A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Pomegranate Material Recovery
2.2. Characterization of PPP
2.2.1. Proximate Analysis and Determination of DF
2.2.2. Determination of Total Phenolic Content (TPC)
2.2.3. Determination of Punicalagin Content
2.2.4. Determination of the Antioxidant Capacity (AC)
2.2.5. PPP Shelf-Life Study
2.3. Preparation of Bread Formulations Incorporating PPP
2.3.1. Characterization of Bread Formulations
Rheological properties of Bread Doughs and Physical Properties of Baked Bread
Proximate Analysis and Determination of DF
Determination of TPC
Microbiological Analysis
Sensorial Analysis
2.4. Clinical Assay
Calculation of Glycemic Index of Bread
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PPP
3.1.1. Proximate Analysis and DF
3.1.2. Total Phenolic and Punicalagin Content
3.1.3. Antioxidant Capacity
3.2. PPP Shelf-Life Study (TPC Stability, Microbiological Quality, and Color)
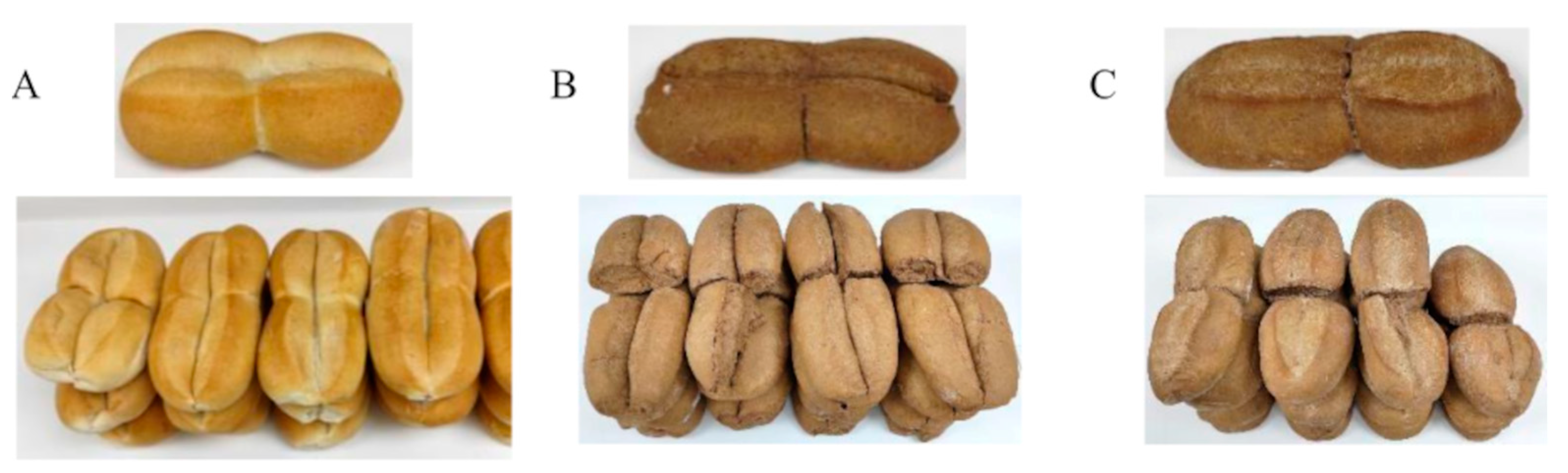
3.3. Characterization of Bread Formulations
3.3.1. Proximate Analysis and DF of the Bread
3.3.2. Rheological Parameters of the Bread Doughs and Physical Properties of Bread
3.3.3. Total Phenolic Compound
3.3.4. Microbiological and Sensory Analyses
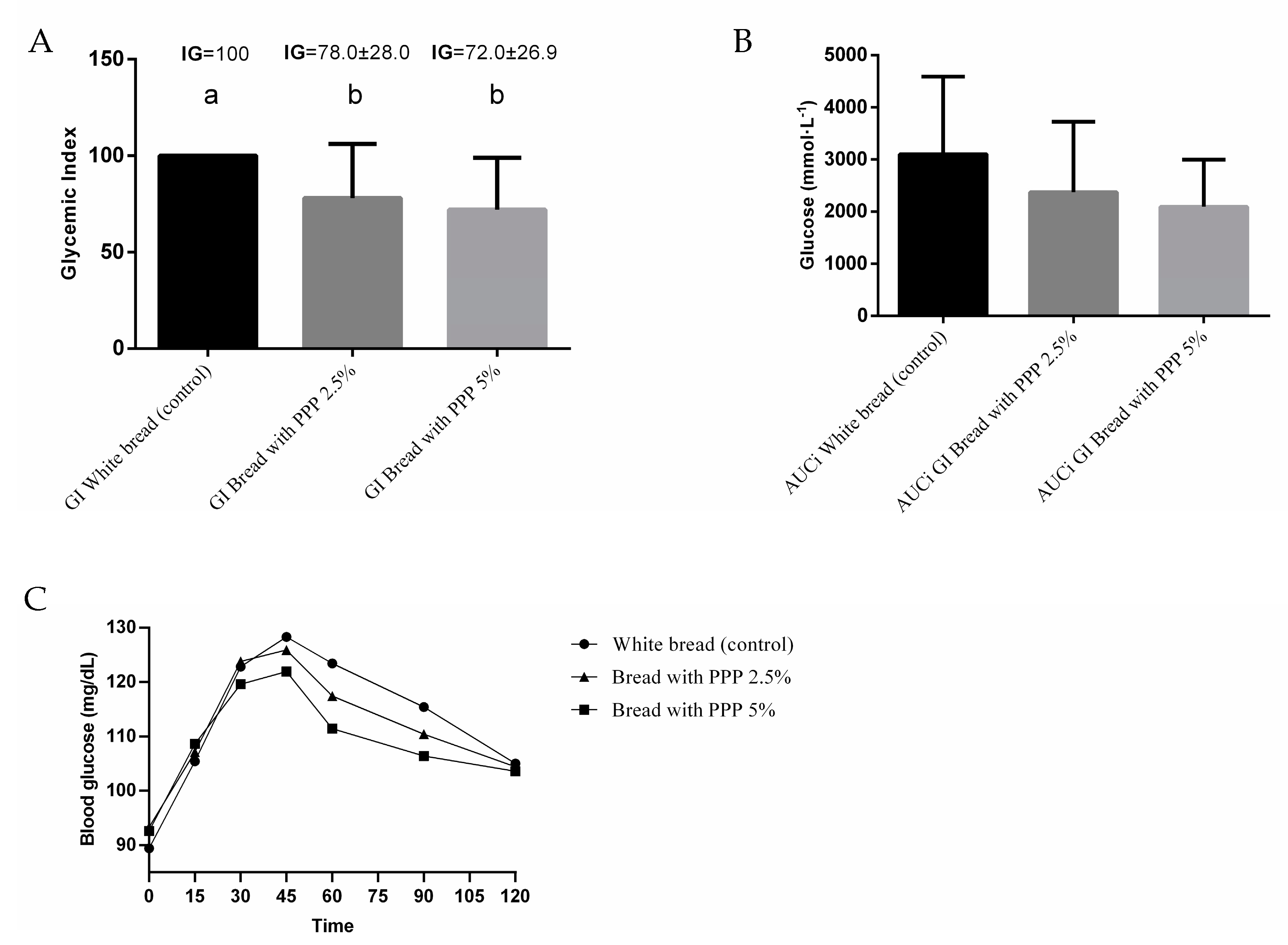
3.4. Clinical Study
Glycemic Index of Bread and Glucose Response
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–577. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of Isolated Soluble Fiber Supplementation on Body Weight, Glycemia, and Insulinemia in Adults with Overweight and Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nut. 2017, 106, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Liu, Y.; Lin, X.; Fang, Y.; Cui, J.; Wan, J. Dietary Fiber Intake and Risk of Metabolic Syndrome: A Meta-Analysis of Observational Studies. Clin. Nut. 2018, 37, 1935–1942. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-Analyses. J. Chirop. Med. 2017, 16, 289–299. [Google Scholar] [CrossRef]
- Institute of Medicine (Ed.) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.M. Bread Fortification. In Bread and Its Fortification: Nutrition and Health Benefits; Bajerska, J., El Sheikha, A.F., Eds.; Taylor y Francis Group: New York, NY, USA, 2015; ISBN 978-0-429-17255-7. [Google Scholar]
- Sharma, S.; Sharma, N.; Sharma, R.; Handa, S. Formulation of Functional Multigrain Bread and Evaluation of Their Health Potential. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 4120–4126. [Google Scholar] [CrossRef]
