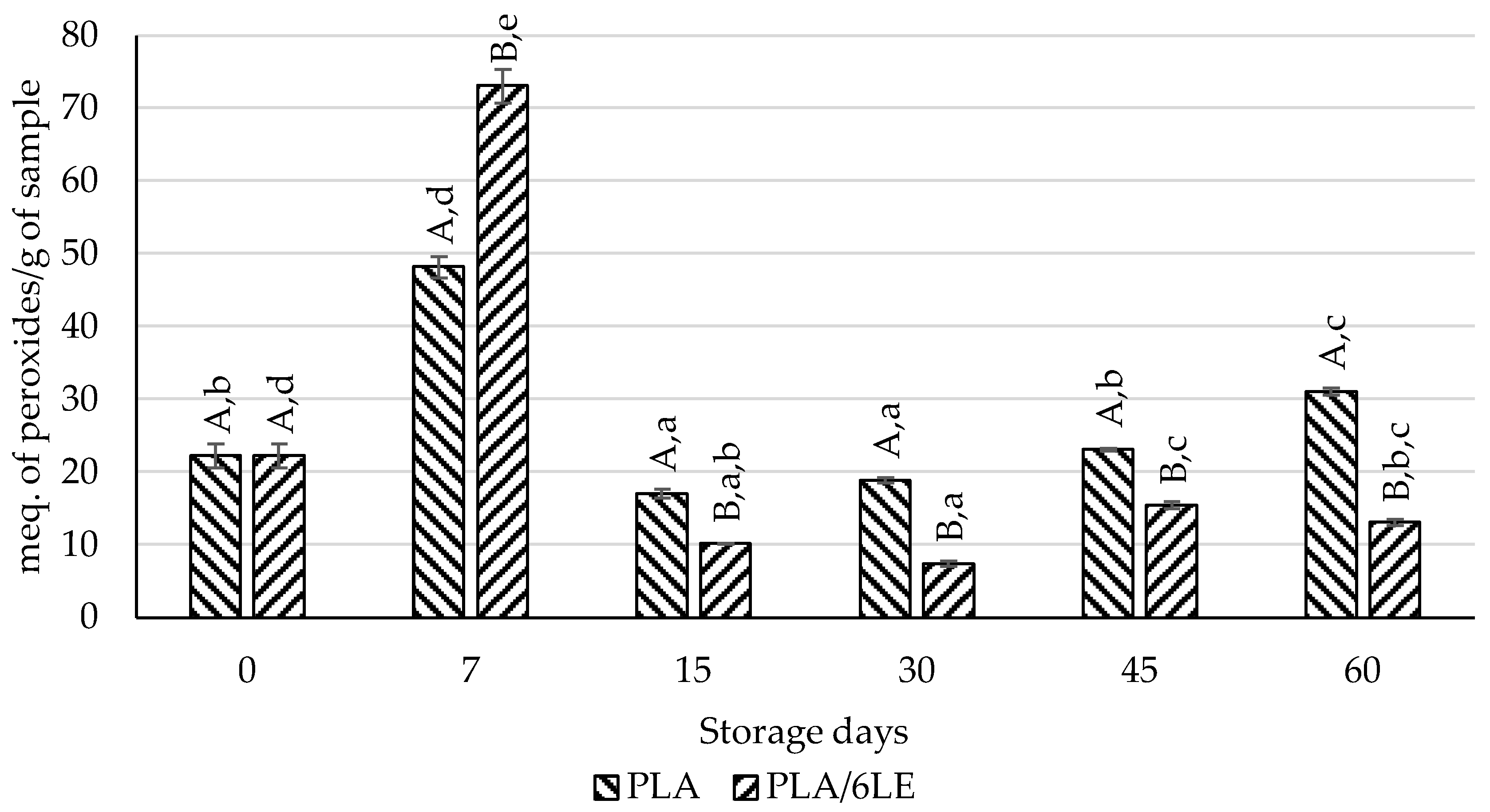
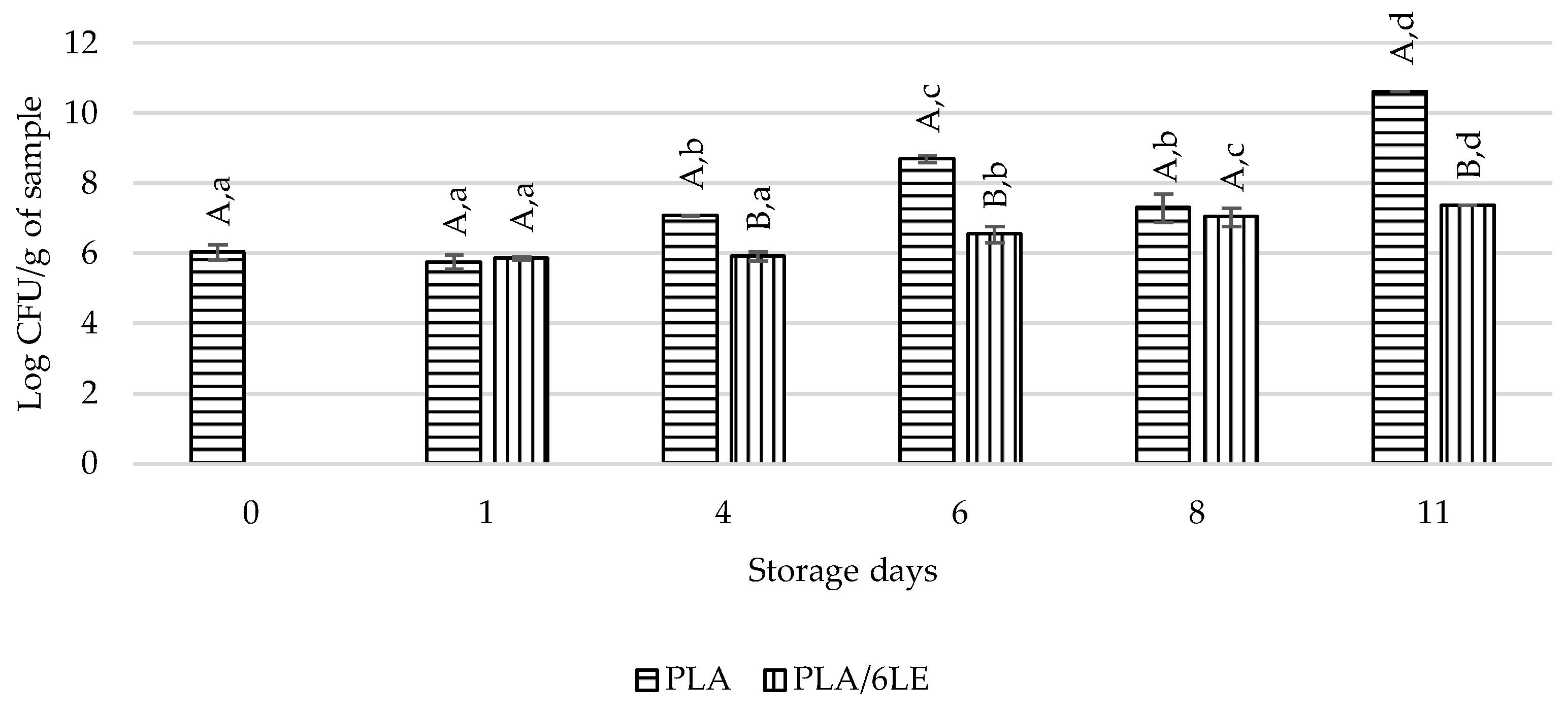
3.5. Lipid Oxidation and Microbial Evaluation of Beef Meat Packaged with PLA/6LE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and Intelligent Food Packaging: Legal Aspects and Safety Concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Day, B.P.F. Active Packaging. In Food Packaging Technology; Coles, R., McDowell, D., Kirwan, M.J., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2003; ISBN 978-1-405-14771-2. [Google Scholar]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Active Edible Packaging. Encyclopedia 2021, 1, 360–370. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Edible Active Coating Systems for Food Purposes. In Releasing Systems in Active Food Packaging, Preparation and Applications; Jafari, S.M., Sanches Silva, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 253–299. ISBN 9783030902995. [Google Scholar]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of Antimicrobial Food Packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; de Melo, N.R.; Andrade, M.; Azevedo, G.; Machado, A.V.; Carvalho-Costa, D.; Sanches-Silva, A. Whey Protein Active Films Incorporated with a Blend of Essential Oils: Characterization and Effectiveness. Packag. Technol. Sci. 2018, 31, 27–40. [Google Scholar] [CrossRef]
- Malpass, D.B. Introduction to Polymers of Ethylene. In Introduction to Industrial Polyethylene: Properties, Catalysts, Process; Scrivener Publishing LLC: Beverly, MA, USA, 2010; ISBN 978-0-470-62598-9. [Google Scholar]
- Piergiovanni, L.; Limbo, S. Plastic Packaging Materials. In Food Packaging: Principles and Practice; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–49. ISBN 9781439862414. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 3–8. [Google Scholar] [CrossRef]
- Vilarinho, F.; Andrade, M.; Buonocore, G.G.; Stanzione, M.; Vaz, M.F.; Sanches Silva, A. Monitoring Lipid Oxidation in a Processed Meat Product Packaged with Nanocomposite Poly(Lactic Acid) Film. Eur. Polym. J. 2018, 98, 362–367. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Choong, P.F.M.; Dass, C.R. Recent Developments in Liposomes, Microparticles and Nanoparticles for Protein and Peptide Drug Delivery. Peptides 2010, 31, 184–193. [Google Scholar] [CrossRef]
