Comparison of Soluble Dietary Fibers Extracted from Ten Traditional Legumes: Physicochemical Properties and Biological Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of SDFs from Different Traditional Legumes
2.3. Structural Characterization of SDFs from Different Traditional Legumes
2.4. Evaluation of Biological Functions of SDFs from Different Traditional Legumes
2.5. Statistical Analysis
3. Results and Discussion
3.1. Approximate Chemical Components of SDFs from Ten Selected Traditional Legumes
3.2. Molecular Weights, Crystalline Characteristics, and Thermal Characteristics of SDFs from Ten Selected Traditional Legumes
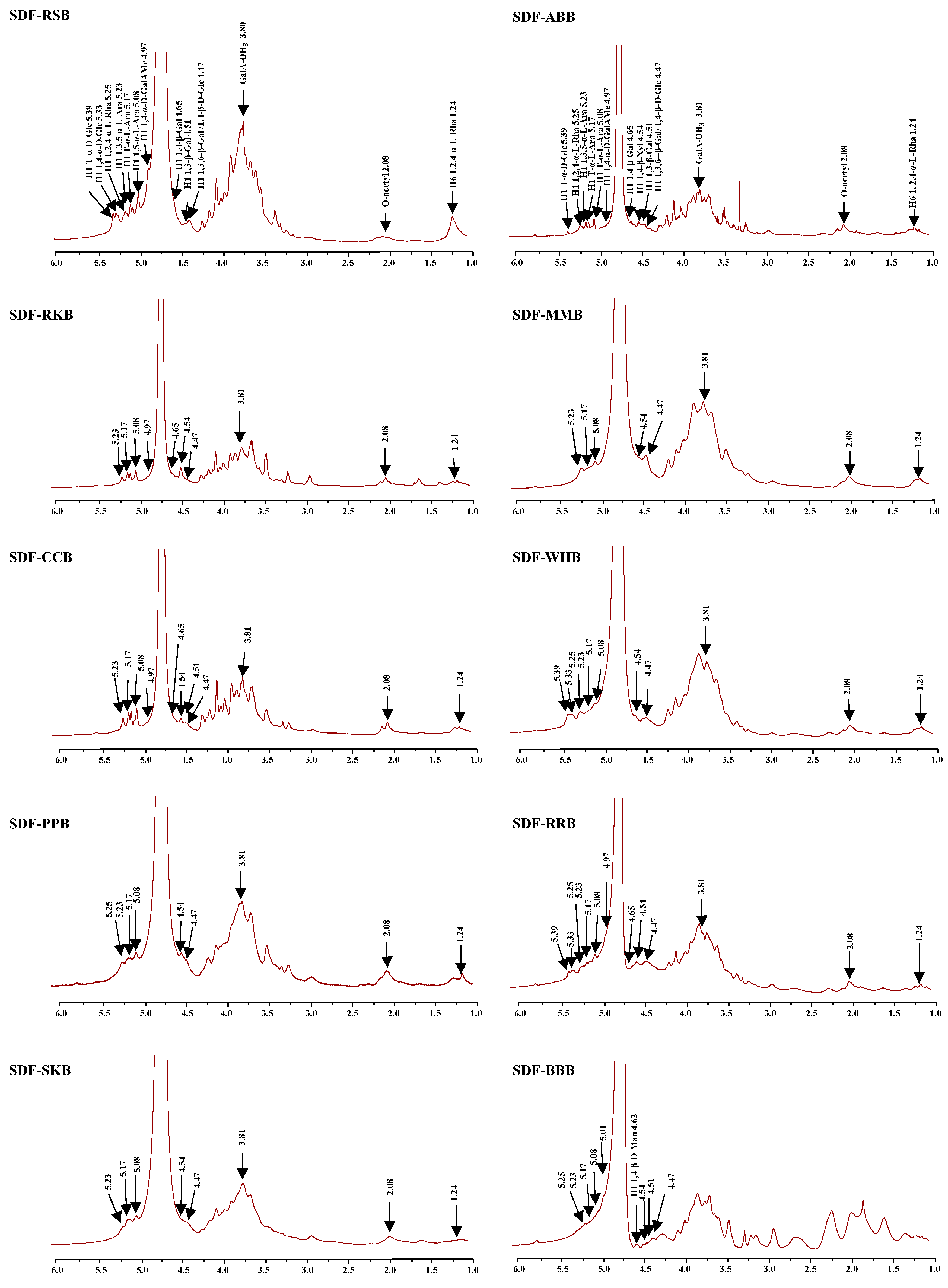
3.3. Monosaccharide Compositions, FT-IR Spectra, and 1D NMR Spectra of SDFs from Ten Selected Traditional Legumes

3.4. Antioxidant Effects of SDFs from Ten Selected Traditional Legumes
3.5. Antiglycation Activities of SDFs from Different Traditional Legumes
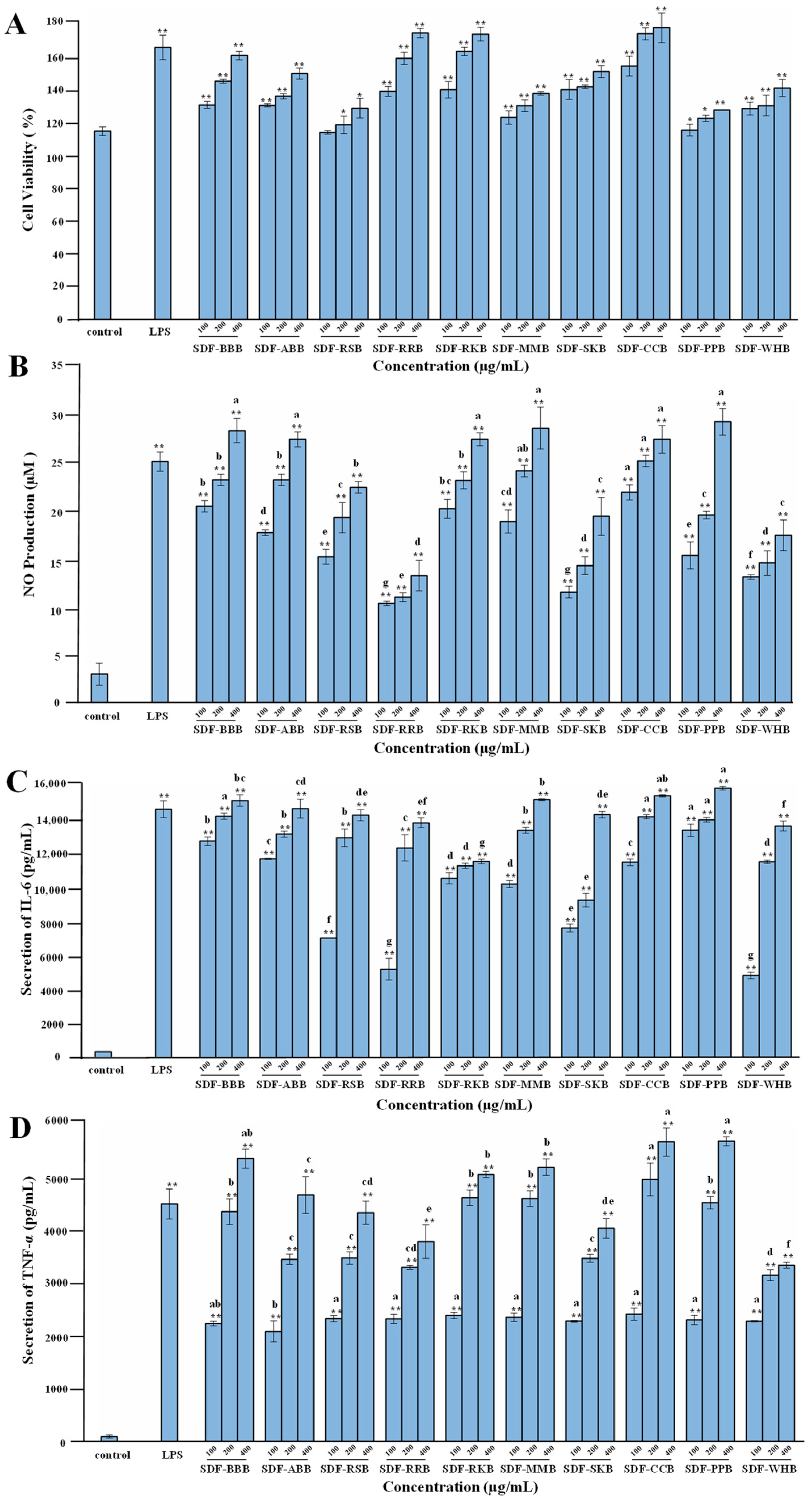
3.6. Immunoregulatory Activities of SDFs from Different Traditional Legumes
3.7. Prebiotic Potential of SDFs from Different Traditional Legumes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, S.X.; Shah, B.R.; Li, J.; Liang, H.S.; Zhan, F.C.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Khorasaniha, R.; Olof, H.; Voisin, A.; Armstrong, K.; Wine, E.; Vasanthan, T.; Armstrong, H. Diversity of fibers in common foods: Key to advancing dietary research. Food Hydrocoll. 2023, 139, 108495. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Dietary fiber intake and total mortality: A meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2014, 180, 565. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Yao, B.; Fang, H.; Xu, W.; Yan, Y.; Xu, H.; Liu, Y.; Mo, M.; Zhang, H.; Zhao, Y. Dietary fiber intake and risk of type 2 diabetes: A dose–response analysis of prospective studies. Eur. J. Epidemiol. 2014, 29, 79. [Google Scholar] [CrossRef]
- Song, W.; Sun, S.; Wu, T.; Yang, R.; Tian, S.; Xu, C.; Jiang, B.; Yuan, S.; Hou, W.; Wu, C.; et al. Geographic distributions and the regionalization of soybean seed compositions across China. Food Res. Int. 2023, 164, 112364. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Mata, A.; Corke, H.; Gan, R.Y.; Fang, Y.P. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Xu, X.; Yuan, Z.; Song, H.; Yang, L.; Zhu, D. Structure/function relationships of bean polysaccharides: A review. Crit. Rev. Food Sci. 2023, 63, 330–344. [Google Scholar] [CrossRef]