- Malik, H. Optimisation of Process for Development of Nutritionally Enriched Multigrain Bread. J. Food Process. Technol. 2016, 7. [Google Scholar] [CrossRef]
- Marinopoulou, A.; Papadakis, D.; Petridis, D.; Papageorgiou, M. Monitoring Staling of Packaged Sliced Pan Breads: Physicochemical Properties, Sensory Evaluation, and Consumer Preference. J. Culin. Sci. Technol. 2020, 18, 396–412. [Google Scholar] [CrossRef]
- Egea, M.B.; De Sousa, T.L.; Dos Santos, D.C.; De Oliveira, J.; Guimaraes, R.; Yoshiara, L.; Lemes, A. Application of Soy, Corn, and Bean By-products in the Gluten-free Baking Process: A Review. Food Bioprocess Technol. 2023, 16, 1429–1450. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Sustainable Development Goals. United Nations. Available online: https://www.un.org/sustainabledevelopment/sustainableconsumption-production/ (accessed on 14 June 2023).
- Brito, T.; Ferreira, M.; Fai, A. Utilization of Agricultural By-Products: Bioactive Properties and Technological Applications. Food Rev. Int. 2020, 38, 1305–1329. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture. Moving Forward on Food Loss and Waste Reduction; CC BY-NC-S.; FAO: Rome, Italy, 2019; ISBN 9789251317891. [Google Scholar]
- Venkitasamy, C.H.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 181–216. ISBN 9780128141380. [Google Scholar]
- Wang, Z. Extract of Phenolics from Pomegranate Peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of Phenolic Compounds in Different Parts of Pomegranate (Punica Granatum) Fruit by HPLC-PDA-ESI/MS and Evaluation of Their Antioxidant Activity: Application to Different Italian Varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Abolila, R. Effect of Adding Pomegranate Peels and Seeds Powder on Quality Properties of Pan Bread. Ann. Agric. Sci. Moshtohor 2019, 57, 705–714. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Babiker, W.A.; Elhardallou, S.B.; Elkhalifa, E.A.; Veettil, V.N. Influence of Enrichment of Wheat Bread with Pomegranate (Punica Granatum L) Peels by-Products. Int. J. Food Sci. Nutr. Eng. 2016, 6, 9–13. [Google Scholar]
- Sayed-Ahmed, E.F. Evaluation of Pomegranate Peel Fortified Pan Bread on Body Weight Loss. Int. J. Food Sci. 2014, 3, 411. [Google Scholar] [CrossRef]
- Mehder, A.O.A. Pomegranate Peels Effectiveness in Improving the Nutritional, Physical and Sensory Characteristics of Pan Bread. Curr. Sci. Int. 2013, 2, 8–14. [Google Scholar]
