Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum sibiricum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Reagents
2.2. Extraction Methods
2.2.1. Pretreatment Process
2.2.2. Hot Water Extraction
2.2.3. Alkali-Assisted Extraction
2.2.4. Ultrasonic-Assisted Extraction
2.2.5. Enzyme-Assisted Extraction
2.2.6. Microwave-Assisted Extraction
2.2.7. Freeze–Thaw-Assisted Extraction
2.3. Characterization of PSPs
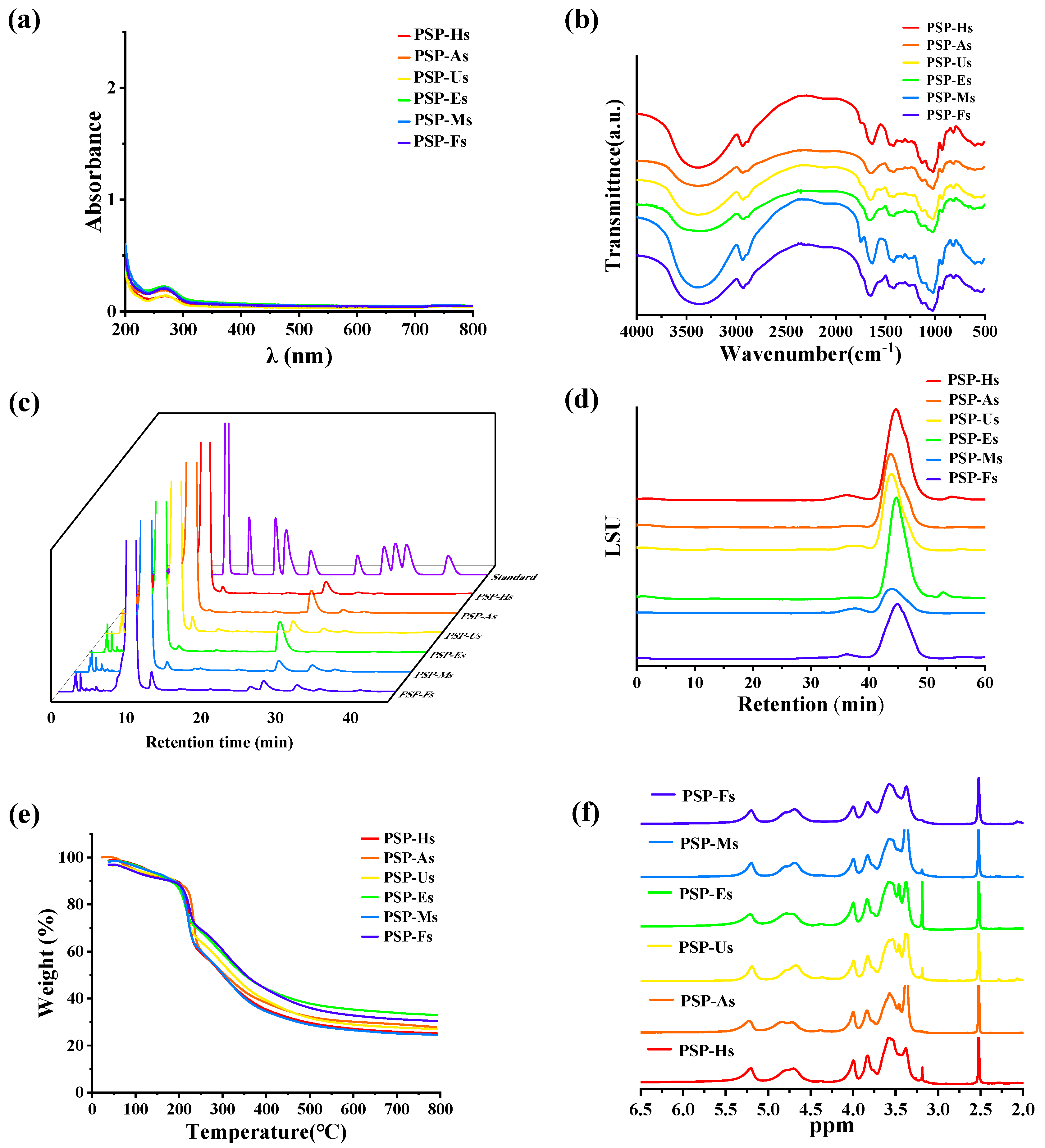
2.3.1. UV-Vis Spectra and FT-IR Spectra Analyses of PSPs
2.3.2. Analysis of Monosaccharide Composition and Average Molecular Weight (Mw) of PSPs
2.3.3. TGA of PSPs
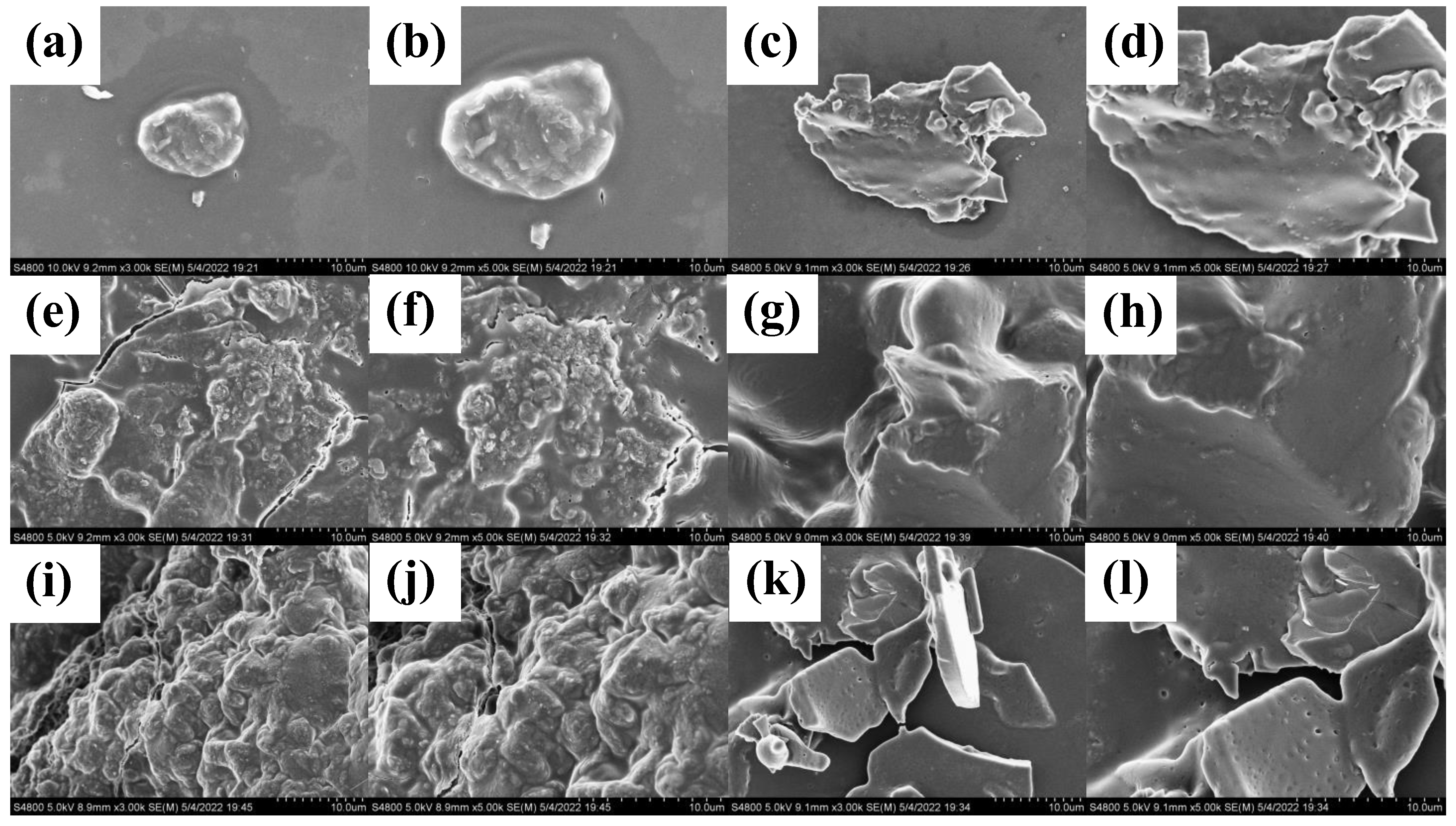
2.3.4. SEM Analysis of PSPs
2.3.5. NMR Analysis of PSPs
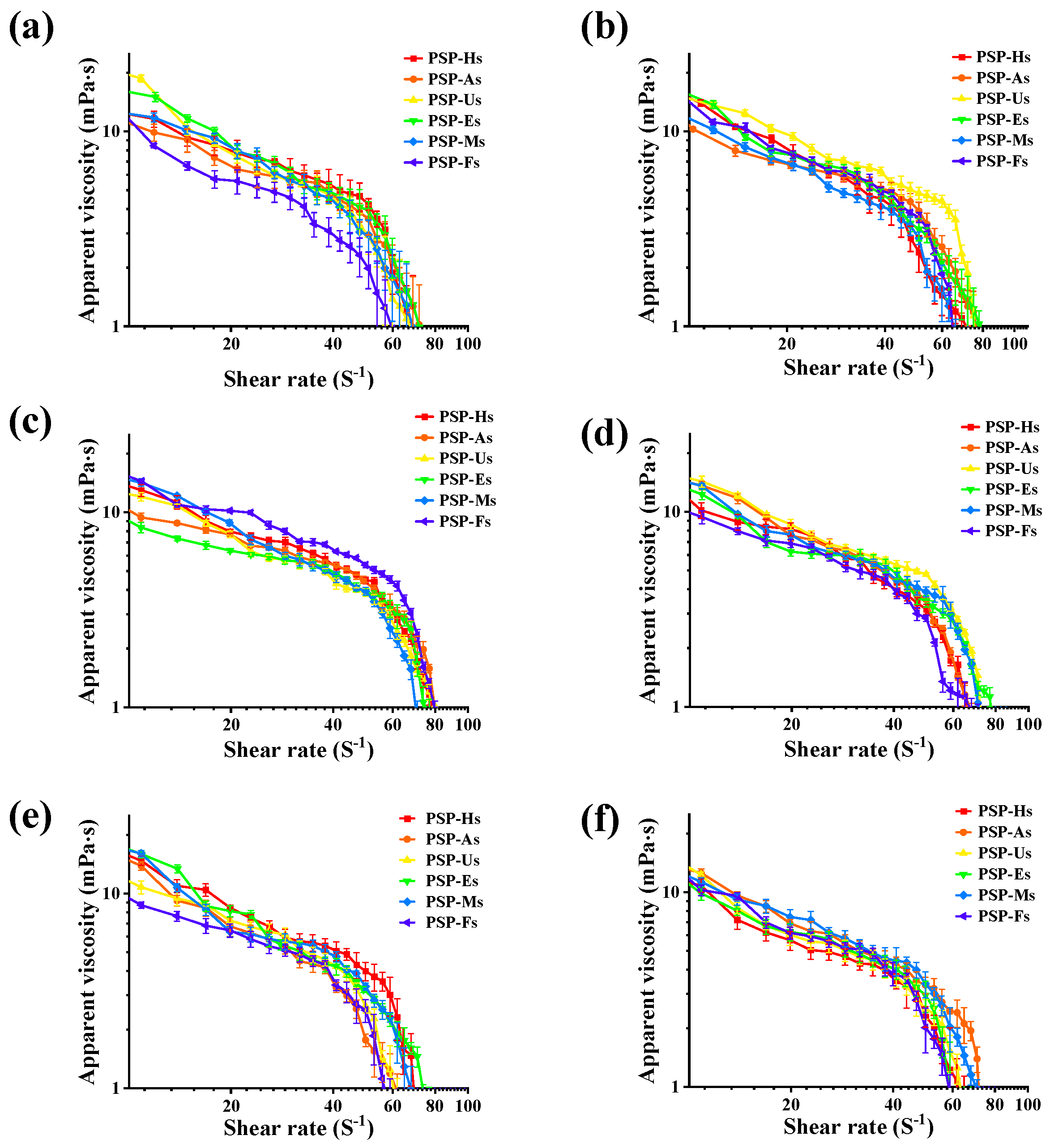
2.3.6. Rheological Property Analysis
2.4. Evaluation of Biological Properties of PSPs
2.4.1. In Vitro Antioxidant Activity
2.4.2. In Vitro Hypolipidemic Activity
2.4.3. In Vitro Immunomodulatory Activity
2.5. Statistical Analysis
3. Results and Analysis
3.1. Characterization
3.1.1. Compositions and Extraction Rates of PSPs
3.1.2. Functional Group Composition of PSPs
3.1.3. Analysis of Monosaccharide Composition and Average Mw of PSPs
3.1.4. Thermal Stability Analysis of PSPs
3.1.5. SEM Analysis of PSPs
3.1.6. NMR Analysis of PSPs
3.1.7. Steady Shear Flow Behavior
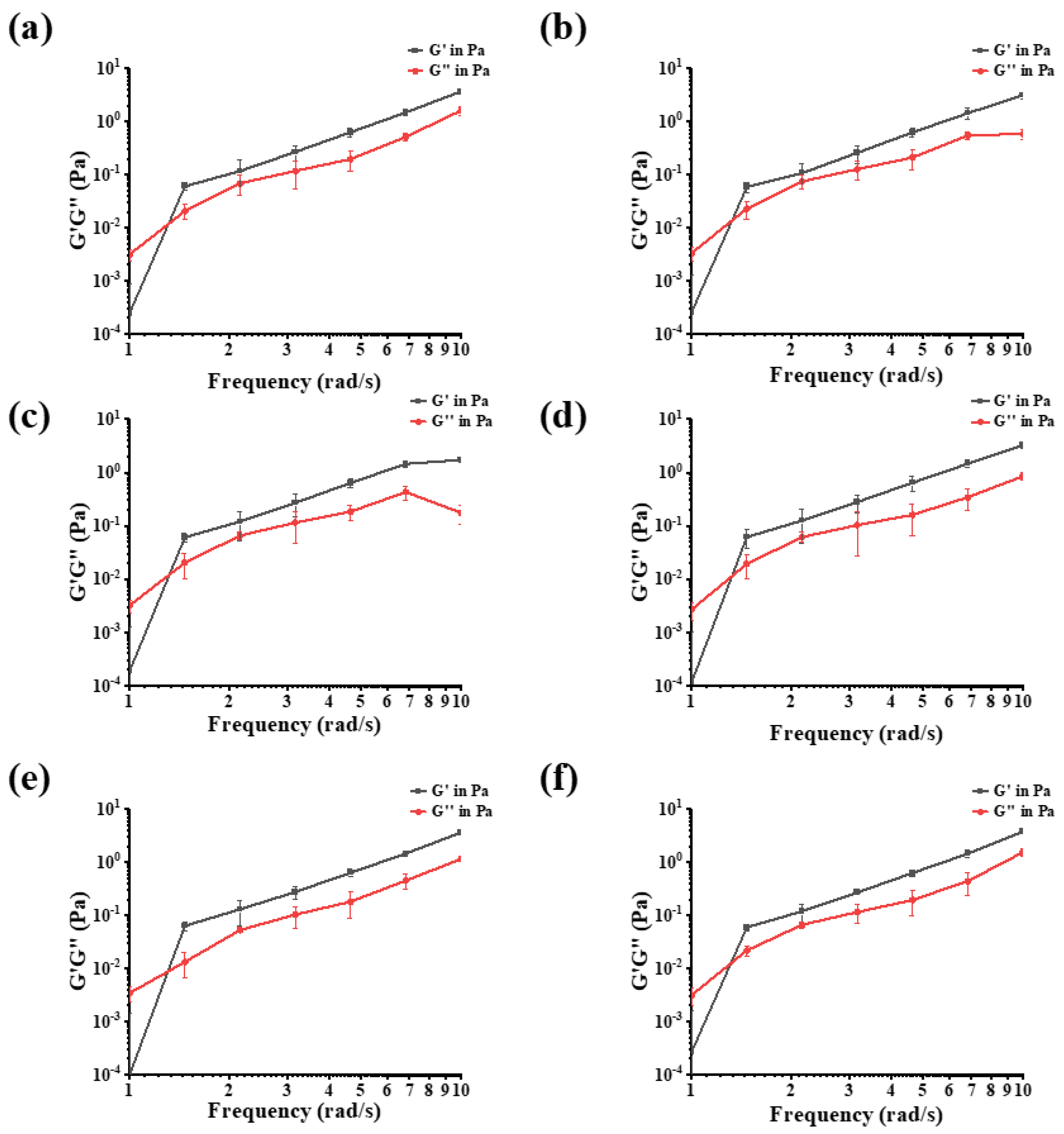
3.1.8. Frequency Sweep Measurements
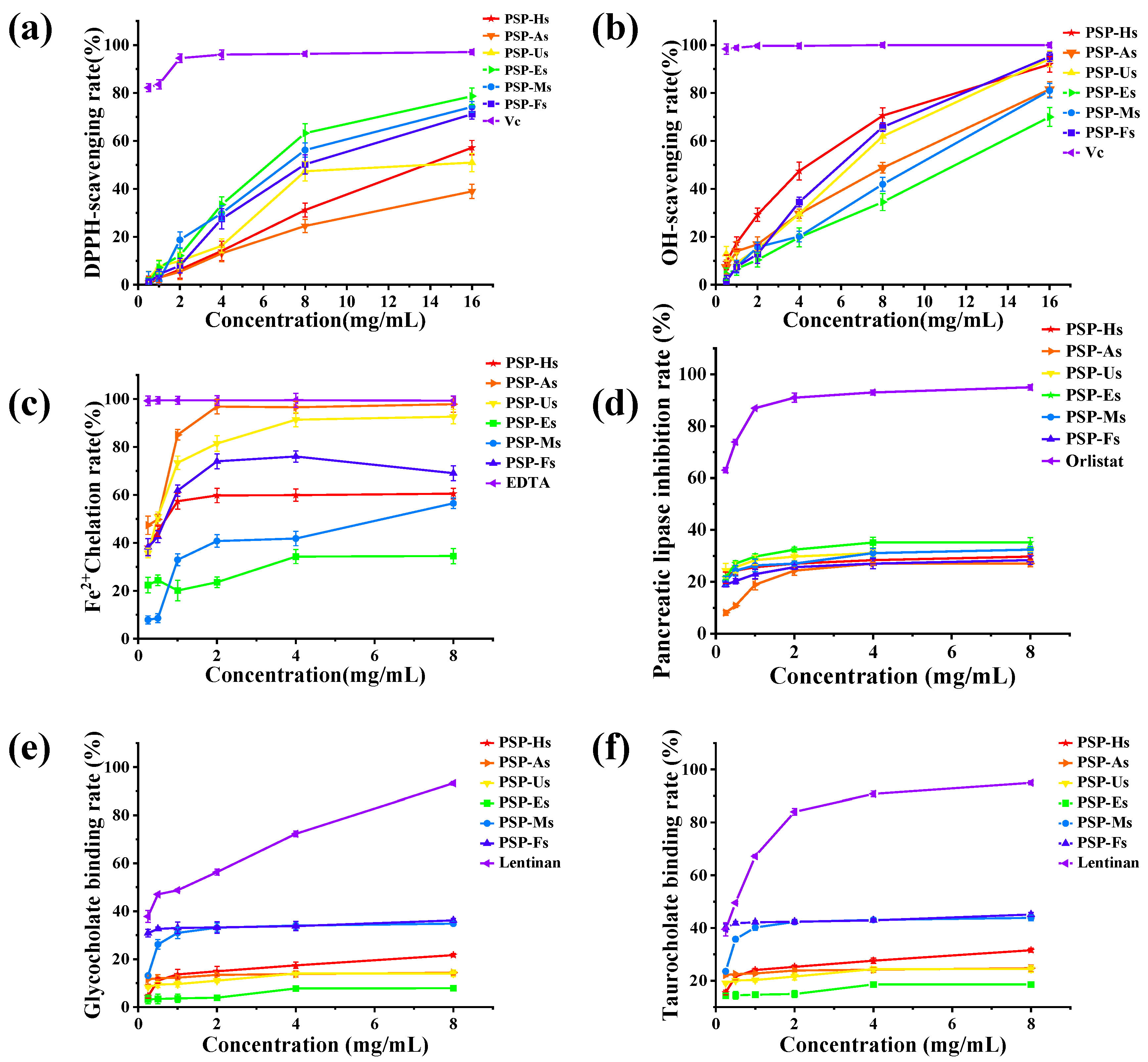
3.1.9. Antioxidant Activity of PSPs
3.1.10. Hypolipidemic Activity of PSPs
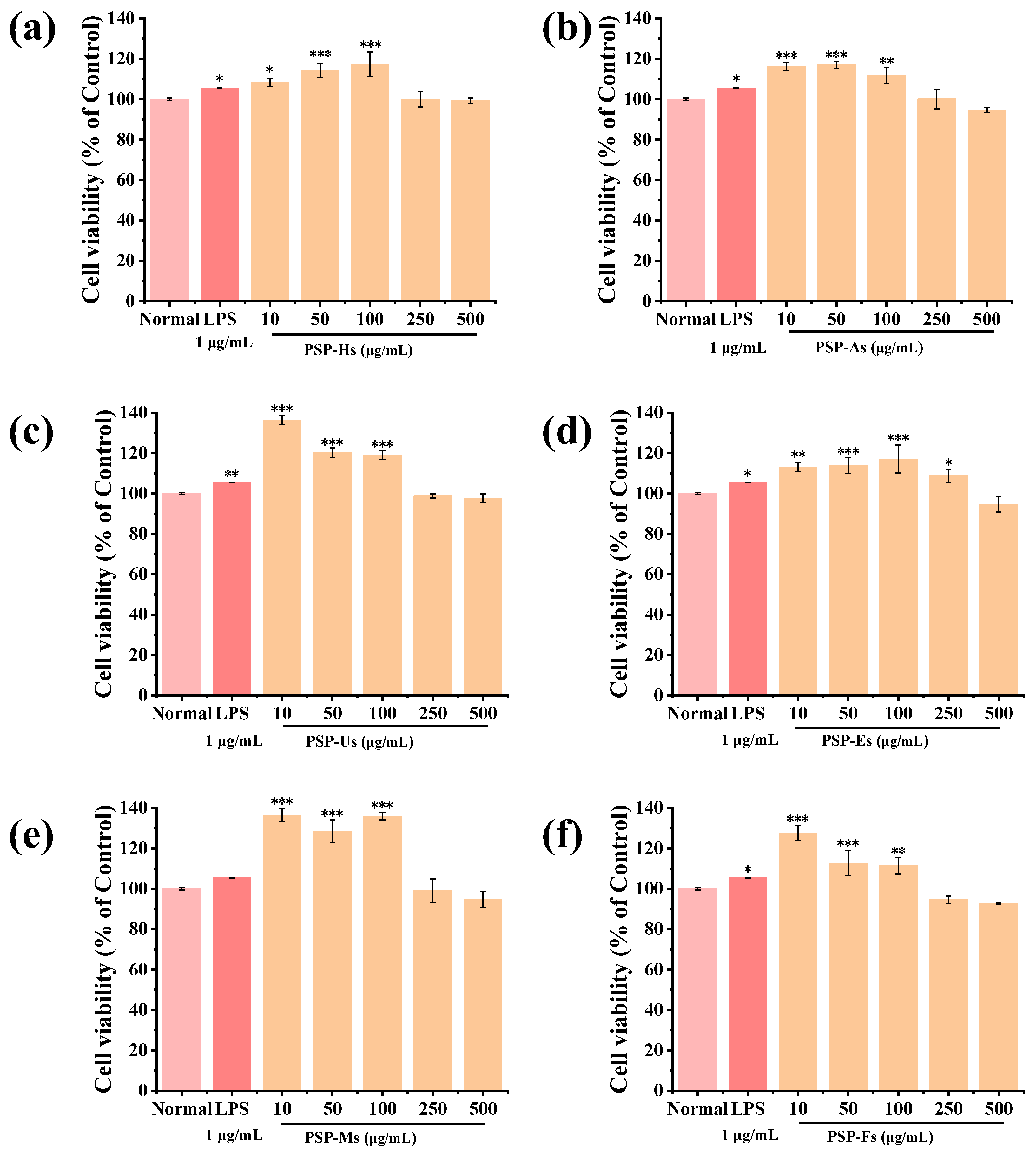
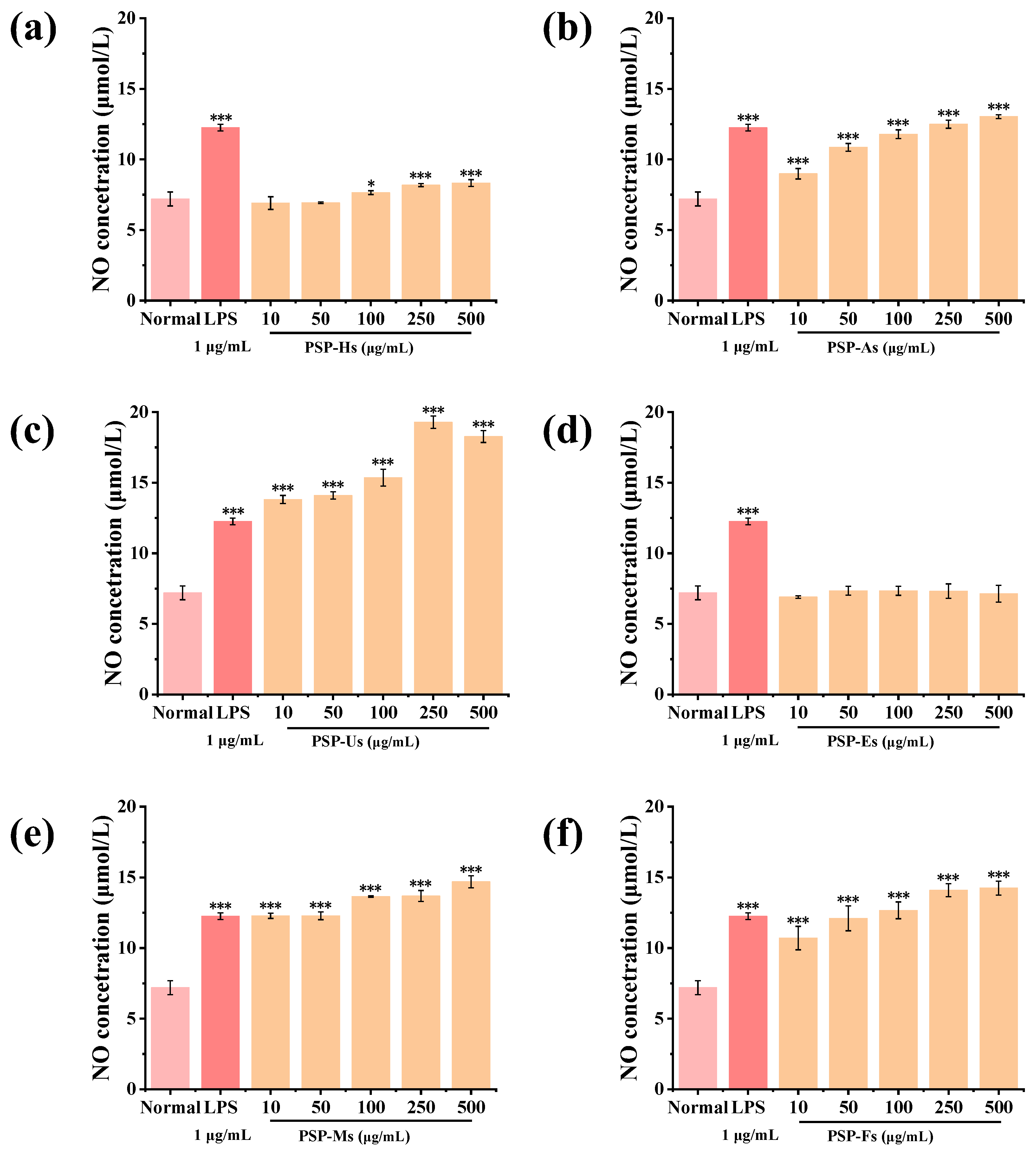
3.1.11. Immunomodulatory Activity of PSPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, C.L. Yunnan genuine medicinal material—Polygonatum kingianum. J. Dali Univ. 2022, 7, 102. [Google Scholar]
