Techno-Functional Properties of Burgers Fortified by Wild Garlic Extract: A Reconsideration
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Freshly Squeezed Wild Garlic Extract
2.3. LC-MS/MS Analyses
2.4. Sample Preparation for Qualitative Analysis
2.5. GC-MS Analyses
2.6. Antibacterial Activity, Minimum Inhibitory Concentrations and Minimum Bactericidal Concentration Values
2.7. Burger Preparation
- CON = 0;
- PS-I = 15 ppm = 15 mg allicin/kg of BU = 1.32 mL FSWGE/kg of BU;
- PS-II = 50 ppm = 50 mg allicin/kg of BU = 4.40 mL FSWGE/kg of BU;
- PS-III = 100 ppm = 100 mg allicin/kg of BU = 8.79 mL FSWGE/kg of BU.
2.8. Determination of Total Phenolics and Antioxidant Capacity in Raw Burgers
2.9. Determination of Technological Properties and Proximate Composition
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of WG Extracts
3.2. Antibacterial Activity
3.3. Total Phenolics and Antioxidant Capacity in Raw Burgers
3.4. Technological Properties and Proximate Composition of Burgers
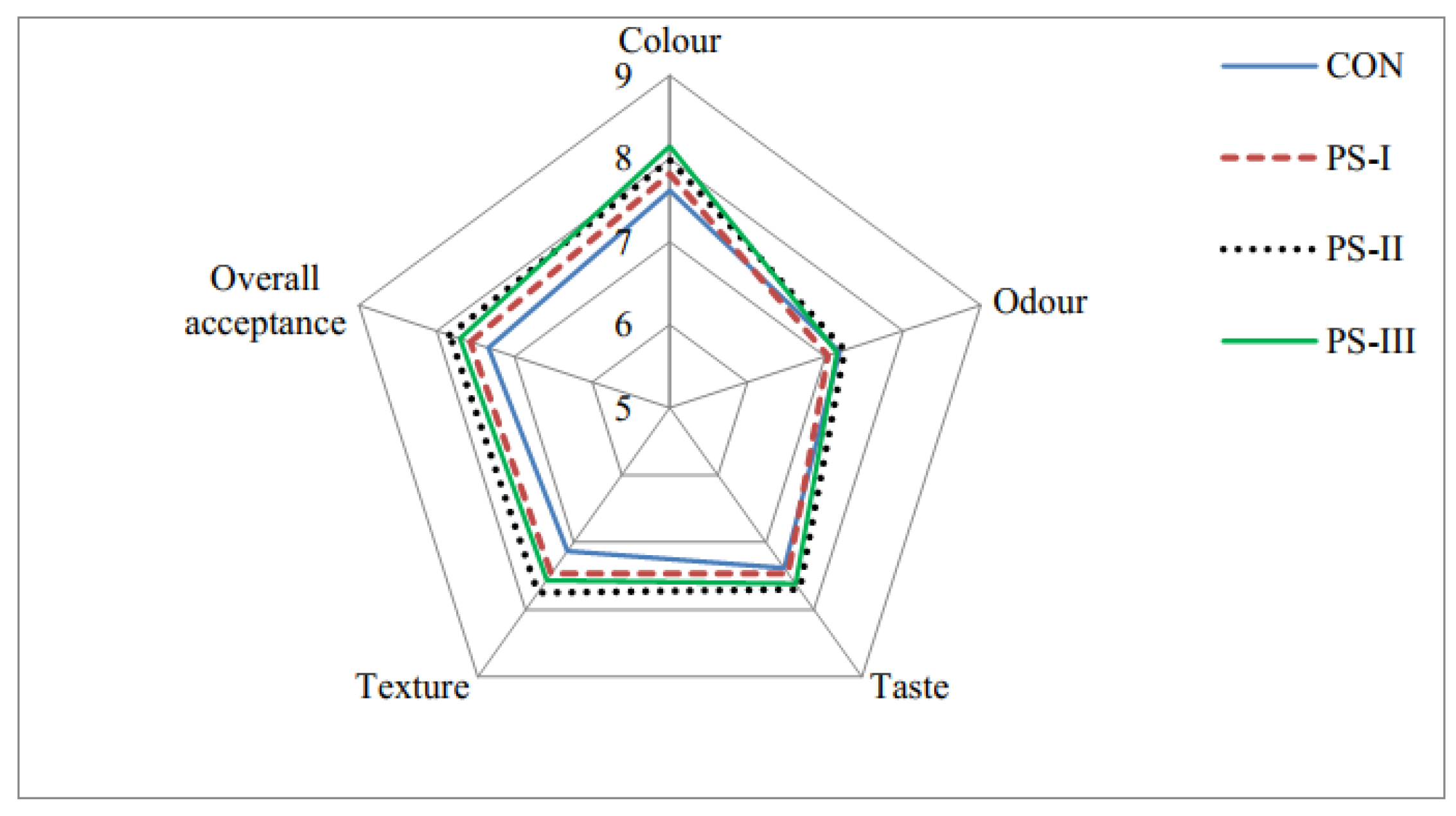
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhaskar, R.; Ola, M. Junk food: Impact on health. J. Drug Deliv. Sci. Technol. 2012, 2, 67–73. [Google Scholar] [CrossRef]
- Bhushan, C.; Taneja, S.; Khurana, A. Centre for Science and Environment, New Delhi, Burden of Packaged Food on Schoolchildren: Based on the CSE Survey ‘Know Your Diet’. Available online: https://www.jstor.org/stable/resrep38038 (accessed on 20 January 2019).