- Wu, G.J.; Liu, D.; Wan, Y.J.; Huang, X.J.; Nie, S.P. Comparison of hypoglycemic effects of polysaccharides from four legume species. Food Hydrocoll. 2019, 90, 299–304. [Google Scholar] [CrossRef]
- Kan, L.J.; Nie, S.P.; Hu, J.L.; Wang, S.N.; Bai, Z.Y.; Wang, J.Q.; Zhou, Y.M.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, R.; Nanda, V. Proximate composition, nutritional profile and health benefits of legumes—A review. Legume Res. 2018, 41, 325–332. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Angeles, J.G.C.; Villanueva, J.C.; Uy, L.Y.C.; Mercado, S.M.Q.; Tsuchiya, M.C.L.; Lado, J.P.; Angelia, M.R.N.; Bercansil-Clemencia, M.C.M.; Estacio, M.A.C.; Torio, M.A.O. Legumes as functional food for cardiovascular disease. Appl. Sci. 2021, 11, 5475. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Meng, J.X.; Huang, J.X.; Wu, G.J.; Zuo, S.; Nie, S.P. Comparative study on antidiabetic function of six legume crude polysaccharides. Int. J. Biol. Marcomol. 2020, 154, 25–30. [Google Scholar] [CrossRef]
- Xie, J.H.; Song, Q.Q.; Yu, Q.; Chen, Y.; Hong, Y.Z.; Shen, M.Y. Dietary polysaccharide from Mung bean [Vigna radiate (Linn.) Wilczek] skin modulates gut microbiota and short-chain fatty acids in mice. Int. J. Food Sci. Technol. 2022, 57, 2581–2589. [Google Scholar] [CrossRef]
- Jayamanohar, J.; Devi, P.B.; Kavitake, D.; Priyadarisini, V.B.; Shetty, P.H. Prebiotic potential of water extractable polysaccharide from red kidney bean (Phaseolus vulgaris L.). LWT 2019, 101, 703–710. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, P.; Zhu, Y.; Gao, Y.; Ren, G. Antioxidant and immunoregulatory activity of polysaccharides from adzuki beans (Vigna angularis). Food Res. Int. 2015, 77, 251–256. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.; Li, H.; Ren, Y.; Xiao, Y.; Song, Q.; Chen, Y.; Yu, Q.; et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocoll. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.Y.; Ren, G.X. Immunoregulatory activities of polysaccharides from mung bean. Carbohyd. Polym. 