New Hydrolyzable Tannin with Potent Antioxidant and α-Glucosidase Inhibitory Activity from Black Tea Produced from Camellia taliensis
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure
2.2. Chemicals and Reagents
2.3. Materials
2.4. HPLC and LC-MS Analysis
2.5. Extraction and Isolation
2.6. Compound 1
2.7. Antioxidant Activity
2.8. α-Glucosidase Inhibitory Activity
3. Results and Discussion
3.1. HPLC and LC-MS Analysis
3.2. Identification of Compounds 1–32
3.3. Antioxidant Activity
3.4. α-Glucosidase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.-Q.; He, M.-Z.; Wang, R.-Y.; Zhou, D.-P.; Liu, X.-H.; Li, J.-H.; Yang, G.-R. An overview of Camellia taliensis. J. Anhui Agric. Sci. 2022, 50, 12–17. (In Chinese) [Google Scholar]
- Zhu, L.-F.; Xu, M.; Zhu, H.-T.; Wang, D.; Yang, S.-X.; Yang, C.-R.; Zhang, Y.-J. New flavan-3-ol dimer from green tea produced from Camellia taliensis in the Ai-Lao mountains of southwest China. J. Agric. Food Chem. 2012, 60, 12170–12176. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, M.; Zhu, H.-T.; Zhang, M.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. Theaflavoids A-C, new flavan-3-ols with potent α-glucosidase inhibitory activity from Yunnan black tea ‘Jin-Ya’. LWT-Food Sci. Technol. 2022, 168, 113918. [Google Scholar] [CrossRef]
- Li, N.; Zhu, H.-T.; Wang, D.; Zhang, M.; Yang, C.-R.; Zhang, Y.-J. New flavoalkaloids with potent α-glucosidase and acetylcholinesterase inhibitory activities from Yunnan black tea ‘Jin-Ya’. J. Agric. Food Chem. 2020, 68, 7955–7963. [Google Scholar] [CrossRef] [PubMed]
- Indrawati, R.; Zubaidah, E.; Sutrisno, A.; Limantara, L.; Yusuf, M.M.; Brotosudarmo, T.H.P. Visible light-induced antibacterial activity of pigments extracted from dregs of green and black teas. Scientifica 2021, 2021, 5524468. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsuo, Y. Production mechanisms of black tea polyphenols. Chem. Pharm. Bull. 2020, 68, 1131–1142. [Google Scholar] [CrossRef]
- Collier, P.D.; Bryce, T.; Mallows, R.; Thomas, P.E.; Frost, D.J.; Korver, O.; Wilkins, C.K. The theaflavins of black tea. Tetrahedron 1973, 29, 125–142. [Google Scholar] [CrossRef]
- Bryce, T.; Collier, P.D.; Mallows, R.; Thomas, P.E.; Frost, D.J.; Wilkins, C.K. Three new theaflavins from black tea. Tetrahedron Lett. 1972, 13, 463–466. [Google Scholar] [CrossRef]