- Altunkaya, A.; Hedegaard, R.V.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Antioxidant capacity versus chemical safety of wheat bread enriched with pomegranate peel powder. Food Funct. 2023, 4, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Tharshini, G.; Sangwan, V. Formulation and Chemical Characterization of Bread Supplemented with Pomegranate Peel Powder. Int. J. Commun. Syst. 2018, 6, 386–390. [Google Scholar]
- Palak, T.; Ranu, P.; Anisha, V. Sensory Analysis of Pomegranate Peel Powder in the Development of Value Added Food Products. Int. J. Sci. Res. 2020, 9, 414–417. [Google Scholar]
- Zare, M.; Goli, A.H.; Karimifar, M.; Tarraji, M.; Rezaei, A.; Amani, R. Effect of bread fortification with pomegranate peel powder on glycemic indicators, antioxidant status, inflammation and mood in patients with type 2 diabetes: Study protocol for a randomized, triple-blind, and placebo-controlled trial. J. Diabetes Metab. Disord. 2023, 22, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Özpak Akkuş, Ö.; Metin, U.; Çamlık, Z. The Effects of Pomegranate Peel Added Bread on Anthropometric Measurements, Metabolic and Oxidative Parameters in Individuals with Type 2 Diabetes: A Double-Blind, Randomized, Placebo-Controlled Study. Nutr. Res. Pract. 2022, 17, e17. [Google Scholar]
- Association Official Analytical Chemist [AOAC]. Official Methods of Analysis. AOAC Publications: Rockville, MD, USA, 2005. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Lee, R.; Henning, S.M.; Heber, D. Absence of Pomegranate Ellagitannins in the Majority of Commercial Pomegranate Extracts: Implications for Standardization and Quality Control. J. Agric. Food Chem. 2009, 57, 7395–7400. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Ana. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH.Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Andrews, W.H.; Jacobson, A.; Andrews, W.H.; Jacobson, A.; Ge, B.; Zhang, G.; Hammack, T. Salmonella. In Bacteriological Analytical Manual (BAM); FDA: Silver Spring, MD, USA, 2023; pp. 1–33. [Google Scholar]
- FAO. The Role of the Glycemic Index in Food Choice. 1998. Available online: https://www.fao.org/3/W8079E/w8079e0a.htmol (accessed on 5 March 2023).
- Wolever, T.M.; Brand-Miller, J.C.; Abernethy, J.; Astrup, A.; Atkinson, F.; Axelsen, M.; Björck, I.; Brighenti, F.; Brown, R.; Brynes, A.; et al. Measuring the Glycemic Index of Foods: Interlaboratory Study. Am. J. Clin. Nutr. 2008, 87, 247S–257S. [Google Scholar] [CrossRef]