- Wang, Y.; Dong, P.; Jin, C.Z.; Kang, J.H.; Hu, Y.H.; Wang, Z. Analysis of polysaccharide composition and antioxidant activity of Polygonatum polysaccharides. Genom. Appl. Biol. 2019, 38, 253–261. [Google Scholar] [CrossRef]
- Chen, H.; Feng, S.S.; Sun, Y.J.; Hao, Z.Y.; Feng, W.S.; Zheng, X.K. Research progress on chemical constituents and pharmacological activities of three medicinal Polygonatum chinensis. Chin. Herb. Med. 2015, 46, 2329–2338. [Google Scholar]
- Ren, H.M.; Zhang, J.L.; Deng, Y.L.; Ye, X.W.; Xia, L.T.; Liu, M.M.; Liu, Y.; Chen, Y.; Zhang, Q.; Wang, T. Analysis of chemical components before and after preparation of Polygonatum cyrtonema based on UPLC-Q-TOF-MS. Chin. J. Exp. Formulas 2021, 27, 110–121. [Google Scholar] [CrossRef]
- Zhao, X.; Patil, S.; Qian, A.; Zhao, C.H. Bioactive compounds of Polygonatum sibiricum—Therapeutic effect and biological activity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 26–37. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.Y.; Huang, G.L. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Sania, Z.; Moazzam, R.K.; Muhammad, A.S.; Abid, A.M.; Muhammad, K.I.K.; Muhammad, N.; Anees, A.K.; Ahmad, D.; Rana, M.A. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in foods and food-related matrices. Food Rev. Int. 2020, 38, 1166–1196. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.F.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Jia, S.Y.; Li, F.; Liu, Y.; Ren, H.T.; Gong, G.L.; Wang, Y.Y.; Wu, S.H. Effects of extraction methods on the antioxidant activities of polysaccharides from Agaricus blazei Murrill. Int. J. Biol. Macromol. 2013, 62, 66–69. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.P.; Huang, Q.; You, L.J.; Liu, R.H.; Zhao, M.M.; Fu, X.; Lou, Z.G. A comparison study on polysaccharides extracted from: Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef]
