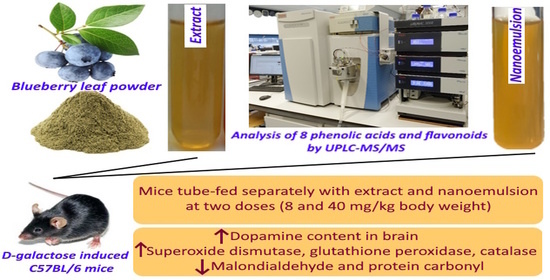
Analysis of Phenolic Acids and Flavonoids in Rabbiteye Blueberry Leaves by UPLC-MS/MS and Preparation of Nanoemulsions and Extracts for Improving Antiaging Effects in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.3. Analysis of Individual Phenolic Acid and Flavonoid by UPLC-MS/MS
2.4. Quantitation of Individual Phenolic Acid and Flavonoid
2.5. Preparation of Rabbiteye Blueberry Leaf Nanoemulsion
2.6. Determination of Nanoemulsion Characteristics
2.6.1. Particle Size and Polydispersity Index
2.6.2. Zeta Potential
2.6.3. Transmission Electron Microscopy
2.6.4. Encapsulation Efficiency
2.7. Stability of Nanoemulsion
2.8. Animal Experiment
- Control group (C): mice were provided with sterilized water by subcutaneous injection once a day for the first 6 weeks and then tube feeding with sterilized water, starting at the 7th week once a day for another 6 weeks.
- Induction group (D): mice were provided with D-galactose at a dose of 300 mg/kg bw by subcutaneous injection once a day for the first 6 weeks and then tube feeding with sterilized water starting at the 7th week once a day for another 6 weeks.
- Low-dose extract (LE): mice were provided with D-galactose at a dose of 300 mg/kg bw by subcutaneous injection once a day for the first 6 weeks and then tube feeding with blueberry extract (dissolved in 0.2 mL of deionized water) at a dose of 8 mg/kg bw containing 0.2 mg of four phenolic acids starting at the 7th week once a day for another 6 weeks.
- High-dose extract (HE): mice were provided with D-galactose at a dose of 300 mg/kg bw by subcutaneous injection once a day for the first 6 weeks and then tube feeding with blueberry extract (dissolved in 0.2 mL of deionized water) at a dose of 40 mg/kg bw containing 1 mg of four phenolic acids starting at the 7th week once a day for another 6 weeks.
- Low-dose nanoemulsion (LN): mice were provided with D-galactose at a dose of 300 mg/kg bw by subcutaneous injection once a day for the first 6 weeks and then tube feeding with 0.2 mL of nanoemulsion at a dose of 8 mg/kg bw containing 0.2 mg of four phenolic acids starting at the 7th week once a day for another 6 weeks.
- High-dose nanoemulsion (HN): mice were provided with D-galactose at a dose of 300 mg/kg bw by subcutaneous injection once a day for the first 6 weeks and then tube feeding with 0.2 mL of nanoemulsion at a dose of 40 mg/kg bw containing 1 mg of four phenolic acids starting at the 7th week once a day for another 6 weeks.