- Singh, S.A.; Dhanasekaran, D.; Ganamurali, N.L.P.; Sabarathinam, S. Junk food-induced obesity—A growing threat to youngsters during the pandemic. Obes. Med. 2021, 26, 100364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Fuhrman, J. The hidden dangers of fast and processed food. Am. J. Lifestyle Med. 2018, 12, 375–381. [Google Scholar] [CrossRef]
- WHO The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 April 2020).
- Patibandla, G.; Yamani, L. Fast food and soft drink consumption pattern in medical students and its association with overweight and obesity. Glob. J. Med. Stud. 2021, 1, 9–14. [Google Scholar] [CrossRef]
- da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Analyt. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A. Nutritional ethnobotany in Europe: From emergency foods to healthy folk cuisines and contemporary foraging trends. In Mediterranean Wild Edible Plants; Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 33–56. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Varela, P.; Peschel, A.O. Consumers’ categorization of food ingredients: Do consumers perceive them as ‘clean label’ producers expect? An exploration with projective mapping. Food Qual. Prefer. 2019, 71, 117–128. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Barba, F.J.; Lorenzo, J.M.; Penna, A.L.B.; Barretto, A.C.S. New strategies for the development of innovative fermented meat products: A review regarding the incorporation of probiotics and dietary fibers. Food Rev. Int. 2019, 35, 467–484. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, D.Y.; Kim, O.Y.; Kang, H.J.; Kim, H.S.; Hur, S.J. Overview of studies on the use of natural antioxidative materials in meat products. Food Sci. Anim. Resour. 2020, 40, 863–880. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, T.K.; Choi, H.D.; Jung, S.; Choi, Y.S. Technological strategy of clean label meat products. Food Life 2020, 1, 13–20. [Google Scholar] [CrossRef]
- Heck, R.T.; Vendruscolo, R.G.; de Araújo Etchepare, M.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Lorenzo, J.M.; Wagner, R.; Campagnol, P.C.B. Is it possible to produce a low-fat burger with a healthy n-6/n-3 PUFA ratio without affecting the technological and sensory properties? Meat Sci. 2017, 130, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Inetianbor, J.; Yakubu, J.; Ezeonu, S. Effects of food additives and preservatives on man: A review. AJST 2016, 6, 1118–1135. [Google Scholar]
- Lee, N.-K.; Paik, H.-D. Status, antimicrobial mechanism, and regulation of natural preservatives in livestock food fystems. Food Sci. Anim. Resour. 2016, 36, 547–557. [Google Scholar] [CrossRef]
- Sharif, Z.I.M.; Mustapha, F.A.; Jai, J.; Yusof, N.; Zaki, N.A.M. Review on methods for preservation and natural preservatives for extending the food longevity. Chem. Eng. Res. Bull. 2017, 19, 145–153. [Google Scholar] [CrossRef]
- Antonini, E.; Torri, L.; Piochi, M.; Cabrino, G.; Melia, M.A.; Bellis, R.D. Nutritional, antioxidant and sensory properties of functional beef burgers formulated with chia seeds and goji puree, before and after in vitro digestion. Meat Sci. 2020, 161, 108021. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramella, M.; Munekata, P.E.S.; Pateiro, M.; Franco, D.; Campagnol, P.C.B.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Physicochemical composition and nutritional properties of deer burger enhanced with healthier oils. Foods 2020, 9, 571. [Google Scholar] [CrossRef]
- Ziegler, V.; Ugalde, M.L.; Veeck, I.A.; Barbosa, F.F. Nutritional enrichment of beef burgers by adding components of non-conventional food plants. Braz. J. Food Technol. 2020, 23, e2019030. [Google Scholar] [CrossRef]