- Wang, X.T.; Li, P.Y.; Wu, L.F.; Wu, Y.J.; Zheng, Y.G.; Liu, C.Y.; Yan, Y.P.; Jing, S.S. Optimization of the extraction process of Anemarrhena asphodeloides polysaccharide complex enzyme and its immune activity. Food Ind. Technol. 2022, 43, 218–227. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, C.H.; Huang, H.H. Characterization of crude polysaccharides from Hericium erinaceus by different extraction methods and comparison of antioxidant activity. Food Ind. Technol. 2017, 38, 80–86. [Google Scholar] [CrossRef]
- Zhao, M.M.; Zou, L.W.; You, L.J. Effects of extraction methods on properties of lentinan. South China Univ. Technol. (Nat. Sci. Ed.) 2013, 41, 26–33. [Google Scholar]
- Pei, R.N.; Zhai, H.L.; Qi, B.; Yang, X.Q. Response surface optimization of complex enzymatic extraction of crude polysaccharide from stone cauliflower. South Aquat. Sci. 2019, 15, 88–95. [Google Scholar]
- Tan, Q.Y.; Yuan, Y.J.; Wang, D.; Jiang, L.L.; Huang, H.; Peng, J. Effects of different extraction methods on Dendrobium officinale polysaccharide and hypoglycemic in vitro. Food Technol. 2019, 44, 202–206. [Google Scholar] [CrossRef]
- Jing, Y.S.; Cui, X.L.; Chen, Z.Y.; Huang, L.J.; Song, L.Y.; Liu, T.; Lv, W.J.; Yu, R.M. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohyd. Polym. 2014, 1002, 288–296. [Google Scholar] [CrossRef]
- Guo, H.; Fu, M.X.; Zhao, Y.X.; Li, H.; Li, H.B.; Wu, D.T.; Gan, R.Y. The Chemical, Structural, and Biological Properties of Crude Polysaccharides from Sweet Tea (Lithocarpus litseifolius (Hance) Chun) Based on Different Extraction Technologies. Foods 2021, 10, 1779. [Google Scholar] [CrossRef]