2.9. Determination of Activities of Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px), and Catalase (CAT) in Mice Liver
2.10. Determination of Dopamine, Malondialdehyde (MDA), and Protein Carbonyl (PC) Contents in Mice Brain
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Solvent Variety on Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in Rabbiteye Blueberry Leaves
3.2. Analysis of Phenolic Acids and Flavonoids in Rabbiteye Blueberry Leaves by UPLC-MS/MS
3.3. Method Validation and Quantitation
3.4. Characteristics of Rabbiteye Blueberry Nanoemulsion
3.5. Animal Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebenezer, P.J.; Wilson, C.B.; Wilson, L.D.; Nair, A.R. The anti-inflammatory effects of blueberries in an animal model of post-traumatic stress disorder (PTSD). PLoS ONE 2016, 11, e0160923. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Li, C.; Feng, J.; Huang, W.Y.; An, X.T. Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vaccinium ashei) in Nanjing. J. Agric. Food Chem. 2013, 61, 523–531. [Google Scholar] [CrossRef]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating properties. Exp. Eye Res. 2016, 145, 258–268. [Google Scholar] [CrossRef]
- Li, H.; Park, H.M.; Ji, H.S.; Han, J.; Kim, S.K.; Park, H.Y.; Jeong, T.S. Phenolic-enriched blueberry-leaf extract attenuates glucose homeostasis, pancreatic β-cell function, and insulin sensitivity in high-fat diet–induced diabetic mice. Nutr. Res. 2020, 73, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, H.; Nagao, K.; Higa, K.; Shirouchi, B.; Inoue, N.; Hidaka, F.; Kai, T.; Yanagita, T. Effect of Vaccinium ashei reade leaves on angiotensin converting enzyme activity in vitro and on systolic blood pressure of spontaneously hypertensive rats in vivo. Biosci. Biotechnol. Biochem. 2007, 71, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and their nanoformulations: Protective effects against human diseases. Life 2022, 12, 1639. [Google Scholar] [CrossRef]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhou, C.L.; Chen, F.P.; Han, D.; Wang, C.Y.; Li, J.X.; Chi, Z.; Liu, C.G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Gladyshev, V.N. Aging: Progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 2016, 15, 594–602. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ying, Y.; Yun, J.; Guoyao, W.; Kaiji, S.; Zhaolai, D.; Zhenlong, W. Dietary L-methionine restriction decreases oxidative stress in porcine liver mitochondria. Exp. Gerontol. 2015, 65, 35–41. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Mitochondrial dysfunction in brain aging: Role of oxidative stress and cardiolipin. Neurochem. Int. 2011, 58, 447–457. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W.; Schipper, H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 2007, 6, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Yang, D.; Zhang, W.; Lu, Y.; Zhang, M.; Wang, L.; Li, X.; Zhou, L.; Wu, Q.; Pan, W.; et al. Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 85, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Liu, F.; Gao, Z.; Kong, D.; Hu, X.; Shi, D.; Bao, Z.; Yu, Z. The anti-inflamm-aging and hepatoprotective effects of huperzine A in D-galactose-treated rats. Mech. Ageing Dev. 2013, 134, 89–97. [Google Scholar] [CrossRef]
- Kao, T.H.; Huang, C.W.; Chen, B.H. Functional components in Luffa cylindrica and their effects on anti-inflammation of macrophage cells. Food Chem. 2012, 135, 386–395. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsai, T.Y.; Chen, B.H. Effects of black garlic extract and nanoemulsion on the deoxy corticosterone acetate-salt induced hypertension and its associated mild cognitive impairment in rats. Antioxidants 2021, 10, 1611. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, B.H. A comparative study on improving streptozotocin-induced Type 2 diabetes in rats by hydrosol, extract and nanoemulsion prepared from cinnamon leaves. Antioxidants 2023, 12, 29. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Chen, B.H. A comparative study on inhibition of breast cancer cells and tumors in mice by carotenoid extract and nanoemulsion prepared from sweet potato (Ipomoea batatas L.) peel. Pharmaceutics 2022, 14, 980. [Google Scholar] [CrossRef]
- Cho, E.; Park, J.; Hwang, E.M.; Kim, H.W.; Park, J.Y. 14-3-3γ haploinsufficiency leads to altered dopamine pathway and Parkinson’s disease-like motor incoordination in mice. Mol. Brain 2023, 16, 2. [Google Scholar] [CrossRef]
- Fan, R.; Schrott, L.M.; Snelling, S.; Felty, J.; Graham, D.; McGauly, P.L.; Arnold, T.; Korneeva, N.L. Carbonyl-protein content increases in brain and blood of female rats after chronic oxycodone treatment. BMC Neurosci. 2020, 21, 4. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). SAS® 9.4 Output Delivery System: User’s Guide, Fifth Edition; SAS Institute Inc.: Cary, NC, USA, 2019. [Google Scholar]
- Hikmawanti, N.P.E.; Fatmawati, S.; Asri, A.W. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012060. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crops Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Xiaoyong, S.; Luming, C. Phenolic constituents, antimicrobial and antioxidant properties of blueberry leaves (V5). J. Food Nutr. Res. 2014, 2, 973–979. [Google Scholar] [CrossRef]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, L.H.; Huang, W. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Cezarotto, V.S.; Giacomelli, S.R.; Vendruscolo, M.H.; Vestena, A.S.; Cezarotto, C.S.; Da Cruz, R.C.; Maurer, L.H.; Ferreira, L.M.; Emanuelli, T.; Cruz, L. Influence of harvest season and cultivar on the variation of phenolic compounds composition and antioxidant properties in Vaccinium ashei leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef]
- TFDA. Analytical Method Validation in Food Chemistry; Taiwan Food and Drug Administration: Taipei, Taiwan, 2021.