- Valantin, M.A.; Aubron-Olivier, C.; Ghosn, J.; Laglenne, E.; Pauchard, M.; Schoen, H.; Bousquet, R.; Katz, P.; Costagliola, D.; Katlama, C. Polylactic Acid Implants (New-Fill)® to Correct Facial Lipoatrophy in HIV-Infected Patients: Results of the Open-Label Study VEGA. Aids 2003, 17, 2471–2477. [Google Scholar] [CrossRef]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and Grape By-Products and Their Active Compounds: Are They a Valuable Source for Food Applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus Waste Derived Nutra-/Pharmaceuticals for Health Benefits: Current Trends and Future Perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Bravo, L.; Saura-Calixto, F. High Dietary Fibre Powders from Orange and Lime Peels: Associated Polyphenols and Antioxidant Capacity. Food Res. Int. 1996, 29, 757–762. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of Phenolic Profile and Antioxidant Capacity of Different Fruit Part from Lemon (Citrus Limon Burm.) Cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Spáčil, Z.; Nováková, L.; Solich, P. Analysis of Phenolic Compounds by High Performance Liquid Chromatography and Ultra Performance Liquid Chromatography. Talanta 2008, 76, 189–199. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations (FAO) FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 31 July 2018).
- Baldi, A.; Rosen, R.T.; Fukuda, E.K.; Ho, C.-T. Identification of Nonvolatile Components in Lemon Peel by High-Performance Liquid Chromatography with Confirmation by Mass Spectrometry and Diode-Array Detection. J. Chromatogr. A 1995, 718, 89–97. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus Limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Horowitz, R.M.; Gentili, B. Flavonoid Compounds of Citrus. III. Isolation and Structure of Eriodictyol Glycoside. J. Am. Chem. Soc. 1960, 82, 2803–2806. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K.; Morimitsu, Y.; Osawa, T. Isolation of C -Glucosylflavone from Lemon Peel and Antioxidative Activity of Flavonoid Compounds in Lemon Fruit. J. Agric. Food Chem. 1997, 45, 4619–4623. [Google Scholar] [CrossRef]