2016, 139, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Hu, J.L.; Li, J.; Wang, J.; Zhang, X.Y.; Wu, X.Y.; Li, X.; Guo, Z.B.; Zou, L.; Wu, D.T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food Res. Int. 2023, 163, 112166. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; He, Y.; Yuan, Q.; Wang, S.P.; Gan, R.Y.; Hu, Y.C.; Zou, L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocoll. 2022, 132, 107897. [Google Scholar] [CrossRef]
- Yu, B.; Wang, M.; Teng, B.; Veeraperumal, S.; Cheung, P.C.; Zhong, S.; Cheong, K.L. Partially Acid-Hydrolyzed Porphyran Improved Dextran Sulfate Sodium-Induced Acute Colitis by Modulation of Gut Microbiota and Enhancing the Mucosal Barrier. J. Agric. Food Chem. 2023, 71, 7299–7311. [Google Scholar] [CrossRef]
- Wu, D.T.; Fu, M.X.; Guo, H.; Hu, Y.C.; Zheng, X.Q.; Gan, R.Y.; Zou, L. Microwave-assisted deep eutectic solvent extraction, structural characteristics, and biological functions of polysaccharides from sweet tea (Lithocarpus litseifolius) leaves. Antioxidants 2022, 11, 1578. [Google Scholar] [CrossRef]
- Wu, D.-T.; He, Y.; Fu, M.-X.; Gan, R.-Y.; Hu, Y.-C.; Peng, L.-X.; Zhao, G.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Hu, X.; Chen, Y.; Xie, J.; Ao, T.; Wang, H.; Xie, J.; Yu, Q. Elucidation of the interaction effect between dietary fiber and bound polyphenol components on the anti-hyperglycemic activity of tea residue dietary fiber. Food Funct. 2022, 13, 2710–2728. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Zheng, Y.; Zhang, X.; He, Z.; Wang, Z.; Wang, Y.; Xie, J.; Chen, Y.; Yu, Q. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity in in vitro digestion and colonic fermentation. Food Funct. 2020, 11, 6652–6665. [Google Scholar] [CrossRef]
- Liu, S.; Jia, M.; Chen, J.; Wan, H.; Dong, R.; Nie, S.; Xie, M.; Yu, Q. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019, 93, 284–292. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.N.; Zhou, X.J.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y.L. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT 2021, 145, 111313. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ahmed, M.I.; Ma, M.; Wang, H. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from Medemia argun fruit. Int. J. Biol. Macromol. 