- Shii, T.; Tanaka, T.; Watarumi, S.; Matsuo, Y.; Miyata, Y.; Tamaya, K.; Tamaru, S.; Tanaka, K.; Matsui, T.; Kouno, I. Polyphenol composition of a functional fermented tea obtained by tea-rolling processing of green tea and loquat leaves. J. Agric. Food Chem. 2011, 59, 7253–7260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, H.-M.; Du, Y.-M.; Yan, R.-A.; Ou, S.-Y.; Chen, T.-F.; Wang, Y.; Zhou, L.-X.; Fu, L. C-Geranylated flavanones from YingDe black tea and their antioxidant and α-glucosidase inhibition activities. Food Chem. 2017, 235, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Watarumi, S.; Fujieda, M.; Kouno, I. New black tea polyphenol having -ethyl-2 pyrrolidinone moiety derived from tea amino acid theanine: Isolation, characterization and partial synthesis. Food Chem. 2005, 93, 81–87. [Google Scholar] [CrossRef]
- Wang, W.; Tang, X.; Hua, F.; Ling, T.-J.; Wan, X.-C.; Bao, G.-H. Camellimidazole A–C, three methylene-bridged dimeric imidazole alkaloids from Keemun black Tea. Org. Lett. 2018, 20, 2672–2675. [Google Scholar] [CrossRef]
- Yang, C.-R.; Zhang, Y.-J.; Gao, D.-F.; Chen, K.-K.; Jiang, H.-J. The evaluation of germplasm resource of Camellia taliensis and origin of cultivated Camellia sinensis var. assamica. Tea Sci. Technol. 2008, 1–4. (In Chinese) [Google Scholar]
- Ming, T.-L. A revision of Camellia Sect. Thea. Acta Bot. Yunnanica 1992, 14, 115–132. (In Chinese) [Google Scholar]
- Jiang, H.-B.; Wang, Y.-G.; Tang, Y.-C.; Song, W.-X.; Li, Y.-Y.; Ji, P.-Z.; Huang, X.-Q. Investigation of wild tea plant (Camellia taliensis) germplasm resource from Yunnan, China. Southwest China J. Agric. Sci. 2009, 22, 1153–1157. (In Chinese) [Google Scholar]
- Zhu, L.-F.; Dong, H.-Z.; Yang, S.-X.; Zhu, H.-T.; Xu, M.; Zeng, S.-F.; Yang, C.-R.; Zhang, Y.-J. Chemical compositions and antioxidant activity of essential oil from green tea produced from Camellia taliens·is (Theaceae) in Yuanjiang, southwestern China. Plant Divers. Resour 2012, 34, 409–416. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, D.-W.; Yang, J.-B.; Yang, S.-X.; Kato, K.; Luo, J.-P. Genetic diversity and domestication origin of tea plant Camellia taliensis (Theaceae) as revealed by microsatellite markers. BMC Plant Biol. 2014, 14, 14. [Google Scholar] [CrossRef]
- Gao, D.-F.; Zhang, Y.-J.; Yang, C.-R.; Chen, K.-K.; Jiang, H.-J. Phenolic antioxidants from green tea produced from Camellia taliensis. J. Agric. Food Chem. 2008, 56, 7517–7521. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-H.; Zhu, H.-T.; Yan, H.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. C-8 N-ethyl-2-pyrrolidinone-substituted flavan-3-ols from the leaves of Camellia sinensis var. pubilimba. J. Agric. Food Chem. 2018, 66, 7150–7155. [Google Scholar] [CrossRef] [PubMed]