- Tsai, Y.J.; Chen, B.H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar]
- Cerda-Opazo, P.; Gotteland, M.; Oyarzun-Ampuero, F.A.; Garcia, L. Design, development and evaluation of nanoemulsion containing avocado peel extract with anticancer potential: A novel biological active ingredient to enrich food. Food Hydrocoll. 2021, 111, 106370. [Google Scholar] [CrossRef]
- Govindan, S.; Johnson, E.E.R.; Christopher, J.; Shanmugam, J.; Thirumalairaj, V.; Gopalan, J. Antioxidant and anti-aging activities of polysaccharides from Calocybe indica var. APK2. Exp. Toxicol. Pathol. 2016, 68, 329–334. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Tsai, S.J.; Yin, M.C. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by D-galactose. Food Chem. Toxicol. 2012, 50, 3198–3205. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Piechal, A.; Blecharz-Klin, K.; Pyrzanowska, J.; Joniec-Maciejak, I.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Administration of protocatechuic acid affects memory and restores hippocampal and cortical serotonin turnover in rat model of oral D-galactose-induced memory impairment. Behav. Brain Res. 2019, 368, 111896. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Yu, J.; Yang, J.; Meng, F.; Jiang, X.; Zhu, C. Protective effects of enzyme degradation extract from Porphyra yezoensis against oxidative stress and brain injury in d-galactose-induced ageing mice. Br. J. Nutr. 2020, 123, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Colantoni, A.; Idilman, R.; de Maria, N.; Duffner, L.A.; Van Thiel, D.H.; Witte, P.L.; Kovacs, E.J. Evidence of oxidative injury during aging of the liver in a mouse model. J. Am. Aging Assoc. 2001, 24, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kuang, Z.; Zhang, J.; Zhao, X.; Pu, P.; Yan, J. The preventive effect of Apocynum venetum polyphenols on D-galactose-induced oxidative stress in mice. Exp. Ther. Med. 2020, 19, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wei, Y.; Chen, J.; Chen, X.; Zhang, Y. Anti-aging and redox state regulation effects of A-type proanthocyanidins-rich cranberry concentrate and its comparison with grape seed extract in mice. J. Funct. Foods 2017, 30, 63–73. [Google Scholar] [CrossRef]
- Li, J.J.; Mo, L.; Song, J.L. Improvement effect of Ficus vasculosa ethanol extract on D-galactose-induced mice aging. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Yu, X.; Yang, M.; Dong, J.; Shen, R. Comparative analysis of the antioxidant capacities and phenolic compounds of oat and buckwheat vinegars during production processes. J. Food Sci. 2018, 83, 844–853. [Google Scholar] [CrossRef]
- Hedden, T.; Gabrieli, J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef]