- Voća, S.; Šic Žlabur, J.; Fabek Uher, S.; Peša, M.; Opačić, N.; Radman, S. Neglected potential of wild garlic (Allium ursinum L.)—Specialized metabolites content and antioxidant capacity of wild populations in relation to location and plant phenophase. Horticulturae 2022, 8, 24. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, L.; Singh, S. A review on medicinal plants having antioxidant potential. Indian J. Res. Pharm. Biotechnol. 2013, 1, 404–409. [Google Scholar]
- Radulović, N.S.; Miltojević, A.B.; Stojković, M.B.; Blagojević, P.D. New volatile sulfur-containing compounds from wild garlic (Allium ursinum L., Liliaceae). Food Res. Int. 2015, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Djurdjevic, L.; Dinic, A.; Pavlovic, P.; Mitrovic, M.; Karadzic, B.; Tesevic, V. Allelopathic potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 32, 533–544. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Was, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.R.; Veljković, M.; Stojanović, N.M.; Gočmanac-Ignjatović, M.; Mihailov-Krstev, T.; Branković, S.; Radenković, M. Influence of different wild-garlic (Allium ursinum) extracts on the gastrointestinal system: Spasmolytic, antimicrobial and antioxidant properties. J. Pharm. Pharmacol. J. 2017, 69, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Soković Bajić, S.; Jovanović, Ž.; Matić, I.; Tolinački, M.; Popović, D.; Samardžić, J. Antioxidant and antiproliferative activity of Allium ursinum and their associated microbiota during simulated in vitro digestion in the presence of food matrix. Front. Microbiol. 2020, 11, 601616. [Google Scholar] [CrossRef]
- Rankovic, M.; Krivokapic, M.; Bradic, J.; Petkovic, A.; Zivkovic, V.; Sretenovic, J.; Tomovic, M. New insight into the cardioprotective effects of Allium ursinum L. extract against myocardial ischemia-reperfusion injury. Front. Physiol. 2021, 12, 690696. [Google Scholar] [CrossRef]
- Coulston, A.M.; Rock, C.L.; Monsen, E.R. Nutrition in the Prevention and Treatment of Disease; Academic Press: Orlando, FL, USA, 2001. [Google Scholar]
- Wu, H.; Dushenkov, S.; Ho, C.-T.; Sang, S. Novel acetylated flavonoid glycosides from the leaves of Allium ursinum. Food Chem. 2009, 115, 592–595. [Google Scholar] [CrossRef]
- Gîtin, L.; Dinică, R.; Parnavel, R. The influence of extraction method on the apparent content of bioactive compounds in Romanian Allium spp. leaves. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 93–97. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Characterization and content of flavonol derivatives of Allium ursinum L. plant. J. Agric. Food Chem. 2013, 61, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Piatkowska, E.; Kopeć, A.; Leszczynska, T. Basic chemical composition, content of micro and macro elements and antioxidant activity of different varieties of garlic’s leaves polish origin. ZYWNOSC—Nauka Technol. Jakosc 2015, 98, 181–192. [Google Scholar]
- Lachowicz, S.; Oszmiański, J.; Wiśniewski, R. Determination of triterpenoids, carotenoids, chlorophylls, and antioxidant capacity in Allium ursinum L. at different times of harvesting and anatomical parts. Eur. Food Res. Technol. 2018, 244, 1269–1280. [Google Scholar] [CrossRef]
- Śmiecińska, K.; Gugołek, A.; Kowalska, D. Effects of garlic (Allium sativum L.) and ramsons (Allium ursinum L.) on lipid oxidation and the microbiological quality, physicochemical properties and sensory attributes of rabbit meat burgers. Animals 2022, 12, 1905. [Google Scholar] [CrossRef]
- Spencer, E.H.; Frank, E.; McIntosh, N.F. Potential effects of the next 100 billion hamburgers sold by McDonald’s. Am. J. Prev. Med. 2005, 28, 379–381. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. 2021. Available online: http://sweetgum.nybg.org/ih (accessed on 11 January 2023).