- Akuru, E.A.; Oyeagu, C.E.; Mpendulo, T.C.; Rautenbach, F.; Oguntibeju, O.O. Effect of Pomegranate (Punica Granatum L.) Peel Powder Meal Dietary Supplementation on Antioxidant Status and Quality of Breast Meat in Broilers. Heliyon 2020, 6, e05709. [Google Scholar] [CrossRef]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of Pomegranate Peel Supplementation on Nutritional, Organoleptic and Stability Properties of Cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.C.; Bera, M.B.; Kumar, P. Nutritional Composition of Detanninated and Fresh Pomegranate Peel Powder. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 7, 38–42. [Google Scholar] [CrossRef]
- Rowayshed, G.; Emad, A.; Mohamed; Aboulfadl, M. Nutritional and Chemical Evaluation for Pomegranate (Punica Granatum L.) Fruit Peel and Seeds Powders by Products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- Topkaya, C.; Isik, F. Effects of Pomegranate Peel Supplementation on Chemical, Physical, and Nutritional Properties of Muffin Cakes. J. Food Process. Preserv. 2019, 43, e13868. [Google Scholar] [CrossRef]
- Urganci, U.; Isik, F. Quality Characteristics of Biscuits Fortified with Pomegranate Peel. Akademik Gıda 2021, 19, 10–20. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of Pomegranate Peel from 12 Cultivars: Dietary Fibre Composition, Antioxidant Capacity and Functional Properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef]
- Srivastava, P.; Indrani, D.; Singh, R.P. Effect of Dried Pomegranate (Punica Granatum) Peel Powder (DPPP) on Textural, Organoleptic and Nutritional Characteristics of Biscuits. Int. J. Food Sci. Nutr. 2014, 65, 827–833. [Google Scholar] [CrossRef]
- Galaz, P.; Valdenegro, M.; Ramírez, C.; Nuñez, H.; Almonacid, S.; Simpson, R. Effect of Drum Drying Temperature on Drying Kinetic and Polyphenol Contents in Pomegranate Peel. J. Food Eng. 2017, 208, 19–27. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica Granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal Activity of Pomegranate Peel Extract against Fusarium Wilt of Tomato. Eur. J. Plant Pathol. 2017, 147, 229–238. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; Hinojosa, A.; Robert, P.; Escalona, V. Extraction and Microencapsulation of Bioactive Compounds from Pomegranate (Punica Granatum Var. Wonderful) Residues. Int. J. Food Sci. Technol. 2017, 52, 1452–1462. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised Water Extraction of Polyphenols from Pomegranate Peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Khalil, A.A.; Khan, M.R.; Shabbir, M.A.; Khalil-ur-Rahman. In Vitro Antioxidant Activity and Punicalagin Content Quantification of Pomegranate Peel Obtained as Agro-Waste after Juice Extraction. Pak. J. Agric. Sci. 2018, 55, 197–201. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Saidur, R.; Al-Sulaiman, F.A. A Review for Phase Change Materials (PCMs) in Solar Absorption Refrigeration Systems. Renew. Sust. Energ. Rev. 2017, 76, 105–137. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, X.; Shi, L.; Shalimu, D.; Ma, C.; Liu, Y. Phenolic Profiles and Antioxidant Activities of Six Chinese Pomegranate (Punica Granatum L.) Cultivars. Int. J. Food Prop. 2017, 20, S94–S107. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound Assisted Extraction of Bioactive Compounds from Pomegranate (Punica Granatum L.) Peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Kennas, A.; Amellal-Chibane, H. Comparison of Five Solvents in the Extraction of Phenolic Anti-Oxidants from Pomegranate (Punica Granatum L.) Peel. N. Afr. J. Food Nutr. Res. 2019, 3, 140–147. [Google Scholar] [CrossRef]
- Elfalleh, W.; Hannachi, H.; Tlili, N.; Yahia, Y.; Nasri, N.; Ferchichi, A. Total Phenolic Contents and Antioxidant Activities of Pomegranate Peel, Seed, Leaf and Flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Al-Rawahi, A.S.; Rahman, M.S.; Guizani, N.; Essa, M.M. Chemical Composition, Water Sorption Isotherm, and Phenolic Contents in Fresh and Dried Pomegranate Peels. Dry. Technol. 2013, 31, 257–263. [Google Scholar] [CrossRef]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate Peel Phenolics: Microencapsulation, Storage Stability and Potential Ingredient for Functional Food Development. LWT Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Tomadoni, B.; Cassani, L.; Viacava, G.; Moreira, M.D.R.; Ponce, A. Effect of Ultrasound and Storage Time on Quality Attributes of Strawberry Juice. J. Food Process Eng. 2017, 40, e12533. [Google Scholar] [CrossRef]
- Bakkalbaşi, E.; Menteş, Ö.; Artik, N. Food Ellagitannins–Occurrence, Effects of Processing and Storage. Crit. Rev. Food Sci. Nutr. 2008, 49, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of Polyphenols and Anthocyanins from Pomegranate (Punica Granatum) by Spray Drying: Encapsulation of Polyphenols and Anthocyanins. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Ministerio de Salud de Chile. Reglamento Sanitario de los Alimentos, DTO; N°977/96; Ministerio de Salud de Chile: Santiago, Chile, 2019. [Google Scholar]