2020, 155, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.L.; Zhang, L.Y.; Wang, H.J.; Yu, W.J.; Li, Q.H. Structural diversity and physicochemical properties of polysaccharides isolated from pumpkin (Cucurbita moschata) by different methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Popa, V.I. Chapter 13—Hemicelluloses: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 289–304. [Google Scholar]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, J.A.; Liu, X.; Li, X.J.; Sun, X.B.; Kou, Y.X.; Li, D.S.; Liu, Y.F.; Zhang, H.; Wu, Y. Pectic polysaccharides from Biluochun Tea: A comparative study in macromolecular characteristics, fine structures and radical scavenging activities in vitro. Int. J. Biol. Macromol. 2022, 195, 598–608. [Google Scholar] [CrossRef]
- Song, H.; Han, L.; Zhang, Z.; Li, Y.; Yang, L.; Zhu, D.; Wang, S.; He, Y.; Liu, H. Structural properties and bioactivities of pectic polysaccharides isolated from soybean hulls. LWT 2022, 170, 114079. [Google Scholar] [CrossRef]
- Sardarodiyan, M.; Arianfar, A.; Sani, A.M.; Naji-Tabasi, S. Antioxidant and antimicrobial activities of water-soluble polysaccharide isolated from Balangu seed (Lallemantia royleana) gum. J. Anal. Sci. Technol. 2019, 10, 17. [Google Scholar] [CrossRef]
- Song, Q.Q.; Jiang, L.; Yang, X.Q.; Huang, L.X.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J.H. Physicochemical and functional properties of a water-soluble polysaccharide extracted from Mung bean (Vigna radiate L.) and its antioxidant activity. Int. J. Biol. Macromol. 2019, 138, 874–880. [Google Scholar] [CrossRef]
- Wu, D.-T.; Wang, J.; Li, J.; Hu, J.-L.; Yan, H.; Zhao, J.; Zou, L.; Hu, Y.-C. Physicochemical properties and biological functions of soluble dietary fibers isolated from common and Tartary buckwheat sprouts. LWT 2023, 183, 114944. [Google Scholar] [CrossRef]
- Jia, W.; Guo, A.A.; Zhang, R.; Shi, L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.M.S.; Ye, Z.P.; Abdin, M.; Hamed, Y.S.; Chen, G.J.; Zeng, X.X. Immunomodulatory activity in vitro and in vivo of polysaccharides from Kabuli Chickpea (Cicer arietinum L.) hull. Food Technol. Biotechnol. 2020, 58, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.O.; Xu, X.Y.; Li, H.B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]





| Sample Codes | Legumes | Growing Regions |
|---|---|---|
| ABB | Adzuki bean (Vigna angularis) | Ganzhou, Jiangxi, China |
| RRB | Red bean (Vigna angularis) | Chouyang, Liaoning, China |