- Pignatelli, A.; Belluzzi, O. Dopaminergic neurones in the main olfactory bulb: An overview from an electrophysiological perspective. Front. Neuroanat. 2017, 11, 7. [Google Scholar] [CrossRef]
- Zhou, X.; Pardue, M.T.; Iuvone, P.M.; Qu, J. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.M.; Gaskill, P.J. Where is dopamine and how do immune cells see it?: Dopamine-mediated immune cell function in health and disease. J. Neuroimmune Pharmacol. 2020, 15, 114–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Villar, V.A.M.; Jose, P.A.; Zeng, C. Renal dopamine receptors and oxidative stress: Role in hypertension. Antioxid. Redox Signal. 2021, 34, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Meder, D.; Herz, D.M.; Rowe, J.B.; Lehéricy, S.; Siebner, H.R. The role of dopamine in the brain-lessons learned from Parkinson’s disease. Neuroimage 2019, 190, 79–93. [Google Scholar] [CrossRef]
- Mereu, M.; Contarini, G.; Buonaguro, E.F.; Latte, G.; Managò, F.; Iasevoli, F.; de Bartolomeis, A.; Papaleo, F. Dopamine transporter (DAT) genetic hypofunction in mice produces alterations consistent with ADHD but not schizophrenia or bipolar disorder. Neuropharmacology 2017, 121, 179–194. [Google Scholar] [CrossRef]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behavior. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef]
- Maia, T.V.; Conceição, V.A. Dopaminergic disturbances in Tourette syndrome: An integrative account. Biol. Psychiatry 2018, 84, 332–344. [Google Scholar] [CrossRef]
- Ahn, S.; Seo, E.; Kim, K.; Lee, S.J. Controlled cellular uptake and drug efficacy of nanotherapeutics. Sci. Rep. 2013, 3, 1997. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Kashani, A.S.; Packirisamy, M. Cancer-nano-interaction: From cellular uptake to mechanobiological responses. Int. J. Mol. Sci. 2021, 22, 9587. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.D.; Liang, Y.R.; Chen, B.H. Effects of tanshinone nanoemulsion and extract on inhibition of lung cancer cells A549. Nanotechnology 2016, 27, 495101. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.F.S.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticle and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar]







| 30% EtOH | 50% EtOH | 70% EtOH | |
|---|---|---|---|
| TPC b | 132.80 ± 3.92 A | 136.19 ± 4.04 A | 116.39 ± 6.27 B |
| TFC c | 206.19 ± 5.51 A | 194.66 ± 1.34 B | 164.19 ± 6.48 B |
| Species | TPC c | TFC d | Species | TPC b | TFC c |
|---|---|---|---|---|---|
| 1. Gulf coast1 | 32.43 ± 0.05 A,l | 13.12 ± 2.61 B,k | 11. GGM | 82.70 ± 1.77 A,f | 22.72 ± 0.66 B,h |
| 2. Sharp blue | 66.31 ± 4.69 A,h | 51.25 ± 1.57 B,b | 12. Tifblue | 119.52 ± 1.04 A,b | 37.83 ± 0.63 B,d |