- Haggett, P.; Gunawardena, K.A. Determination of population thresholds for settlement functions by the reed-muench method. Prof. Geogr. 1964, 16, 6–9. [Google Scholar] [CrossRef]
- Peng, Z.-C.; Hao, J.; Pan, X.-G.; Ye, X.-S.; Li, X.-X.; Yin, W.-F.; Zhang, W.-K.; Xu, J.-K. Isolation and identification of chemical constituents from fruit of Cornus officinalis. Chin Trad Herb Drugs 2021, 52, 4480–4486. (In Chinese) [Google Scholar]
- Santos, S.C.; Pereira, M.O.A.; Santos, K.B.; Ferri, P.H. Bioactive compounds of fruit parts of three Eugenia uniflora biotypes in four ripening stages. Chem. Biodivers. 2021, 18, e2100704. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-S.; Hao, Z.-Y.; Zheng, X.-K.; Kuang, H.-X. Chemical constituents from leaves of Celastrus gemmatus Loes. Yao Xue Xue Bao Acta Pharm. Sin. 2007, 42, 625–630. (In Chinese) [Google Scholar]
- Yagi, K.; Goto, K.; Nanjo, F. Identification of a major polyphenol and polyphenolic composition in leaves of Camellia irrawadiensis. Chem. Pharm. Bull 2009, 57, 1284–1288. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Fang, Z.-G.; Masayuki, Y. Chemical constituents from Canavalia gladiata. J. Shenyang Pharm. Univ. 2007, 24, 676–678. (In Chinese) [Google Scholar]
- Liang, Y.; Xu, M.; Tang, C.; Fu, K.; Li, X.; Song, Y.; Zhang, J.; Wang, Z. The study of chemical components in Qishiwei Zhenzhu pills and its anti-apoptotic mechanism in cerebral ischemic based on LC-MS and network pharmacology. J. Ethnopharmacol. 2023, 302, 115891. [Google Scholar] [CrossRef]
- Li, C.-W.; Dong, H.-J.; Cui, C.-B. The synthesis and antitumor activity of twelve galloyl glucosides. Molecules 2015, 20, 2034–2060. [Google Scholar] [CrossRef]
- Guo, H.-L.; Zhou, J.-Y. Isolation and struture elucidation of glycosides in n-butanol extracts from rhizome of Periploca calophylla. China J. Chin. Mater Med. 2005, 30, 44–46. (In Chinese) [Google Scholar]
- Zhang, Y.; Deng, S.; Li, X.-X.; Chen, Y.; Han, L.-F.; Wang, T. Isolation and identification of constituents from Leonurus japonicus II. Chin J. Med. Chem. 2013, 23, 480–485. (In Chinese) [Google Scholar]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Zang, E.-H.; Chen, Z.-W.; Zhang, C.-H.; Li, M.-H. Chemical constituents of Physochlaina physaloides (L.) G. Don (Solanaceae). Biochem. Syst. Ecol. 2021, 98, 104332. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Tsai, T.H.; Lin, L.C. Prenylflavonol, acylated flavonol glycosides and related compounds from Epimedium sagittatum. Phytochemistry 2007, 68, 2455–2464. [Google Scholar] [CrossRef]