- Zhou, Y.; Dhital, S.; Zhao, C.; Ye, F.; Chen, J.; Zhao, G. Dietary Fiber-Gluten Protein Interaction in Wheat Flour Dough: Analysis, Consequences and Proposed Mechanisms. Food Hydrocoll. 2021, 111, 106203. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Lozano Sanchez, J.; Borras-Linares, I.; Guiducci, A.; Muscolino, E.; Giacomazza, D.; Sanfilippo, T.; Guggino, R.; Bulone, D.; et al. Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment. Biology 2022, 11, 416. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic Index of Foods: A Physiological Basis for Carbohydrate Exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International Tables of Glycemic Index and Glycemic Load Values 2021: A Systematic Review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Rampersaud, G.C.; Valim, M.F. 100% Citrus Juice: Nutritional Contribution, Dietary Benefits, and Association with Anthropometric Measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef]
- Jimenez-Cruz, A.; Loustaunau-López, V.M.; Bacardi-Gascon, M. The Use of Low Glycemic and High Satiety Index Food Dishes in Mexico: A Low Cost Approach to Prevent and Control Obesity and Diabetes. Nutr. Hosp. 2006, 21, 353–356. [Google Scholar]
- Bornet, F.R.J.; Jardy-Gennetier, A.-E.; Jacquet, N.; Stowell, J. Glycaemic Response to Foods: Impact on Satiety and Long-Term Weight Regulation. Appetite 2007, 49, 535–553. [Google Scholar] [CrossRef]
- Mayer, J. Glucostatic Mechanism of Regulation of Food Intake. N. Engl. J. Med. 1953, 249, 13–16. [Google Scholar] [CrossRef]
- Wolever, T.M.; Mehling, C. Long-Term Effect of Varying the Source or Amount of Dietary Carbohydrate on Postprandial Plasma Glucose, Insulin, Triacylglycerol, and Free Fatty Acid Concentrations in Subjects with Impaired Glucose Tolerance. Am. J. Clin. Nutr. 2003, 77, 612–621. [Google Scholar] [CrossRef]
- Elichalt, M.; Russo, M.; Vázquez, D.; Suburú, G.; Tihista, H.; Godiño, M. Lípidos, Sodio y Fibra Dietética En Harina de Trigo y Pan Artesanal En Uruguay: Aporte Nutricional Según Recomendaciones Para Distintos Grupos de Población. Rev. Chil. Nutr 2017, 44, 71–78. [Google Scholar] [CrossRef]
- Li, Y.O.; Komarek, A.R. Dietary Fibre Basics: Health, Nutrition, Analysis, and Applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Tosh, S.M.; Bordenave, N. Emerging Science on Benefits of Whole Grain Oat and Barley and Their Soluble Dietary Fibers for Heart Health, Glycemic Response, and Gut Microbiota. Nutr. Rev. 2020, 78, 13–20. [Google Scholar] [CrossRef]
- Willett, W.; Manson, J.; Liu, S. Glycemic Index, Glycemic Load, and Risk of Type 2 Diabetes. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jian, C. Sustainable plant-based ingredients as wheat flour substitutes in bread making. npj Sci. Food 2022, 6, 49. [Google Scholar] [CrossRef]
- Amicarelli, V.; Lombardi, M.; Varese, E.; Bux, C. Material flow and economic cost analysis of the Italian artisan bread production before and during the Russia-Ukraine conflict. Environ. Impact Assess. Rev. 2023, 101, 107101. [Google Scholar] [CrossRef]