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–175. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 2, 1231–1237. [Google Scholar] [CrossRef]
- ISO 1442:1997; Meat and Meat Products—Determination of Moisture Content. International Organization for Standardization: Geneva, Switzerland, 1997.
- ISO 937:1978; Meat and Meat Products. Determination of Nitrogen Content. International Organization for Standardization: Geneva, Switzerland, 1978.
- ISO 1443:1973; Meat and Meat Products. Determination of Total Fat Content. International Organization for Standardization: Geneva, Switzerland, 1973.
- Poojary, M.M.; Putnik, P.; Bursać Kovačević, D.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barbad, F.J.; Granato, D.; Mallikarjunanb, K.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Phytochemical analysis of Allium fistulosum L. and A. ursinum L. Dig. J. Nanomater. Biost. 2013, 8, 457–467. [Google Scholar]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the antioxidant activity of Nigella sativa L. and Allium ursinum extracts in a cellular model of doxorubicin-induced cardiotoxicity. Molecules 2020, 25, 5259. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Di Gioia, F.; Polyzos, N.; Tzortzakis, N. Natural antioxidants, health effects and bioactive properties of wild allium species. Curr. Pharm. Des. 2020, 26, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Gođevac, D.; Vujisić, L.; Mojović, M.; Ignjatović, A.; Spasojević, I.; Vajs, V. Evaluation of antioxidant capacity of Allium ursinum L. volatile oil and its effect on membrane fluidity. Food Chem. 2008, 107, 1692–1700. [Google Scholar] [CrossRef]
- Vidović, S.; Tomšik, A.; Vladić, J.; Jokić, S. Supercritical carbon dioxide extraction of Allium ursinum: Impact of temperature and pressure on the extracts chemical profile. Chem. Biodivers. 2021, 18, e2100058. [Google Scholar] [CrossRef]
- Schmitt, B.; Schulz, H.; Storsberg, J.; Keusgen, M. Chemical characterization of Allium ursinum L. depending on harvesting time. J. Agric. Food Chem. 2005, 53, 7288–7294. [Google Scholar] [CrossRef]
- Błażewicz-Woźniak, M.; Michowska, A. The growth, flowering and chemical composition of leaves of three ecotypes of Allium ursinum L. Acta Agrobot. 2011, 64, 171–180. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, A.R.; Han, J. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Contr. 2013, 29, 112–120. [Google Scholar] [CrossRef]
- Sapunjieva, T.; Alexieva, I.; Mihaylova, D.; Popova, A. Antimicrobial and antioxidant activity of extracts of Allium ursinum L. J. BioSci. Biotechnol. 2012, SE/ONLINE, 143–145. [Google Scholar]
- Synowiec, A.; Gniewosz, M.; Zieja, I.; Baczek, K.; Przybyl, J. The comparison of antimicrobial properties of ramson (Allium ursinum) extracts. Zesz. Probl. Postep. Nauk Roln. 2010, 553, 203–209. (In Polish) [Google Scholar]
- Bagiu, R.V.; Vlaicu, B.; Butnariu, M. Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). Int. J. Mol. Sci. 2012, 13, 1426–1436. [Google Scholar] [CrossRef]
- de Sousa Lima, C.M.; Fujishima, M.A.T.; de Paula Lima, B.; Mastroianni, P.C.; de Sousa, F.F.O.; da Silva, J.O. Microbial contamination in herbal medicines: A serious health hazard to elderly consumers. BMC Complement. Med. Ther. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Kurćubić, V.S.; Stajić, S.B.; Dmitrić, M.P.; Miletić, N.M. Food safety assessment of burger patties with added herbal plant material. Fleischwirtschaft 2022, 102, 73–78, ISSN 0015-363X. [Google Scholar]