- Tao, L.; Huang, J.; Zhao, Y.; Li, C. Chemical constituents in Buddleja albiflora. China J. Chin. Mater. Medica 2009, 34, 3043–3046. (In Chinese) [Google Scholar]
- Hasler, A.; Gross, G.A.; Meier, B.; Sticher, O. Complex flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry 1992, 31, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Devkota, H.P. Antioxidant phenolic constituents from the leaves of Acer ginnala var aidzuense. J. Nat. Remedies 2017, 17, 9–12. [Google Scholar] [CrossRef]
- Li, K.; Li, X.-M.; Wang, B.-G. Chemical constituents from herb of Gueldenstaedtia stenophylla. China J. Chin. Mater Med. 2008, 33, 1711–1713. (In Chinese) [Google Scholar]
- Zhao, A.-H.; Zhao, Q.-S.; Li, R.-T.; Sun, H.-D. Chemical constituents from Clerodendranthus spicatus. Acta Bot. Yunnanica 2004, 26, 563–568. (In Chinese) [Google Scholar]
- Miao, C.-P.; Hu, J.; Zhai, Y.-Z.; Song, F.; Xuan, Q.-C.; Chen, Y.-W.; Wu, S.-H. Identification and secondary metabolites of endophytic fungus PR20 from Paeonia delavayi. Nat. Prod. Res. Dev. 2012, 24, 1339–1442. (In Chinese) [Google Scholar]
- Ahmad, I.; Bashir, A.; Janbaz, K.; Uzair, M.; Ashraf, M. Urease inhibitors and antioxidants from Vernonia cinerascens. J. Chem. Soc. Pak. 2011, 33, 14–117. [Google Scholar]
- Balo, T.; Sapi, A.; Kiss, A.; Raimbaud, E.; Paysant, J.; Cattin, M.-E.; Berger, S.; Kotschy, A.; Faucher, N. Synthesis of thieno[2,3-c]pyridine derived GRK2 inhibitors. Monatsh Chem. 2022, 1–19. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J.H.; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Kuczkowiak, U.; Petereit, F.; Nahrstedt, A. Hydroxycinnamic acid derivatives obtained from a commercial crataegus extract and from authentic Crataegus spp. Sci. Pharm. 2014, 82, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, K.; Guo, Z.; Zhao, Y.; Cheng, F.; Zhang, H. Chemical constituents from leaves of Boehmeria nivea. China J. Chin. Mater. Medica 2010, 35, 1432–1434. (In Chinese) [Google Scholar]
- Wu, Z.-J.; Ouyang, M.-A.; Yang, C.-R. Polyphenolic constituents of Salvia sonchifolia. Acta Bot. Yunnanica 1999, 21, 393–398. (In Chinese) [Google Scholar]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.E.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Silva Bolzani, V.D.; Siqueira Silva, D.H.; Ishii, H.; Berova, N.; Nakanishi, K. The absolute configuration of 1-(3′,4′-dihydroxycinnamoyl)cyclopentane-2,3-diol from the amazonian tree Chimarrhis turbinata. J. Nat. Prod. 2006, 69, 1046–1050. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Park, S.; Na Kim, Y.; Kwak, H.J.; Jeong, E.J.; Kim, S.H. Estrogenic activity of constituents from the rhizomes of Rheum undulatum Linné. Bioorganic Med. Chem. Lett. 2018, 28, 552–557. [Google Scholar] [CrossRef]