| MMB | Mung bean (Vigna radiata) | Baicheng, Jilin, China |
| RKB | Red kidney bean (Phaseolus vulgaris) | Songyuan, Jilin, China |
| CCB | Common bean (Phaseolus vulgaris) | Beijing, China |
| SKB | Speckled kidney bean (Phaseolus vulgaris) | Lijiang, Yunnan, China |
| BBB | Black bean (Glycine max) | Chouyang, Liaoning, China |
| RSB | Red sword bean (Canavalia gladiata) | Shouguang, Shandong, China |
| WHB | White hyacinth bean (Lablab purpureus) | Kunming, Yunnan, China |
| PPB | Pea (Pisum sativum) | Ganzhou, Jiangxi, China |
| SDF-RSB | SDF-ABB | SDF-RKB | SDF-MMB | SDF-CCB | SDF-WHB | SDF-PPB | SDF-RRB | SDF-SKB | SDF-BBB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total polysaccharides (mg/100 mg) | 91.22 ± 0.56 ab | 90.49 ± 0.38 ab | 90.49 ± 0.15 ab | 86.07 ± 2.01 c | 86.69 ± 2.34 c | 89.36 ± 2.05 b | 93.81 ± 5.71 a | 85.86 ± 1.46 c | 88.04 ± 2.20 c | 77.58 ± 4.39 d |
| Total uronic acids (mg/100 mg) | 17.50 ± 0.64 b | 11.33 ± 0.38 e | 22.75 ± 0.66 a | 16.10 ± 0.35 c | 11.79 ± 0.49 e | 14.07 ± 0.56 d | 16.01 ± 0.85 c | 16.65 ± 0.58 bc | 15.68 ± 0.31 c | 13.20 ± 0.59 d |
| Total phenolics (mg GAE/g) | 8.7 ± 0.27 f | 23.32 ± 0.69 b | 12.03 ± 0.0.50 e | 15.77 ± 0.77 d | 7.13 ± 0.04 g | 6.51 ± 0.33 g | 3.92 ± 0.15 h | 27.18 ± 0.26 a | 18.81 ± 0.34 c | 27.89 ± 1.23 a |
| Total proteins (mg/100 mg) | 3.12 ± 0.07 d | 2.02 ± 0.25 ef | 2.42 ± 0.13 e | 4.51 ± 0.46 c | 2.43 ± 0.08 e | 1.83 ± 0.17 f | 0.14 ± 0.02 g | 4.16 ± 0.06 c | 6.03 ± 0.40 b | 7.88 ± 0.50 a |
| Degree of methylation (%) | 20.27 ± 0.41 d | 11.17 ± 0.41 g | 28.71 ± 0.21 b | 20.04 ± 0.46 d | 19.47 ± 0.31 e | 22.30 ± 0.32 c | 30.43 ± 0.59 a | 10.41 ± 0.17 h | 14.63 ± 0.19 f | 2.67 ± 0.43 i |
| SDF-RSB | SDF-ABB | SDF-RKB | SDF-MMB | SDF-CCB | SDF-WHB | SDF-PPB | SDF-RRB | SDF-SKB | SDF-BBB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | ||||||||||
| 106 (Da) | 1.325 ± 0.007 f | 2.207 ± 0.013 b | 2.493 ± 0.047 a | 1.821 ± 0.012 e | 1.856 ± 0.027 e | 2.156 ± 0.013 c | 2.016 ± 0.017 d | - | - | - |
| Relative peak areas (%) | 19.6 | 11.8 | 35.3 | 11.7 | 37.4 | 12.3 | 18.9 | - | - | - |
| 105 (Da) | 1.232 ± 0.008 g | 1.284 ± 0.010 f | 2.744 ± 0.054 b | 1.449 ± 0.011 e | 1.705 ± 0.034 c | 1.119 ± 0.008 h | 1.506 ± 0.015 d | 1.059 ± 0.014 i | 4.606 ± 0.030 a | 1.543 ± 0.015 d |