- Fullerton, M.; Khatiwada, J.; Johnson, J.U.; Davis, S.; Williams, L.L. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Escherichia coli O157:H7 isolated from food, veterinary, and clinical samples. J. Med. Food 2011, 14, 950–956. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Yin, M.-C.; Cheng, W.-S. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003, 63, 23–28. [Google Scholar] [CrossRef]
- Stupar, A.; Šarić, L.; Vidović, S.; Bajić, A.; Kolarov, V.; Šarić, B. Antibacterial potential of Allium ursinum extract prepared by the green extraction method. Microorganisms 2022, 10, 1358. [Google Scholar] [CrossRef]
- Li, K.; Guan, G.; Zhu, J.; Wu, H.; Sun, Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control 2019, 96, 234–243. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Putnoky, S.; Caunii, A.; Butnariu, M. Study on the stability and antioxidant effect of the Allium ursinum watery extract. Chem. Cent. J. 2013, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Šarić, L.; Bertoni, S.; Protti, M.; Albertini, B.; Mercolini, L.; Passerini, N. Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Res. Int. 2018, 119, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmianski, J.; Wisniewski, R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. J. Food Process. Preserv. 2016, 41, e12921. [Google Scholar] [CrossRef]
- Harris, C.S.; Mo, F.; Migahed, L.; Chepelev, L.; Haddad, P.S.; Wright, J.S.; Bennett, S.A.L. Plant phenolics regulate neoplasic cell growth and survival: A quantitative structure- activity and biochemical analysis. Can. J. Physiol. Pharmacol. 2007, 85, 1124–1138. [Google Scholar] [CrossRef]
- Hiyasat, B.; Sabha, D.; Grötzinger, K.; Kempfert, J.; Rauwald, J.-W.; Mohr, F.W.; Dhein, S. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Choi, J.W.; Nam, J.H.; Park, H.J. Anti-ulcerogenic effects of the flavonoid-rich fraction from the extract of Orostachys japonicus in mice. J. Med. Food 2007, 10, 702–706. [Google Scholar] [CrossRef]
- Lien, A.P.H.; He, H.; Chuong, P.H. Green tea and health: An overview. J. Food Agric. Environ. 2008, 6, 6–13. [Google Scholar]
- Rietz, B.; Isensee, H.; Strobach, H.; Makdessi, S.; Jacob, R. Cardioprotective actions of Wild garlic (Allium ursinum) in ischemia and reperfusion. Mol. Cell. Biochem. 1993, 119, 143–150. [Google Scholar] [CrossRef]
- Asdaq, S.M.; Inamdar, M.N. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M.A. Review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Omar, S.H. Garlic and cardiovascular diseases. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Xu, X.Y.; Song, G.Q.; Yu, Y.Q.; Ma, H.Y.; Ma, L.; Jin, Y.N. Apoptosis and G2/M arrest induced by Allium ursinum (ramson) watery extract in an AGS gastric cancer cell line. Onco Targets Ther. 2013, 25, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Parvu, A.E.; Parvu, M.; Vlase, L.; Miclea, P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory effects of Alium schoenoprasum L. leaves. J. Physiol. Pharmacol. 2014, 65, 309–315. [Google Scholar] [PubMed]