- Matsuo, Y.; Iki, M.; Okubo, C.; Saito, Y.; Tanaka, T. Conformationally flexible ellagitannins: Conformational analysis of davidiin and punicafolin via DFT calculation of 1H NMR coupling constants. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Tanaka, T.; Iwata, H.; Sakamoto, S.; Hirose, Y.; Kouno, I. Ellagitannins and lignan glycosides from Balanophora japonica (Balanophoraceae). Chem. Pharm. Bull. 2005, 53, 339–341. [Google Scholar] [CrossRef]
- Li, C.-H.; Yang, X.-D.; Zhao, J.-F.; Li, L. The chemical constituents of Breynia rostrata. Yao Xue Xue Bao Acta Pharm. Sin. 2006, 41, 125–127. (In Chinese) [Google Scholar]
- Saijo, R.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. LXXXIV. Isolation and characterization of five new hydrolyzable tannins from the bark of Mallotus japonicus. Chem. Pharm. Bull. 1989, 37, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Matsuo, Y.; Saito, Y.; Tanaka, T. Formation of dehydrohexahydroxydiphenoyl esters by oxidative coupling of galloyl esters in an aqueous medium involved in ellagitannin biosynthesis. Chem.Asian J. 2021, 16, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. C. Reaction of dehydrohexahydroxydiphenic acid esters with bases, and its application to the structure determination of pomegranate tannins, granatins A and B. Chem. Pharm. Bull. 1990, 38, 2424–2428. [Google Scholar] [CrossRef]
- Yang, X.; Tomas-Barbera, F.A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 2019, 67, 5394–5404. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure-activity relationship study. Drug Chem. Toxicol. 2016, 40, 416–424. [Google Scholar] [CrossRef]
- Tanaka, T.; Zhang, H.; Jiang, Z.-H. Relationship between hydrophobicity and structure of hydrolyzable tannins, and association of tannins with crude drug constituents in aqueous solution. Chem. Pharm. Bull. 1997, 45, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
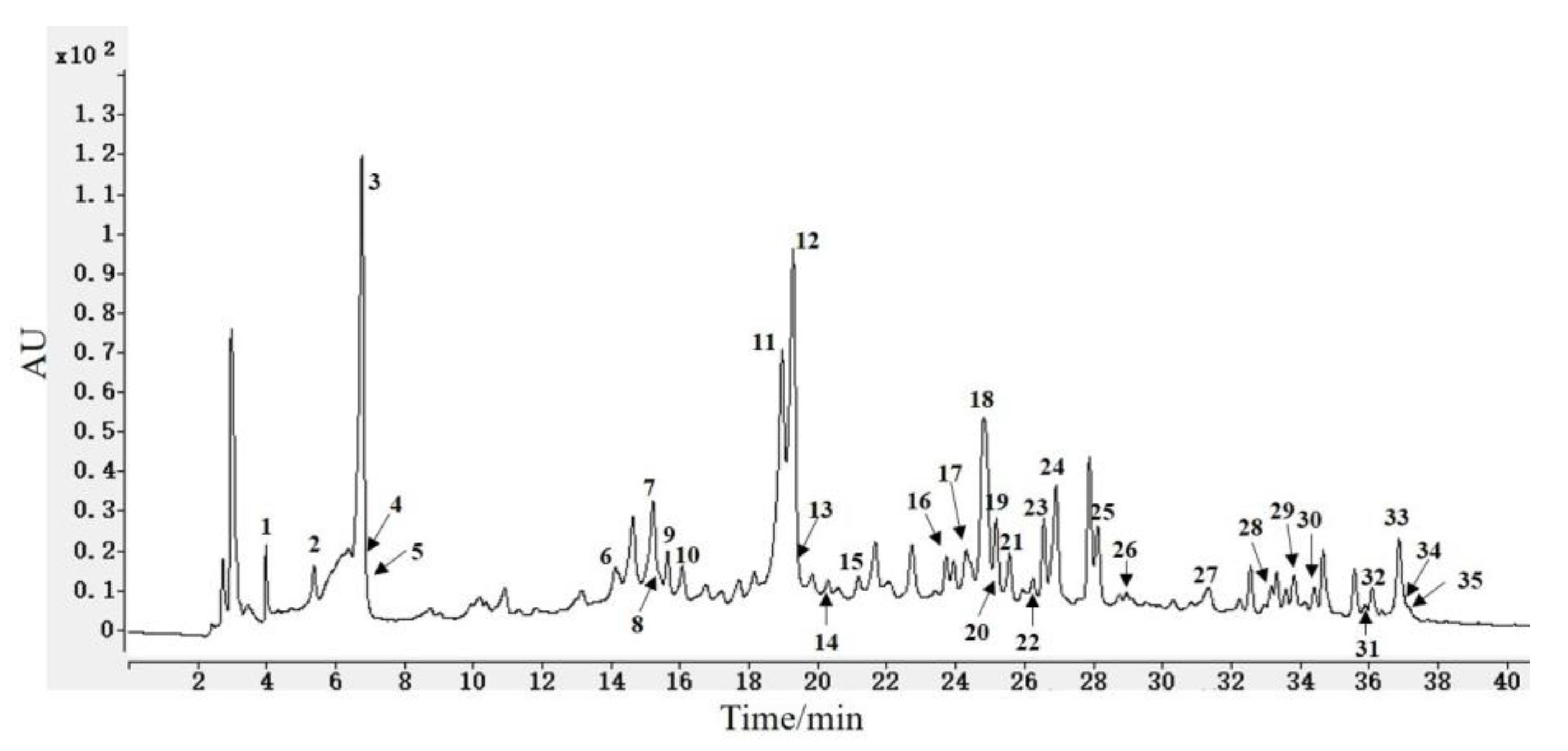
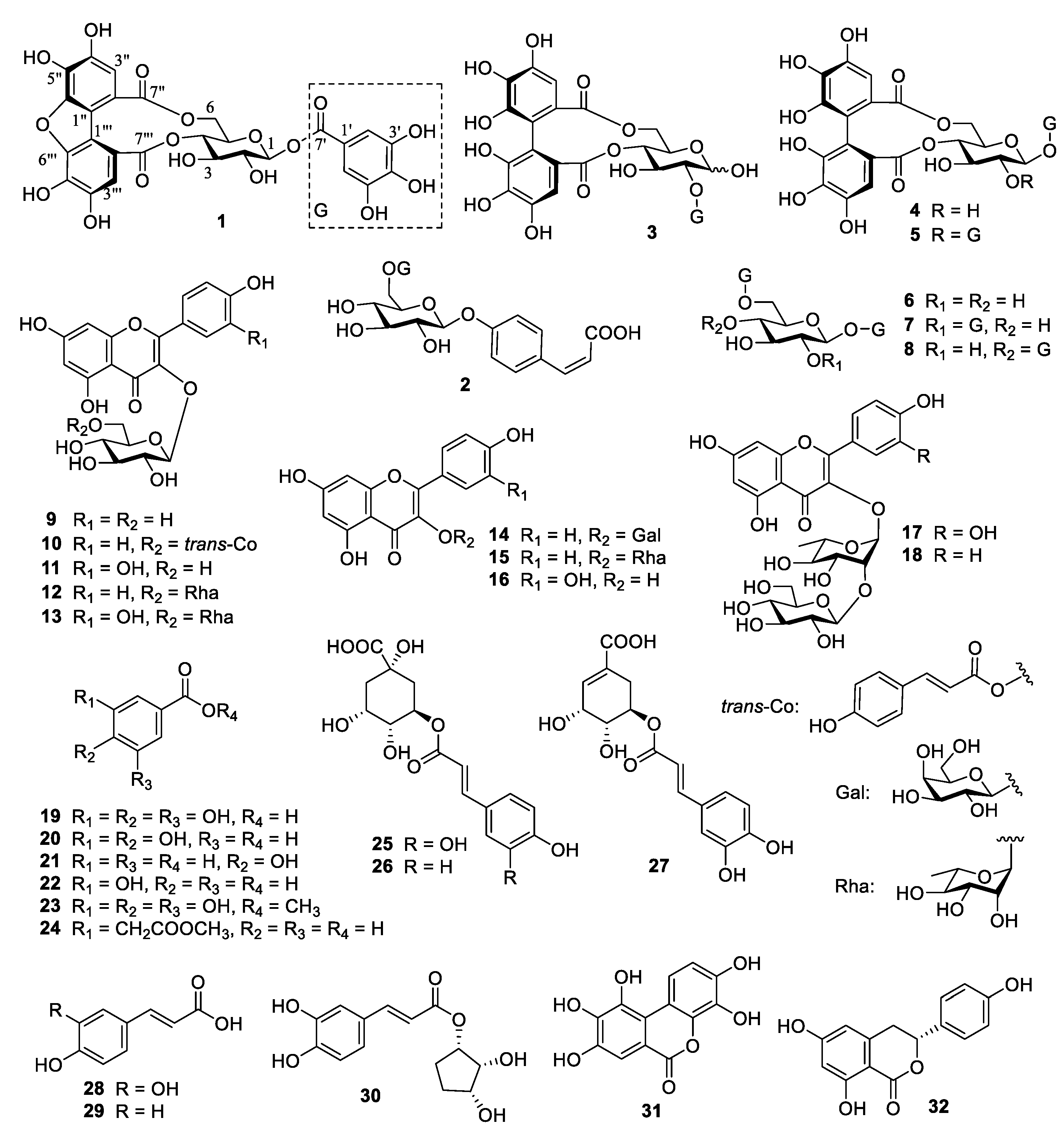
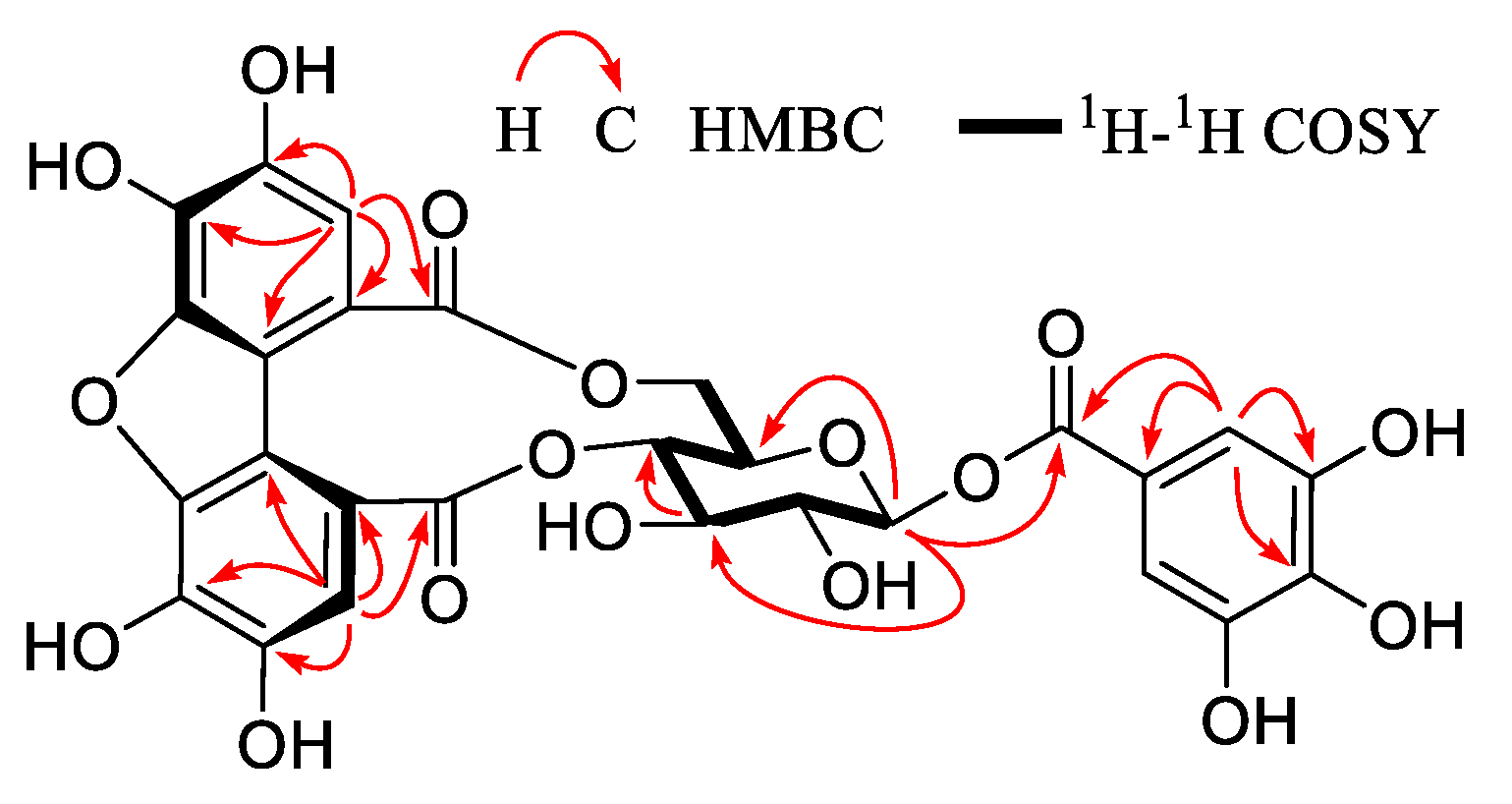
| Peak | tR (min) | MW | MS− | MS2− | MS+ | Compounds |
|---|---|---|---|---|---|---|
| 1 | 4.04 | 174 | 173[M − H]− | 127, 87, 45 | Theanine | |
| 2 | 5.43 | 332 | 331[M − H]− | 241, 211, 169[M − H − glu]−, 151 | β-Glucogallin | |
| 3 | 6.82 | 170 | 169[M − H]− | 125[M − COO]− | 19 | |