- Jing, Y.S.; Zhang, Y.M.; Cheng, W.J.; Li, M.S.; Hu, B.B.; Zheng, Y.G.; Zhang, D.S.; Wu, L.F. The Synthesis, Characterization, and Protein-Release Properties of Hydrogels Composed of Chitosan-Zingiber offcinale Polysaccharide. Foods 2022, 11, 2747. [Google Scholar] [CrossRef]
- Jing, Y.S.; Cheng, W.J.; Li, M.S.; Zhang, Y.M.; Pang, X.Y.; Qiu, X.Y.; Zheng, Y.G.; Zhang, D.S.; Wu, L.F. Structural Characterization, Rheological Properties, Antioxidant and Anti-Inflammatory Activities of Polysaccharides from Zingiber officinale. Plant Food. Hum. Nutr. 2023, 78, 160–165. [Google Scholar] [CrossRef]
- Jing, Y.S.; Cheng, W.J.; Ma, Y.F.; Zhang, Y.M.; Li, M.S.; Zheng, Y.G.; Zhang, D.S.; Wu, L.F. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Zingiber officinale and its application in synthesis of silver nanoparticles. Front. Nutr. 2022, 9, 917094. [Google Scholar] [CrossRef]
- Jing, Y.S.; Zhang, H.; Zhang, R.J.; Su, L.; Hu, B.B.; Zhang, D.S.; Zheng, Y.G.; Wu, L.F. Multiple fingerprint profiles and chemometrics analysis of polysaccharides from the roots of Glehnia littoralis. Nat. Prod. Commun. 2022, 17, 6. [Google Scholar] [CrossRef]
- Rafe, A.; Razavi, S.M.A. Dynamic viscoelastic study on the gelation of basil seed gum. Int. J. Food Sci. Technol. 2013, 48, 556–563. [Google Scholar] [CrossRef]
- Freitas, R.A.; Gorin, P.A.J.; Neves, J.; Sierakowski, M.R. A rheological description of mixtures of a galactoxyloglucan with high amylose and waxy corn starches. Carbohyd. Polym. 2003, 51, 25–32. [Google Scholar] [CrossRef]
- Wu, D.T.; An, L.Y.; Liu, W.; Hu, Y.C.; Wang, S.P.; Zou, L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022, 156, 111185. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.H.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, F.; Gan, S.; He, Y.N.; Chen, Z.J.; Liu, X.W.; Fu, C.M.; Qu, Y.; Zhang, J.M. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem. Toxicol. 2019, 131, 110539. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, Y.T.; Zong, S.; Zhang, J.Q.; Ye, M. Study on hypoglycemic and hypolipidemic activity of morel exopolysaccharide in vitro. Food Res. Dev. 2020, 41, 39–45. [Google Scholar]