- Mihaylova, D.; Lante, A.; Tinello, F.; Krastanov, A.I. Study on the antioxidant and antimicrobial activities of Allium ursinum L. pressurized-liquid extract. Nat. Prod. Res. 2014, 28, 2000–2005. [Google Scholar] [CrossRef]
- Finotti, E.; Di Majo, D. Influence of solvents on the antioxidant property of flavonoids. Food/Nahrung 2003, 47, 186–187. [Google Scholar] [CrossRef]
- Muñiz-Becerá, S.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J.; Sandoval-Torres, S.; López-Ortíz, A.; Barriada-Bernal, L.G. Modeling of solute transport inside plant tissue during osmotic dehydration of apple. Dry. Technol. 2022, 40, 387–400. [Google Scholar] [CrossRef]
- Naveena, B.M.; Sen, A.R.; Vaithiyanathan, S.; Babji, Y.; Kondaiah, N. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008, 80, 1304–1308. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; True, A.D.; Zhou, L.; Xiong, Y.L. Inhibition of lipid oxidation and rancidity in precooked pork patties by radical-scavenging licorice (Glycyrrhiza glabra) extract. J. Food Sci. 2013, 78, C1686–C1694. [Google Scholar] [CrossRef]
- Patinho, I.; Selani, M.M.; Saldaña, E.; Bortoluzzi, A.C.T.; Rios-Mera, J.D.; da Silva, C.M.; Kushida, M.M.; Contreras-Castillo, C.J. Agaricus bisporus mushroom as partial fat replacer improves the sensory quality maintaining the instrumental characteristics of beef burger. Meat Sci. 2021, 172, 108307. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf-life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; da Silva Barretto, A.C. Red pitaya extract as natural antioxidant in pork patties with total replacement of animal fat. Meat Sci. 2021, 171, 108284. [Google Scholar] [CrossRef]
- Martins, A.J.; Lorenzo, J.M.; Franco, D.; Vicente, A.A.; Cunha, R.L.; Pastrana, L.M.; Cerqueira, M.A. Omega-3 and Polyunsaturated Fatty Acids-Enriched Hamburgers Using Sterol-Based Oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1900111. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Medyński, A.; Pospiech, E.; Kniat, R. Effect of various concentrations of lactic acid and sodium chloride on selected physico-chemical meat traits. Meat Sci. 2000, 55, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Manios, S.G.; Skandamis, P.N. Effect of frozen storage, different thawing methods and cooking processes on the survival of Salmonella spp. and Escherichia coli O157:H7 in commercially shaped beef patties. Meat Sci. 2015, 101, 25–32. [Google Scholar] [CrossRef]
| Compound [Molecular Formula] | RT 1 (min) | Concentration (mg/mL) |
|---|---|---|
| Allicin [C6H10OS2] | 6.21 | 11.375 |
| Ferulic acid [C10H10O4] | 5.53 | 4.259 |
| p-Coumaric acid [C9H8O3] | 4.86 | 1.453 |
| Sinapic acid [C11H12O5] | 5.27 | 1.175 |
| Compound [Molecular Formula] | RT 1 (min) | Area (%) 2 | |
|---|---|---|---|
| 1. | Methyl 2-propenyl trisulfide [C4H8S3] | 13.233 | 21.63 |
| 2. | Diallyl disulfide [C6H10S2] | 10.757 | 13.30 |
| 3. | Diallyl trisulfide [C6H10S3] | 20.305 | 12.87 |
| 4. | Allyl methyl disulfide [C4H8S2] | 5.428 | 8.43 |
| 5. | Dimethyl trisulfide [C2H6S3] | 6.881 | 4.66 |
| 6. | (3-Chlorophenyl)acetylene [C8H5Cl] | 15.602 | 3.80 |
| 7. | 2-Ethylbenzenesulfonamide [C8H11NO2S] | 6.482 | 3.29 |
| 8. | Ethyl vinyl disulfide [C4H8S2] | 23.710 | 3.16 |