| 4 | 6.82 | 126 | 125[M − H]− | Pyrogallol | ||
| 5 | 6.82 | 344 | 343[M − H]− | 345[M + H]+ | Theogallin | |
| 6 | 14.17 | 484 | 483[M − H]− | 357, 313[M − H − gallic acid]−, 271, 210, 169, 125[169 − COO]− | 6 | |
| 7 | 15.31 | 184 | 183[M − H]− | 169[M − CH3]−, 125[M − COOCH3]− | 23 | |
| 8 | 15.55 | 290 | 289[M − H]− | 245[M − H − COO]−, 203[245 − C2H2O]−, 137[1,3A]−, 125[1,4A]−, 85 | (+) Catechin | |
| 9 | 15.70 | 634 | 633[M − H]− | 577, 463[M − H − gallic acid]−, 425, 387, 301[463 − glu]−, 274, 232, 169[gallic acid − H]− | 4 | |
| 10 | 16.12 | 354 | 353[M − H]− | 191[quinic acid − H]−, 179[caffeic acid − H]−, 173[191 − H2O]−, 135, 69 | 25 | |
| 11 | 18.71 | 290 | 289[M − H]− | 245[M − H − COO]−, 205[M − H – 2 · C2H2O]−, 179[M − H − C6H6O2]−, 151[179 − CO]−, 125[1,4A]− | (−)-Epicatechin | |
| 12 | 19.22 | 458 | 457[M − H]− | 411, 331, 305[M − H − C7H4O4]−, 287[M − H − C7H6O5]−, 233, 199, 169[gallic acid − H]−, 125[287(1,4A)]− | (−)-Epigallocatechin-3-O-gallate | |
| 13 | 19.34 | 338 | 337[M − H]− | 191[quinic acid − H]−, 173[191 − H2O]−, 163[coumaric acid − H]−, 155, 137, 119, 93 | 361[M + Na]+ | 26 |
| 14 | 20.36 | 636 | 635[M − H]− | 483[M − galloyl]−, 465[M − H − gallic acid]−, 411, 313[465 − H − galloyl]−, 169[gallic acid − H]−, 125[169 − COO]− | 8 | |
| 15 | 21.24 | 178 | 177[M − H]− | 132, 109, 89, 65 | 5,7-Dihydroxycoumarin | |
| 16 | 23.79 | 610 | 609[M − H]− | 466, 308[glu + rha]−, 301[quercetin]−, 300, 190, 113 | 13 | |
| 17 | 24.37 | 302 | 301[M − H]− | 185, 79 | Not identified | |
| 18 | 24.85 | 464 | 463[M − H]− | 301[quercetin]−, 271, 191, 169 | 11 | |
| 19 | 24.96 | 442 | 441[M − H]− | 331[M − H − C6H6O2]−, 289[M − galloyl]−, 271[M − H − gallic acid]−, 193[331 − C7H6O3]−, 169[gallic acid − H]−, 125[169 − COO]− | 443[M + H]+ | (−)-Epicatechin-3-O-gallate |
| 20 | 25.25 | 464 | 463[M − H]− | 465[M + H]+ | Quercetin 3-O-β-D-galactopyranoside | |
| 21 | 25.63 | 594 | 593[M − H]− | 447[M − rha]−, 285[447 − glu]− | 12 | |
| 22 | 26.31 | 610 | 609[M − H]− | 302[quercetin]− | 17 | |
| 23 | 26.62 | 594 | 593[M − H]− | 561, 494, 453, 413, 365, 321, 285, 230, 159, 125, 93 | 595[M + H]+ | 18 |
| 24 | 27.16 | 448 | 447[M − H]− | 327, 285[M − H − glu]−, 255, 227, 174, 151, 127 | 449[M + H]+ | 9 |
| 25 | 28.21 | 448 | 447[M − H]− | 327, 285[M − H − gal]−, 255, 227, 198, 151, 93 | 449[M + H]+ | 14 |
| 26 | 29.04 | 426 | 425[M − H]− | 273[M − galloyl]−, 255[M − H − gallic acid]−, 229[273 − COO]−, 169[gallic acid − H]−, 151, 125[255(1,4A)]− | (−)-Epiafzelechin 3-O-gallate | |