- Trollman, H.; Jagtap, S.; Garcia-Garcia, G.; Harastani, R.; Colwill, J.; Trollman, F. COVID-19 demand-induced scarcity effects on nutrition and environment: Investigating mitigation strategies for eggs and wheat flour in the United Kingdom. Sustain. Prod. Consum. 2021, 27, 1255–1272. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.; Cortés-García, F.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Sajdakowska, M.; Gębski, J.; Jeżewska-Zychowicz, M.; Królak, M. Consumer Choices in the Bread Market: The Importance of Fiber in Consumer Decisions. Nutrients 2021, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Finley, J.; Hess, J.M.; Ingram, J.; Miller, G.; Peters, C. Toward Healthy Diets from Sustainable Food Systems. Curr. Dev. Nutr. 2020, 4, nzaa083. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Mahmud, M.M.; Abdi, G.; Wanich, U.; Farooqi, M.Q.; Settapramote, N.; Khan, S.; Wani, S.A. New alternatives from sustainable sources to wheat in bakery foods: Science, technology, and challenges. J. Food Biochem. 2022, 46, e14185. [Google Scholar] [CrossRef]
| TPC | Moisture | Ash | Protein | Fat | Carbohydrate | DF | |||
|---|---|---|---|---|---|---|---|---|---|
| TDF | IDF | SDF | |||||||
| mg GAE/g DW | g/100 g | g/100 g DW | g/100 g DW | ||||||
| PPP | 172.4 ± 1.7 | 6.7 ± 0.05 a | 4.2 ± 0.02 a | 5.0 ± 0.10 a | 3.6 ±0.03 a | 30.0 ± 0.8 a | 50.5 ± 0.9 a | 37 ± 0.9 a | 13.3 ± 0.9 a |
| Control bread | 0.5 ± 0.04 | 32.7 ± 0.1 b | 1.87 ± 0.07 b | 12.4 ± 0.04 c | 1.1 ± 0.2 b | 81,2 ± 0.7 b | 3.7 ± 0.1 b | 2.0 ± 0.0 b | 1.7 ± 0.0 b |
| Bread 2.5% PPP | 1.3 ± 0.2 | 32.1 ± 0.4 b | 1.5 ± 0.8 b | 11.9 ± 0.4 bc | 1.2 ± 0.2 b | 75.1 ± 1.1 b | 10.3 ± 0.9 c | 7.1 ± 0.8 c | 3.2 ± 0.3 c |
| Bread 5.0% PPP | 2.2 ± 0.1 | 34.0 ± 0.1 c | 2.0 ± 0.2 b | 11.7 ± 0.5 bc | 1.3 ± 0.2 b | 74.5 ± 1.0 b | 10.4 ± 1.2 c | 7.3 ± 1.0 c | 3.1 ± 0.1 c |
| Bread 7.5% PPP | 3.2 ± 0.1 | 34.4 ± 0.3 c | 2.50 ± 0.01 b | 11.4 ± 0.3 bc | 1.1 ± 0.2 b | 72.9 ± 0.3 b | 12.1 ± 0.9 d | 9.2 ± 1.0 d | 2.9 ± 0.5 c |
| Bread 10% PPP | 3.8 ± 0.06 | 36.1 ± 0.3 d | 2.78 ± 0.08 b | 10.8 ± 0.08 b | 1.2 ± 0.07 b | 70.5 ± 0.3 b | 14.7 ± 0.1 e | 10.5 ± 0.2 e | 4.2 ± 0.1 d |
| Storage Period | Color | TPC | Microbiological Counts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Months | L* (D65) | a*(D65) | b* (D65) | C* (D65) | h° (D65) | mg GAE/g DW | Salmonella spp. (CFU/g) | Filamentous Fungi (CFU/g) | Yeasts (CFU/g) |