- Xu, Y.Q.; Niu, X.J.; Liu, N.Y.; Gao, Y.K.; Wang, L.B.; Xu, G.; Li, X.G.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Fu, M.X.; Gan, R.Y.; Hu, Y.C.; Peng, L.X.; Zhao, G.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhou, H.L.; Li, Y.P.; Wu, M.F.; Yu, M.; Sun, X.S. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zeng, J.S.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.H.; Chen, K.F. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef] [PubMed]
- Rafe, A.; Razavi, S.M.A. Effect of Thermal Treatment on Chemical Structure of Β-Lactoglobulin and Basil Seed Gum Mixture at Different States by ATR-FTIR Spectroscopy. Int. J. Food Prop. 2015, 18, 2652–2664. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A.; et al. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Chansoria, P.; Asif, S.; Polkoff, K.; Chung, J.; Piedrahita, J.A.; Shirwaiker, R.A. Characterizing the Effects of Synergistic Thermal and Photo-Cross-Linking during Biofabrication on the Structural and Functional Properties of Gelatin Methacryloyl (GelMA) Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 5175–5188. [Google Scholar] [CrossRef]
- Li, L.; Liao, B.; Thakur, K.; Zhang, J.; Wei, Z. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2017, 109, 761–771. [Google Scholar] [CrossRef]
- Cheng, J.; He, S.Y.; Wan, Q.; Jing, P. Multiple fingerprinting analyses in quality control of Cassiae Semen polysaccharides. J. Chromatogr. B 2018, 1077–1078, 22–27. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, F.S. Application of nuclear magnetic resonance spectroscopy in the analysis of polysaccharide structure. Food Drug 2007, 8, 39–43. [Google Scholar]