- Miyake, Y.; Suzuki, E.; Ohya, S.; Fukumoto, S.; Hiramitsu, M.; Sakaida, K.; Osawa, T.; Furuichi, Y. Lipid-Lowering Effect of Eriocitrin, the Main Flavonoid in Lemon Fruit, in Rats on a High-Fat and High-Cholesterol Diet. J. Food Sci. 2006, 71, S633–S637. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chuang, Y.-C.; Hsu, H.-W. The Flavonoid, Carotenoid and Pectin Content in Peels of Citrus Cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Sanches, V.L.; Cunha, T.A.; Viganó, J.; de Souza Mesquita, L.M.; Faccioli, L.H.; Breitkreitz, M.C.; Rostagno, M.A. Comprehensive Analysis of Phenolics Compounds in Citrus Fruits Peels by UPLC-PDA and UPLC-Q/TOF MS Using a Fused-Core Column. Food Chem. X 2022, 14, 100262. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of Active Films Utilizing Antioxidant Compounds Obtained from Tomato and Lemon By-Products for Use in Food Packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Séndon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods 2021, 10, 272. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of Rosemary and Thyme Extracts for Incorporation into a Whey Protein Based Film. LWT-Food Sci. Technol. 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an Antioxidant Polylactic Acid (PLA) Film Prepared with α-Tocopherol, BHT and Polyethylene Glycol Using Film Cast Extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) NO. 10/2011. Off. J. Eur. Union 2011, L 012, 1–89. [Google Scholar]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus Linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Brown, H.; Williams, J. Packaged Product Quality and Shelf Life. In Food Packaging Technology; Coles, R., McDowell, D., Kirwan, M.J., Eds.; Blackwell Publishing, Ltd.: Hoboken, NJ, USA, 2003; pp. 65–94. [Google Scholar]
- USDA. USDA FoodData Central. Available online: https://fdc.nal.usda.gov/index.html (accessed on 1 May 2020).
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA Test by an Extractive Method Applied to ‘Paté’. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Castro, F.V.R.; Andrade, M.A.; Sanches Silva, A.; Vaz, M.F.; Vilarinho, F. The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo Salar L.). Foods 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Instituto Português da Qualidade. NP 1819: Edible Fats and Oils. Determination of p-Anisidine Value; Instituto Português da Qualidade: Caparica, Portugal, 1984. [Google Scholar]