| Relative peak areas (%) | 49.3 | 53.3 | 30.9 | 44.2 | 37.0 | 73.1 | 65.6 | 33.5 | 23.1 | 8.3 |
| 104 (Da) | 1.346 ± 0.054 e | 2.309 ± 0.062 c | 4.798 ± 0.211 a | 1.618 ± 0.038 d | 4.165 ± 0.141 b | 2.329 ± 0.073 c | 4.932 ± 0.138 a | 0.8007 ± 0.054 f | 1.736 ± 0.046 d | 1.232 ± 0.049 e |
| Relative peak areas (%) | 31.1 | 34.9 | 33.8 | 44.1 | 25.6 | 14.6 | 15.5 | 66.5 | 76.9 | 91.7 |
| Monosaccharide and molar ratio | ||||||||||
| Rhamnose | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mannose | 0.11 | 0.54 | 0.77 | 0.72 | 0.29 | 0.35 | 0.36 | 0.49 | 0.92 | 3.37 |
| Glucuronic acid | 0.24 | 0.59 | 0.82 | 0.97 | 0.66 | 0.61 | 0.50 | 0.60 | 0.75 | 0.54 |
| Galacturonic acid | 1.11 | 1.93 | 3.57 | 2.36 | 2.11 | 2.17 | 2.06 | 1.17 | 4.28 | 1.14 |
| Glucose | 2.80 | 3.03 | 1.29 | 2.67 | 0.84 | 1.04 | 1.52 | 1.43 | 2.21 | 1.11 |
| Galactose | 1.16 | 3.70 | 5.20 | 6.72 | 4.20 | 4.26 | 3.85 | 4.36 | 4.84 | 5.46 |
| Xylose | 0.17 | 1.26 | 4.00 | 1.64 | 3.57 | 1.42 | 0.95 | 1.35 | 3.02 | 0.42 |
| Arabinose | 1.68 | 3.37 | 6.77 | 3.74 | 8.02 | 3.26 | 3.10 | 2.64 | 8.08 | 2.55 |
| Fucose | - | - | 1.78 | - | 2.17 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Wan, J.; Li, W.; Li, J.; Guo, W.; Zheng, X.; Gan, R.-Y.; Hu, Y.; Zou, L. Comparison of Soluble Dietary Fibers Extracted from Ten Traditional Legumes: Physicochemical Properties and Biological Functions. Foods 2023, 12, 2352. https://doi.org/10.3390/foods12122352
Wu D, Wan J, Li W, Li J, Guo W, Zheng X, Gan R-Y, Hu Y, Zou L. Comparison of Soluble Dietary Fibers Extracted from Ten Traditional Legumes: Physicochemical Properties and Biological Functions. Foods. 2023; 12(12):2352. https://doi.org/10.3390/foods12122352
Chicago/Turabian StyleWu, Dingtao, Jiajia Wan, Wenxing Li, Jie Li, Wang Guo, Xiaoqin Zheng, Ren-You Gan, Yichen Hu, and Liang Zou. 2023. "Comparison of Soluble Dietary Fibers Extracted from Ten Traditional Legumes: Physicochemical Properties and Biological Functions" Foods 12, no. 12: 2352. https://doi.org/10.3390/foods12122352
APA StyleWu, D., Wan, J., Li, W., Li, J., Guo, W., Zheng, X., Gan, R.-Y., Hu, Y., & Zou, L. (2023). Comparison of Soluble Dietary Fibers Extracted from Ten Traditional Legumes: Physicochemical Properties and Biological Functions. Foods, 12(12), 2352. https://doi.org/10.3390/foods12122352