- Tao, A.E. Physicochemical Properties, Activity Screening and Quality Evaluation of Polygonatum kingianum Polysaccharides. Master’s Thesis, Dali University, Dali, China, 2019. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.Z.; Sun, B.G.; Zhao, M.M. A comparison study on polysaccharides extracted from: Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef]
- Nie, X.R.; Li, H.Y.; Du, G.; Lin, S.; Hu, R.; Li, H.Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.L.; Wang, F.H.; Xu, J.J.; Tang, X.Z.; Li, N.Y. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef]
- Zheng, C.; Dong, Q.; Chen, H.; Cong, Q.; Ding, K. Structural characterization of a polysaccharide from Chrysanthemum morifolium flowers and its antioxidant activity. Carbohydr. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Wang, W.S.; Song, X.L.; Gao, Z.; Zhao, H.J.; Wang, X.X.; Liu, M.; Jia, L. Anti-hyperlipidemic, antioxidant and organic protection effects of acidic-extractable polysaccharides from Dictyophora indusiata. Int. J. Biol. Macromol. 2019, 129, 281–292. [Google Scholar] [CrossRef]
- Xue, Z.H.; Gao, X.D.; Jia, Y.N.; Wang, Y.J.; Lu, Y.P.; Zhang, M.; Panichayupakaranant, P.; Chen, H.X. Structure characterization of high molecular weight soluble dietary fiber from mushroom Lentinula edodes (Berk.) Pegler and its interaction mechanism with pancreatic lipase and bile salts. Int. J. Biol. Macromol. 2020, 153, 1281–1290. [Google Scholar] [CrossRef]
- Xu, W.; Mohan, A.; Pitts, N.L.; Udenigwe, C.; Mason, B. Bile acid-binding capacity of lobster shell-derived chitin, chitosan and chitooligosaccharides. Food Biosci. 2020, 33, 100476. [Google Scholar] [CrossRef]