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Álvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.Ó.; Monahan, F.J. Effect of an Active Packaging with Citrus Extract on Lipid Oxidation and Sensory Quality of Cooked Turkey Meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef]
- Maru, V.R.; Gupta, S.; Ranade, V.; Variyar, P.S. Pullulan or Chitosan Based Active Coating by Incorporating Polyphenols from Lemon Peel in Raw Poultry Meat. J. Food Sci. Technol. 2021, 58, 3807–3816. [Google Scholar] [CrossRef]
- Kerrihard, A.L.; Pegg, R.B.; Sarkar, A.; Craft, B.D. Update on the Methods for Monitoring UFA Oxidation in Food Products. Eur. J. Lipid Sci. Technol. 2015, 117, 1–14. [Google Scholar] [CrossRef]
- Dermiş, S.; Can, S.; Doğru, B. Determination of Peroxide Values of Some Fixed Oils by Using the MFOX Method. Spectrosc. Lett. 2012, 45, 359–363. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nawar, W.W. Lipids. In Food Chemistry; Fennema, O.R., Ed.; Taylor & Francis: Abingdon, UK, 1996; pp. 139–244. [Google Scholar]
- Andrade, M.A.; Barbosa, C.H.; Santos, R.R.; Vilarinho, F.; Silva, A.S.; Ramos, F. Application of Releasing Systems in Active Packaging of Meat Products. In Releasing Systems in Active Food Packaging, Preparation and Applications; Jafari, S.M., Silva, A.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 303–352. [Google Scholar]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and Characterization of Grass Carp Collagen-Chitosan-Lemon Essential Oil Composite Films for Application as Food Packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Shelf Life Extension of Ground Chicken Meat Using an Oxygen Absorber and a Citrus Extract. LWT 2012, 49, 21–27. [Google Scholar] [CrossRef]
- Kadhim Hindi, N.K.; Ghani Chabuck, Z.A. Antimicrobial Activity of Different Aqueous Lemon Extracts. J. Appl. Pharm. Sci. 2013, 3, 74–78. [Google Scholar] [CrossRef]
- Sanz-Puig, M.; Pina-Pérez, M.C.; Martínez-López, A.; Rodrigo, D. Escherichia Coli O157:H7 and Salmonella Typhimurium Inactivation by the Effect of Mandarin, Lemon, and Orange by-Products in Reference Medium and in Oat-Fruit Juice Mixed Beverage. LWT-Food Sci. Technol. 2016, 66, 7–14. [Google Scholar] [CrossRef]




| Phenolic Compound | LDPE/4LE ‡ (µg/dm2) | PLA/PEG/4LE (µg/dm2) | PLA/6LE (µg/dm2) | Sig. |
|---|---|---|---|---|