| 27 | 31.45 | 432 | 431[M − H]− | 285[M − H − rha]−, 255, 227 | 15 | |
| 28 | 33.19 | 902 | 901[M − H]− | 755[M − H − rha]−, 603, 447[755 − H − glu-cou]−, 416, 327, 301[447 − rha] − | 903[M + H]+ | Quercetin-3-O-cou-rha-glu-rhamnopyranoside |
| 29 | 33.90 | 580 | 579[M − H]− | Not identified | ||
| 30 | 34.28 | 886 | 885[M − H]− | 739[M − H − rha]−, 609, 577[739 − glu]−, 521, 431[577 − H − cou]−, 408, 285[431 − rha]− | Kaempferol-3-O-cou-rha-glu-rhamnopyranoside | |
| 31 | 35.95 | 594 | 593[M − H]− | 447[M − 2H − cou]−, 285[447 − glu]−, 148 | 10 | |
| 32 | 36.15 | 716 | 715[M − H]− | 563[M − galloyl]−, 545[M − H − gallic acid]−, 502, 407, 319 | Theaflavin-3-gallate | |
| 33 | 36.94 | 868 | 867[M − H]− | 715[M − galloyl]−, 697[M − H − gallic acid]−, 571, 545[715 − gallic acid]−, 483, 441, 372, 257, 169 | Theaflavin-3,3′-digallate | |
| 34 | 37.07 | 716 | 715[M − H]− | 679, 601, 563[M − galloyl]−, 545[M − H − gallic acid]−, 316 | Theaflavin-3′-digallate | |
| 35 | 37.24 | 302 | 301[M − H]− | 257, 179, 151, 107 | 16 |
| No. | 1 | 4 | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| Glucose-1 | 95.7, d | 5.73, d, J = 8.2 Hz | 96.2, s | 5.69, d, J = 8.1 Hz |
| 2 | 74.2, d | 3.65, dd, J = 9.4, 8.2 Hz | 74.7, t | 3.63, dd, J = 9.4, 8.1 Hz |
| 3 | 74.9, d | 3.86, t, J = 9.4 Hz | 76.0, d | 3.74, t, J = 9.4 Hz |
| 4 | 76.2, d | 5.00, m | 73.2, t | 4.87, t, J = 9.4 Hz |
| 5 | 71.7, d | 3.98, m | 73.7, d | 4.06, dd, J = 9.4, 6.5 Hz |
| 6 | 67.6, t | a 4.78, m | 64.3, t | a 5.24, dd, J = 13.3, 6.5 Hz |
| b 4.01, m | b 3.83, dd, J = 13.3, 1.2 Hz | |||
| Galloyl-1′ | 120.4, s | 120.5, s | ||
| 2′,6′ | 110.6, d | 7.12, s | 110.5, d | 7.16, s |
| 3′,5′ | 146.6, s | 146.6, s | ||
| 4′ | 140.6, s | 140.5, s | ||
| 7′ | 166.8, s | 166.8, s | ||
| Acyl-1″ | 115.9, s | 116.8, s | ||
| 2″ | 117.0, s | 126.3, s | ||
| 3″ | 116.2, s | 7.20, s | 108.6, d | 6.71, s |
| 4″ | 145.2, s | 145.9, s | ||
| 5″ | 136.1, s | 137.6, s | ||
| 6″ | 147.6, s | 144.9, s | ||
| 7″ | 170.0, s | 169.6, s | ||
| Acyl-1‴ | 115.4, s | 116.6, s | ||
| 2‴ | 119.8, s | 126.6, s | ||
| 3‴ | 112.1, s | 7.05, s | 108.3, d | 6.57, s |
| 4‴ | 145.5, s | 145.8, s | ||
| 5‴ | 134.0, s | 137.3, s | ||
| 6‴ | 147.7, s | 144.8, s | ||
| 7‴ | 171.5, s | 169.9, s | ||
| Samples | DPPH | ABTS+ | ||
|---|---|---|---|---|
| IC50 (μM) b | Inhibition Ratio (%) c | IC50 (μM) b | Inhibition Ratio (%) c | |
| 1 | 338.9 ± 24.7 | 110.7 ± 2.1 | ||
| 2 | 502.1 ± 14.0 | 400.4 ± 9.6 | ||
| 3 | 941.3 ± 191.0 | 106.0 ± 0.5 | ||
| 4 | 311.5 ± 10.8 | 84.8 ± 0.4 | ||
| 5 | 300.8 ± 79.6 | 75.6 ± 2.1 | ||