| 9. | Allyl-1-propenyl trisulfide [C6H10S3] | 21.374 | 2.58 |
| 10. | Isobutyl isothiocyanate [C5H9NS] | 20.754 | 2.00 |
| 11. | Diallyl tetrasulfide [C6H10S4] | 30.399 | 1.92 |
| 12. | 1-Methyl-3-(methylamino)-4-pyrazolecarboxamide [C6H10N4O] | 13.604 | 1.56 |
| 13. | Methyl 1-propenyl disulfide [C4H8S2] | 5.982 | 1.51 |
| 14. | 3-Vinyl-4H-1,2-dithiin [C6H8S2] | 15.120 | 1.08 |
| 15. | Dimethyl disulfide [C2H6S2] | 2.700 | 0.92 |
| 16. | 2-Vinyl-4H-1,3-dithiine [C6H8S2] | 16.223 | 0.88 |
| 17. | Dihydro-2(3H)-thiophenthione [C4H6S2] | 11.387 | 0.78 |
| 18. | 3-Sulfanyl-2-(sulfanylmethyl)propanoic acid [C4H8O2S2] | 14.004 | 0.63 |
| 19. | Methyl 2-(propylthio)acetate [C6H12O2S] | 12.104 | 0.36 |
| 20. | Hexathiepane [CH2S6] | 36.888 | 0.34 |
| 21. | 2-Chloro-6-(methoxymethyl)toluene [C9H11ClO] | 34.193 | 0.34 |
| 22. | 1,2-Benzenediamine, N,N’-disulfinyl [C6H4N2O2S2] | 34.858 | 0.32 |
| 23. | Dimethyl tetrasulfide [C2H6S4] | 19.837 | 0.32 |
| 24. | 2,2,4,6,6-Pentamethylheptane [C12H26] | 7.420 | 0.19 |
| 25. | Triphenylboroxin[C18H15B3O3] | 45.592 | 0.15 |
| 26. | 3-Methylbutanal [C5H10O] | 50.582 | 0.14 |
| 27. | Propachlor [C11H14ClNO] | 21.959 | 0.14 |
| 28. | 2,4-Dimethylthiophene [C6H8S] | 5.130 | 0.13 |
| 29. | Dimethyl pentasulfide [C2H6S5] | 33.535 | 0.12 |
| 30. | Diallyl sulfide [C6H10S] | 4.173 | 0.11 |
| 31. | Pyrimidine-2,4(1H,3H)-dione [C4H4N4O3] | 50.932 | 0.09 |
| 32. | 2-Hexylthiirane [C8H16S] | 10.367 | 0.08 |
| 33. | Methyl octadeca-9,12,15-trienoate [C19H32O2] | 52.669 | 0.08 |
| 34. | 1,4,7-Trithionane 1,1-dioxide [C6H12O2S3] | 29.714 | 0.07 |
| 35 | 1,4-Dithiepane-2-butanal, 3-oxo-[C9H14O2S2] | 32.972 | 0.05 |
| 36. | 2,2,4,4-Tetramethyloctane [C12H26] | 8.683 | 0.02 |
| 37. | Methanethiol, N-cyclopropylamidino-, hydrogen thiosulfate [C5H10N2O3S2] | 41.571 | 0.02 |
| 38. | Hexanal [C6H12O] | 3.255 | 0.01 |
| Total Identified | 88.08 | ||
| Tested Bacteria/Strain | MIC (%) | MBC (%) |
|---|---|---|
| 50 | >60 |
| 30 | >50 |
| 30 | 40 |
| 30 | 40 |
| 20 | 30 |
| 30 | 40 |
| Tested Bacteria/Strain | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| 100 | >100 |
| 90 | 90 |
| 90 | >100 |
| 100 | >100 |
| 90 | 100 |
| 50 | 60 |
| Production Series | CON | PS-I | PS-II | PS-III | |
|---|---|---|---|---|---|
| Storage Duration | Cold Storage | ||||
| Total phenolics (mg/100 g of BU) | Day 0 | 81.39 ± 11.77 | 69.30 ± 9.69 | 73.41 ± 3.61 | 71.55 ± 5.63 |
| Day 5 | 76.16 ab ± 5.33 | 60.47 a ± 9.40 | 77.17 ab ± 5.96 | 81.32 b ± 7.63 | |
| Day 10 | 77.78 ± 5.87 | 72.93 ± 3.82 | 72.33 ± 5.94 | 63.65 ± 6.67 | |
| AOX capacity (ABTS, mmol TE/100 g of BU) | Day 0 | 0.420 bcB ± 0.006 | 0.392 abC ± 0.007 | 0.353 a ± 0.006 | 0.442 cB ± 0.037 |
| Day 5 | 0.291 aA ± 0.002 | 0.339 bB ± 0.008 | 0.320 b ± 0.002 | 0.341 bA ± 0.036 | |
| Day 10 | 0.321 abA ± 0.024 | 0.283 aA ± 0.004 | 0.319 ab ± 0.009 | 0.347 bA ± 0.007 | |
| Freeze Storage | |||||
| Total phenolics (mg/100 g of BU) | Day 0 | 81.39 B ± 11.77 | 69.30 ± 9.69 | 73.41 ± 3.61 | 71.55 B ± 5.63 |
| Day 90 | 56.65 bA ± 2.50 | 65.91 b ± 6.60 | 66.17 b ± 4.03 | 40.48 aA ± 2.05 | |
| AOX capacity (ABTS, mmol TE/100 g of BU) | Day 0 | 0.420 bcA ± 0.006 | 0.392 abA ± 0.007 | 0.353 aA ± 0.006 | 0.442 cA ± 0.037 |