| 4-Hydroxybenzoic acid | 9.97 ± 0.32 aA | 3.22 ± 0.83 cdC | 6.50 ± 0.40 eB | *** |
| Caffeic acid | <LOQ | 3.19 ± 0.15 cd | < LOQ | |
| p-coumaric acid | 2.22 ± 0.95 c | 1.27 ± 0.20 d | 2.06 ± 0.11 f | n.s. |
| Ferulic acid | <LOQ | 11.4 ± 4.91 c | 11.0 ± 0.98 d | n.s. |
| Eriocitrin | <LOQ | 21.8 ± 3.17 bA | 13.0 ± 0.85 cB | ** |
| Hesperidin | <LOQ | 49.9 ± 13.3 aA | 26.4 ± 2.1 aB | * |
| Rutin | <LOQ | 4.45 ± 0.87 cdA | 2.95 ± 0.12 fB | * |
| Rosmarinic acid | <LOQ | 4.06 ± 0.74 cd | 3.35 ± 0.11 f | n.s. |
| Naringenin | 3.77 ± 0.16 bC | 22.1 ± 0.42 bB | 27.3 ± 1.9 aA | *** |
| Apigenin | <LOQ | 21.4 ± 0.50 bB | 22.2 ± 0.20 bA | * |
| Sig. | *** | *** | *** | |
| ∑ (µg/dm2) | 15.97 ± 1.33 B | 142.7 ± 24.7 A | 114.9 ± 6.66 A | *** |
| TPC (µg GAE/dm2) | n.d. | 624.6 ± 4.64 | 673.3 ± 35.9 | n.s. |
| RSA (µmol TE/dm2) | n.d. | 1.75 ± 0.02 B | 1.94 ± 0.09 A | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.A.; Barbosa, C.H.; Mariño-Cortegoso, S.; Barbosa-Pereira, L.; Sendón, R.; Buonocore, G.G.; Stanzione, M.; Coelho, A.; Correia, C.B.; Saraiva, M.; et al. LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat. Foods 2023, 12, 2450. https://doi.org/10.3390/foods12132450
Andrade MA, Barbosa CH, Mariño-Cortegoso S, Barbosa-Pereira L, Sendón R, Buonocore GG, Stanzione M, Coelho A, Correia CB, Saraiva M, et al. LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat. Foods. 2023; 12(13):2450. https://doi.org/10.3390/foods12132450
Chicago/Turabian StyleAndrade, Mariana A., Cássia H. Barbosa, Sandra Mariño-Cortegoso, Letricia Barbosa-Pereira, Raquel Sendón, Giovanna G. Buonocore, Mariamelia Stanzione, Anabela Coelho, Cristina Belo Correia, Margarida Saraiva, and et al. 2023. "LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat" Foods 12, no. 13: 2450. https://doi.org/10.3390/foods12132450
APA StyleAndrade, M. A., Barbosa, C. H., Mariño-Cortegoso, S., Barbosa-Pereira, L., Sendón, R., Buonocore, G. G., Stanzione, M., Coelho, A., Correia, C. B., Saraiva, M., Quirós, A. R.-B. d., Vilarinho, F., Khwaldia, K., Silva, A. S., & Ramos, F. (2023). LDPE and PLA Active Food Packaging Incorporated with Lemon by-Products Extract: Preparation, Characterization and Effectiveness to Delay Lipid Oxidation in Almonds and Beef Meat. Foods, 12(13), 2450. https://doi.org/10.3390/foods12132450