| 6 | 217.3 ± 27.4 | 99.7 ± 1.4 | ||
| 7 | 532.1 ± 38.0 | 128.4 ± 0.9 | ||
| 8 | 156.5 ± 26.1 | 77.2 ± 0.7 | ||
| 9 | 0.97 | 0.19 | ||
| 10 | 6.28 | 11.55 | ||
| 11 | 2304.0 ± 372.3 | 322.8 ± 0.6 | ||
| 12 | 8.58 | 4.24 | ||
| 13 | 900.3 ± 63.4 | 265.8 ± 5.4 | ||
| 14 | −0.94 | 4.03 | ||
| 15 | 1.04 | 8.00 | ||
| 16 | 1141.6 ± 206.8 | 206.2 ± 2.6 | ||
| 17 | 2616.0 ± 500.5 | 480.9 ± 4.1 | ||
| 18 | 1.08 | 5.89 | ||
| 19 | 237.2 ± 22.6 | 201.7 ± 3.4 | ||
| 20 | 2119.3 ± 335.7 | 462.0 ± 9.3 | ||
| 21 | 3.32 | 3.21 | ||
| 22 | 7.58 | 7.00 | ||
| 23 | 298.8 ± 32.3 | 187.4 ± 2.7 | ||
| 25 | 455.5 ± 45.5 | 272.0 ± 1.6 | ||
| 26 | 6.92 | 6.82 | ||
| 27 | 722.2 ± 43.2 | 545.1 ± 12.8 | ||
| 28 | 478.7 ± 33.8 | 198.7 ± 4.1 | ||
| 29 | 1.55 | 8.12 | ||
| 30 | 547.5 ± 61.5 | 306.9 ± 3.3 | ||
| 31 | 335.2 ± 24.0 | 107.4 ± 0.5 | ||
| 32 | 3938.3 ± 825.2 | 560.3 ± 3.3 | ||
| Ascorbic acid d | 1197.0 ± 87.7 | |||
| Trolox d | 404.8 ± 6.2 | |||
| Samples | IC50 (μM) b | Inhibition Ratio (%) c |
|---|---|---|
| Quercetin d | 5.75 ± 0.78 | 64.81 ± 3.30 e |
| Acarbose d | 223.30 ± 9.98 | 65.02 ± 1.19 f |
| 1 | 1.77 ± 0.05 | |
| 2 | 18.29 ± 1.33 | |
| 3 | 1.74 ± 0.03 | |
| 4 | 1.96 ± 0.06 | |
| 5 | 0.67 ± 0.04 | |
| 6 | 38.42 ± 1.57 | |
| 7 | 1.96 ± 0.05 | |
| 8 | 2.01 ± 0.02 | |
| 19 | 3.01 ± 1.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Li, N.; Zhu, H.-T.; Zhang, M.; Duan, Z.-H.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. New Hydrolyzable Tannin with Potent Antioxidant and α-Glucosidase Inhibitory Activity from Black Tea Produced from Camellia taliensis. Foods 2023, 12, 2512. https://doi.org/10.3390/foods12132512
Chen M, Li N, Zhu H-T, Zhang M, Duan Z-H, Wang D, Yang C-R, Zhang Y-J. New Hydrolyzable Tannin with Potent Antioxidant and α-Glucosidase Inhibitory Activity from Black Tea Produced from Camellia taliensis. Foods. 2023; 12(13):2512. https://doi.org/10.3390/foods12132512
Chicago/Turabian StyleChen, Min, Na Li, Hong-Tao Zhu, Man Zhang, Zhao-Hong Duan, Dong Wang, Chong-Ren Yang, and Ying-Jun Zhang. 2023. "New Hydrolyzable Tannin with Potent Antioxidant and α-Glucosidase Inhibitory Activity from Black Tea Produced from Camellia taliensis" Foods 12, no. 13: 2512. https://doi.org/10.3390/foods12132512
APA StyleChen, M., Li, N., Zhu, H.-T., Zhang, M., Duan, Z.-H., Wang, D., Yang, C.-R., & Zhang, Y.-J. (2023). New Hydrolyzable Tannin with Potent Antioxidant and α-Glucosidase Inhibitory Activity from Black Tea Produced from Camellia taliensis. Foods, 12(13), 2512. https://doi.org/10.3390/foods12132512