| 0 | 43.2 ± 2.6 a | 14.3 ± 1.8 a | 51.2 ± 3.6 a | 53.2 ± 3.9 a | 74.5 ± 0.9 a | 172.4 ± 1.7 a | ND | ˂10 a | ˂10 a |
| 1 | 43.6 ± 2.8 a | 13.2 ± 1.8 a | 49.0 ± 3.3 a | 50.8 ± 3.7 a | 74.9 ± 0.9 a | 127.4 ± 7.8 ab | ND | ˂10 a | ˂10 a |
| 2 | 43.3 ± 0.9 a | 15.7 ± 0.7 a | 54.7 ± 1.5 a | 56.9 ± 1.7 a | 74.0 ± 0.3 a | 121.0 ± 15.2 b | ND | ˂10 a | ˂10 a |
| 3 | 44.2 ± 0.7 a | 14.2 ± 0.5 a | 52.3 ± 1.0 a | 54.2 ± 1.0 a | 74.8 ± 0.5 a | 147.4 ± 6.0 b | ND | ˂10 a | ˂10 a |
| 4 | 44.9 ± 0.3 a | 15.0 ± 0.0 a | 55.4 ± 0.1 a | 57.4 ± 0.1 a | 74.8 ± 0.1 a | 139.5 ± 8.2 b | ND | ˂10 a | ˂10 a |
| 5 | 44.4 ± 1.3 a | 14.4 ± 1.4 a | 50.5 ± 5.0 a | 52.6 ± 5.2 a | 74.1 ± 0.1 a | 126.1 ± 0.0 b | ND | ˂10 a | ˂10 a |
| 6 | 43.9 ± 0.1 a | 14.9 ± 0.3 a | 52.6 ± 1.0 a | 54.6 ± 1.1 a | 74.2 ± 0.1 a | 129.4 ± 4.3 b | ND | ˂10 a | ˂10 a |
| 7 | 42.6 ± 0.7 a | 15.4 ± 0.4 a | 54.7 ± 0.4 a | 56.9 ± 0.5 a | 74.2 ± 0.3 a | 133.9 ± 5.2 bv | ND | ˂10 a | ˂10 a |
| 8 | 43.5 ± 0.9 a | 14.7 ± 0.8 a | 53.7 ± 0.9 a | 55.7± 2.3 a | 74.5 ± 0.7 a | 173.3 ± 8.5 a | ND | ˂10 a | ˂10 a |
| 9 | 44.5 ± 0.8 a | 14.7 ± 0.9 a | 53.7 ± 0.8 a | 54.9 ± 1.2 a | 74.9 ± 0.5 a | 177.4 ± 7.6 a | ND | ˂10 a | 5 × 102 b |
| Bread Formulation | Average Score | 1–3 N (%) | 4 N (%) | 5–7 N (%) |
|---|---|---|---|---|
| Control bread | 5.9 ± 0.9 a | 1.6 | 0 | 98.4 |
| Bread 2.5% PPP | 6.0 ± 0.7 a | 0 | 0 | 100 |
| Bread 5.0% PPP | 5.9 ± 0.7 a | 0 | 1.6 | 98.4 |
| Bread 7.5% PPP | 5.4 ± 0.9 b | 1.3 | 10.5 | 88.2 |
| Bread 10% PPP | 4.8 ± 1.2 c | 13.6 | 24.2 | 62.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, P.; Bustamante, A.; Echeverría, F.; Encina, C.; Palma, M.; Sanhueza, L.; Sambra, V.; Pando, M.E.; Jiménez, P. A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index. Foods 2023, 12, 2798. https://doi.org/10.3390/foods12142798
García P, Bustamante A, Echeverría F, Encina C, Palma M, Sanhueza L, Sambra V, Pando ME, Jiménez P. A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index. Foods. 2023; 12(14):2798. https://doi.org/10.3390/foods12142798
Chicago/Turabian StyleGarcía, Paula, Andrés Bustamante, Francisca Echeverría, Cristian Encina, Manuel Palma, Leyla Sanhueza, Verónica Sambra, Maria Elsa Pando, and Paula Jiménez. 2023. "A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index" Foods 12, no. 14: 2798. https://doi.org/10.3390/foods12142798
APA StyleGarcía, P., Bustamante, A., Echeverría, F., Encina, C., Palma, M., Sanhueza, L., Sambra, V., Pando, M. E., & Jiménez, P. (2023). A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index. Foods, 12(14), 2798. https://doi.org/10.3390/foods12142798