- Wu, D.T.; Zhao, Y.X.; Guo, H.; Gan, R.Y.; Peng, L.X.; Zhao, G.; Zou, L. Physicochemical and Biological Properties of Polysaccharides from Dictyophora indusiata Prepared by Different Extraction Techniques. Polymers 2021, 13, 2357. [Google Scholar] [CrossRef]
- Zhao, G.H.; Li, Z.X.; Chen, Z.D. Structural analysis and antitumor activity of RDPS-I polysaccharide from Chinese yam. Acta Pharm. Sin. B 2003, 1, 37–41. [Google Scholar] [CrossRef]
- Xue, H.Y.; Li, J.R.; Liu, Y.G.; Gao, Q.; Wang, X.W.; Zhang, J.W.; Tanokura, M.; Xu, Y.L. Optimization of the ultrafiltration-assisted extraction of Chinese yam polysaccharide using response surface methodology and its biological activity. Int. J. Biol. Macromol. 2019, 121, 1186–1193. [Google Scholar] [CrossRef]
| Sample | Extraction Rate (%) | Total Soluble Sugar Content (%) |
|---|---|---|
| PSP-Hs | 14.86 ± 3.14 a | 80.07 ± 2.70 b |
| PSP-As | 9.62 ± 2.02 bc | 78.10 ± 4.33 b |
| PSP-Us | 5.83 ± 2.52 cd | 76.61 ± 1.74 b |
| PSP-Es | 12.00 ± 3.26 ab | 84.64 ± 2.11 a |
| PSP-Ms | 6.71 ± 1.49 cd | 87.14 ± 1.69 a |
| PSP-Fs | 3.40 ± 1.01 d | 77.52 ± 1.28 b |
| Absorption Peak/cm−1 | Functional Group |
|---|---|
| 3276 | -OH stretching vibration absorption peak |
| 2933 | C-H stretching vibration of CH2 group |
| 1743 | C=O vibration absorption peak |
| 1629 | Vibrational absorption peaks induced by O-H/C-O/COO- |
| 1414 | C-H, CH2 bending vibration absorption peaks |
| 1126 | Variation-angle vibration peaks of alcohol hydroxyl groups |
| 1014/929 | Presence of pyranose |
| 875 | Characteristic absorption peaks of β-pyranose |
| 875/813 | Presence of mannose (Man) |
| Sample | Monosaccharide Composition (mol%) | ||||
|---|---|---|---|---|---|
| Man | GalA | Glc | Gal | Xyl | |
| PSP-Hs | 0.212 | 0.086 | 0.559 | 0.143 | - |