| Day 90 | 0.553 B ± 0.015 | 0.563 B ± 0.011 | 0.545 B ± 0.015 | 0.540 B ± 0.011 | |
| CON | PS-I | PS-II | PS-III | ||
|---|---|---|---|---|---|
| Cold Storage | |||||
| WL (%) | Day 1 | 17.01 bB ± 0.50 | 14.26 a ± 1.23 | 13.06 a ± 0.53 | 13.53 a ± 0.19 |
| Day 5 | 14.26 A ± 1.51 | 13.71 ± 0.98 | 12.69 ± 0.70 | 12.77 ± 0.67 | |
| Day 10 | 16.04 bAB ± 1.16 | 13.14 a ± 0.81 | 13.20 a ± 0.87 | 12.98 a ± 1.01 | |
| dR (%) | Day 1 | 19.21 ± 1.65 | 20.04 ± 1.94 | 17.28 ± 0.81 | 18.91 ± 1.36 |
| Day 5 | 18.68 ± 1.44 | 19.62 ± 1.52 | 20.38 ± 2.42 | 20.68 ± 1.67 | |
| Day 10 | 16.70 ± 1.16 | 21.10 ± 1.90 | 20.18 ± 1.54 | 17.76 ± 3.30 | |
| pH raw | Day 1 | 6.47 aAB ± 0.03 | 6.55 ab ± 0.03 | 6.59 bB ± 0.04 | 6.56 abB ± 0.04 |
| Day 5 | 6.57 bB ± 0.04 | 6.49 b ± 0.09 | 6.37 aA ± 0.06 | 6.30 aA ± 0.10 | |
| Day 10 | 6.38 aA ± 0.07 | 6.51 b ± 0.09 | 6.46 abA ± 0.07 | 6.46 abB ± 0.11 | |
| pH grilled | Day 1 | 6.70 B ± 0.05 | 6.70 B ± 0.02 | 6.70 B ± 0.04 | 6.69 B ± 0.03 |
| Day 5 | 6.74 bB ± 0.03 | 6.59 aA ± 0.06 | 6.55 aA ± 0.04 | 6.53 aA ± 0.05 | |
| Day 10 | 6.56 A ± 0.07 | 6.60 A ± 0.07 | 6.57 A ± 0.06 | 6.61 AB ± 0.06 | |
| Freeze Storage | |||||
| WL (%) | Day 1 | 17.01 b ± 0.50 | 14.26 a ± 1.23 | 13.06 a ± 0.53 | 13.53 a ± 0.19 |
| Day 90 | 15.13 b ± 1.26 | 12.73 a ± 0.46 | 12.11 a ± 1.01 | 12.03 a ± 0.78 | |
| dR (%) | Day 1 | 19.21 ± 1.65 | 20.04 ± 1.94 | 17.28 ± 0.81 | 18.91 ± 1.36 |
| Day 90 | 16.48 ± 1.56 | 16.25 ± 1.80 | 15.36 ± 0.95 | 14.05 ± 1.12 | |
| pH raw | Day 1 | 6.47 aA ± 0.03 | 6.55 abA ± 0.03 | 6.59 bA ± 0.04 | 6.56 abA ± 0.04 |
| Day 90 | 6.76 B ± 0.05 | 6.74 B ± 0.07 | 6.74 B ± 0.06 | 6.75 B ± 0.06 | |
| pH grilled | Day 1 | 6.70 A ± 0.05 | 6.70 A ± 0.02 | 6.70 A ± 0.04 | 6.69 A ± 0.03 |
| Day 90 | 6.81 B ± 0.05 | 6.82 B ± 0.04 | 6.84 B ± 0.01 | 6.84 B ± 0.04 | |
| Proximate Composition | |||||
| moisture | Day 1 | 56.72 ± 0.58 | 56.90 ± 0.93 | 57.20 ± 0.56 | 57.15 ± 0.48 |
| protein | 20.75 ± 1.92 | 20.87 ± 0.65 | 19.89 ± 0.48 | 20.90 ± 0.27 | |
| fat | 15.97 ± 0.87 | 16.07 ± 0.83 | 17.37 ± 1.02 | 17.30 ± 0.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurćubić, V.S.; Stajić, S.B.; Miletić, N.M.; Petković, M.M.; Dmitrić, M.P.; Đurović, V.M.; Heinz, V.; Tomasevic, I.B. Techno-Functional Properties of Burgers Fortified by Wild Garlic Extract: A Reconsideration. Foods 2023, 12, 2100. https://doi.org/10.3390/foods12112100
Kurćubić VS, Stajić SB, Miletić NM, Petković MM, Dmitrić MP, Đurović VM, Heinz V, Tomasevic IB. Techno-Functional Properties of Burgers Fortified by Wild Garlic Extract: A Reconsideration. Foods. 2023; 12(11):2100. https://doi.org/10.3390/foods12112100
Chicago/Turabian StyleKurćubić, Vladimir S., Slaviša B. Stajić, Nemanja M. Miletić, Marko M. Petković, Marko P. Dmitrić, Vesna M. Đurović, Volker Heinz, and Igor B. Tomasevic. 2023. "Techno-Functional Properties of Burgers Fortified by Wild Garlic Extract: A Reconsideration" Foods 12, no. 11: 2100. https://doi.org/10.3390/foods12112100
APA StyleKurćubić, V. S., Stajić, S. B., Miletić, N. M., Petković, M. M., Dmitrić, M. P., Đurović, V. M., Heinz, V., & Tomasevic, I. B. (2023). Techno-Functional Properties of Burgers Fortified by Wild Garlic Extract: A Reconsideration. Foods, 12(11), 2100. https://doi.org/10.3390/foods12112100