| 3. Climax | 47.30 ± 0.52 A,j | 15.27 ± 0.38 B,j | 13. Sunshine Blue | 30.01 ± 1.04 A,l | 18.34 ± 0.06 B,i |
| 4. Misty | 104.16 ± 2.88 B,c | 111.36 ± 2.67 A,a | 14. NTU 042 | 22.57 ± 0.94 A,m | 9.12 ± 0.34 B,l |
| 5. O’Neal | 59.16 ± 6.47 A,i | 32.27 ± 1.29 B,e | 15. NTU 03 | 32.12 ± 0.49 A,l | 15.16 ± 0.03 B,j |
| 6. Gloria | 88.51 ± 9.95 A,e | 21.05 ± 1.51 B,h | 16. Gulf coast2 | 38.05 ± 0.71 A.k | 30.20 ± 0.50 B,f |
| 7. Fuku | 77.28 ± 4.00 A,fg | 29.45 ± 0.44 B,f | 17. Onel | 64.37 ± 2.21 A,hi | 37.72 ± 0.63 B,d |
| 8. Powderblue | 93.02 ± 0.49 A,de | 26.95 ± 0.96 B,g | 18. Sunshine | 21.57 ± 0.85 A,m | 14.47 ± 0.44 B,jk |
| 9. Gwl coast | 51.30 ± 1.51 A,j | 43.69 ± 1.68 B,c | 19. NTU 104 (fresh leaves) | 136.09 ± 2.72 A,a | 36.25 ± 1.07 B,d |
| 10. Alice | 73.66 ± 0.71 A,g | 21.83 ± 0.16 B,h | 20. NTU 104 (withered leaves) | 97.20 ± 3.75 A,d | 29.09 ± 0.38 B,f |
| Peak No. | Compound | Retention Time (min) | MS/MS (m/z) b | Content (µg/g) c | ||
|---|---|---|---|---|---|---|
| Standard | Sample | Precursor Ion | Product Ion | |||
| 1 | 5-O-caffeoylquinic acid a | 2.92 | 2.79 | 353 | 191, 179 | 815.8 ± 18.7 |
| 2 | 3-O-caffeoylquinic acid a | 3.18 | 3.07 | 353 | 191, 173 | 6474.2 ± 105.58 |
| 3 | quercetin-3-O-rutinoside a | 4.08 | 3.98 | 609 | 271, 300 | 1036.6 ± 17.2 |
| 4 | quercetin-3-O-galactoside a | 4.38 | 4.26 | 463 | 300 | 1943.9 ± 125.8 |
| 5 | quercetin-3-O-glucoside a | 4.54 | 4.46 | 463 | 301, 271 | 867.2 ± 39.2 |
| 6 | kaempferol 3-O-glucoside a | 5.48 | 5.38 | 447 | 284, 255 | 309.7 ± 15.4 |
| 7 | 3,5-dicaffeoylquinic acid a | 5.54 | 5.48 | 515 | 173, 353 | 195.3 ± 13.1 |
| 8 | 4,5-dicaffeoylquinic acid a | 6.41 | 6.24 | 515 | 191, 353 | 60.8 ± 3.3 |
| Day | Particle Size (nm) a | Polydispersity Index b | Zeta-Potential (mV) b |
|---|---|---|---|
| 0 | 16.0 | 0.214 ± 0.011 A | −53.8 ± 1.9 A |
| 7 | 16.2 | 0.230 ± 0.013 B | −52.2 ± 0.9 A |
| 21 | 15.4 | 0.235 ± 0.007 B | −49.9 ± 0.9 B |
| 30 | 15.7 | 0.249 ± 0.003 B | −43.2 ± 1.1 C |
| 60 | 15.8 | 0.246 ± 0.013 B | −41.6 ± 1.2 C |
| 90 | 15.0 | 0.249 ± 0.007 B | −39.2 ± 1.3 D |
| Temp | Particle Size (nm) a | Polydispersity Index b | Zeta-Potential (mV) b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 1.5 h | 2 h | 0 h | 0.5 h | 1 h | 1.5 h | 2 h | 0 h | 0.5 h | 1 h | 1.5 h | 2 h | |
| 40 °C | 16.0 A | 12.5 BC | 14.3 A | 12.8 A | 14.2 A | 0.214 | 0.223 | 0.237 | 0.258 | 0.263 | −53.8 A | −46.9 A | −42.4 A | −40.5 A | −39.7 A |
| 60 °C | 16.0 A | 11.6 C | 15.0 A | 12.6 A | 12.8 C | 0.214 | 0.235 | 0.241 | 0.249 | 0.271 | −53.8 A | −43.2 B | −38.9 B | −36.2 B | −34.0 B |