| PSP-As | 0.190 | 0.039 | 0.768 | 0.122 | 0.052 |
| PSP-Us | 0.302 | - | 0.516 | - | 0.182 |
| PSP-Es | 0.050 | - | 0.950 | - | - |
| PSP-Ms | 0.165 | - | 0.502 | - | 0.333 |
| PSP-Fs | 0.309 | 0.136 | 0.378 | 0.178 | - |
| Sample | δ1 | δ2 | δ3 | δ4 | δ5 | δ6 | δ7 | δ8 | δ9 | δ10 |
|---|---|---|---|---|---|---|---|---|---|---|
| PSP-Hs | 2.511 | 3.190 | 3.387 | - | 3.558 | 3.841 | 4.007 | 4.693 | 4.819 | 5.202 |
| PSP-As | 2.514 | 3.190 | 3.396 | 3.462 | 3.560 | 3.850 | 4.012 | 4.715 | 4.851 | 5.216 |
| PSP-Us | 2.511 | 3.188 | 3.388 | 3.463 | 3.561 | 3.840 | 4.009 | 4.681 | 4.792 | 5.207 |
| PSP-Es | 2.515 | 3.187 | 3.388 | 3.467 | 3.569 | 3.845 | 4.008 | 4.693 | 4.807 | 5.219 |
| PSP-Ms | 2.516 | 3.185 | 3.385 | 3.463 | 3.567 | 3.842 | 4.005 | 4.689 | 4.805 | 5.199 |
| PSP-Fs | 2.518 | 3.189 | 3.386 | - | 3.569 | 3.843 | 4.000 | 4.680 | 4.812 | 5.212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Yan, M.; Zhang, H.; Liu, D.; Qiu, X.; Hu, B.; Zhang, D.; Zheng, Y.; Wu, L. Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum sibiricum. Foods 2023, 12, 2088. https://doi.org/10.3390/foods12102088
Jing Y, Yan M, Zhang H, Liu D, Qiu X, Hu B, Zhang D, Zheng Y, Wu L. Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum sibiricum. Foods. 2023; 12(10):2088. https://doi.org/10.3390/foods12102088
Chicago/Turabian StyleJing, Yongshuai, Meng Yan, Hao Zhang, Dongbo Liu, Xiaoyue Qiu, Beibei Hu, Danshen Zhang, Yuguang Zheng, and Lanfang Wu. 2023. "Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum sibiricum" Foods 12, no. 10: 2088. https://doi.org/10.3390/foods12102088
APA StyleJing, Y., Yan, M., Zhang, H., Liu, D., Qiu, X., Hu, B., Zhang, D., Zheng, Y., & Wu, L. (2023). Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum sibiricum. Foods, 12(10), 2088. https://doi.org/10.3390/foods12102088