| 80 °C | 16.0 A | 15.2 A | 12.2 B | 11.6 B | 13.4 B | 0.214 | 0.229 | 0.228 | 0.251 | 0.259 | −53.8 A | −39.6 C | −39.6 B | −38.5 A | −31.4 C |
| 100 °C | 16.0 A | 13.1 B | 11.8 B | 11.3 B | 11.2 D | 0.214 | 0.232 | 0.245 | 0.243 | 0.258 | −53.8 A | −37.9 D | −36.6 C | −35.4 B | −31.1 C |
| Group | Body Weight (g) | |||||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
| C | 26.0 ± 2.8 A | 26.7 ± 2.4 B | 27.5 ± 2.0 AB | 28.2 ± 1.9 A | 28.2 ± 2.0 A | 29.0 ± 2.1 A |
| D | 26.4 ± 1.4 A | 27.4 ± 1.7 AB | 27.6 ± 1.4 AB | 27.9 ± 1.6 A | 28.3 ± 1.7 A | 28.7 ± 1.7 A |
| HE | 26.5 ± 1.0 A | 27.6 ± 1.3 AB | 27.3 ± 0.8 B | 27.9 ± 0.8 A | 28.1 ± 1.2 A | 29.0 ± 1.2 A |
| HN | 27.2 ± 0.9 A | 28.1 ± 0.6 AB | 28.4 ± 0.7 AB | 28.8 ± 0.8 A | 28.8 ± 0.8 A | 29.5 ±1.0 A |
| LE | 26.6 ± 0.8 A | 27.5 ± 0.8 AB | 28.1 ± 0.6 AB | 28.5 ± 0.7 A | 28.4 ± 0.6 A | 29.2 ± 0.7 A |
| LN | 28.1 ± 1.6 A | 29.0 ± 1.7 A | 29.3 ± 1.6 A | 29.4 ± 1.6 A | 29.8 ± 1.9 A | 30.5 ± 1.9 A |
| Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | |
| C | 28.6 ± 2.1 A | 29.6 ± 2.0 AB | 29.4 ± 1.6 A | 29.8 ± 1.8 AB | 29.9 ±1.7 AB | 31.4 ± 1.8 AB |
| D | 28.7 ± 1.6 A | 28.9 ± 1.4 A | 28.9 ± 1.4 A | 28.9 ± 1.7 B | 28.7 ± 1.7 B | 30.2 ± 1.7 B |
| HE | 28.7 ± 1.3 A | 29.6 ± 0.9 AB | 29.1 ± 1.3 A | 29.4 ± 1.3 B | 29.6 ± 1.3 AB | 30.6 ± 1.4 B |
| HN | 29.3 ± 1.0 A | 30.3 ± 0.8 AB | 29.8 ± 1.1 A | 30.3 ± 1.0 AB | 30.4 ± 0.9 AB | 31.6 ± 0.7 AB |
| LE | 28.8 ± 0.6 A | 30.1 ± 0.9 AB | 30.1 ± 0.7 A | 30.3 ± 0.9 AB | 30.1 ± 0.8 AB | 30.7 ± 1.0 AB |
| LN | 30.1 ± 2.0 A | 31.2 ± 2.3 B | 30.9 ± 2.1 A | 31.6 ± 2.0 A | 31.2 ± 1.9 A | 32.7 ± 1.9 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-R.; Chen, B.-H. Analysis of Phenolic Acids and Flavonoids in Rabbiteye Blueberry Leaves by UPLC-MS/MS and Preparation of Nanoemulsions and Extracts for Improving Antiaging Effects in Mice. Foods 2023, 12, 1942. https://doi.org/10.3390/foods12101942
Yu H-R, Chen B-H. Analysis of Phenolic Acids and Flavonoids in Rabbiteye Blueberry Leaves by UPLC-MS/MS and Preparation of Nanoemulsions and Extracts for Improving Antiaging Effects in Mice. Foods. 2023; 12(10):1942. https://doi.org/10.3390/foods12101942
Chicago/Turabian StyleYu, Hsin-Rong, and Bing-Huei Chen. 2023. "Analysis of Phenolic Acids and Flavonoids in Rabbiteye Blueberry Leaves by UPLC-MS/MS and Preparation of Nanoemulsions and Extracts for Improving Antiaging Effects in Mice" Foods 12, no. 10: 1942. https://doi.org/10.3390/foods12101942
APA StyleYu, H.-R., & Chen, B.-H. (2023). Analysis of Phenolic Acids and Flavonoids in Rabbiteye Blueberry Leaves by UPLC-MS/MS and Preparation of Nanoemulsions and Extracts for Improving Antiaging Effects in Mice. Foods, 12(10), 1942. https://doi.org/10.3390/foods12101942