Effects of Phytochemicals from Fermented Food Sources in Alzheimer’s Disease In Vivo Experimental Models: A Systematic Review
Abstract
1. Introduction
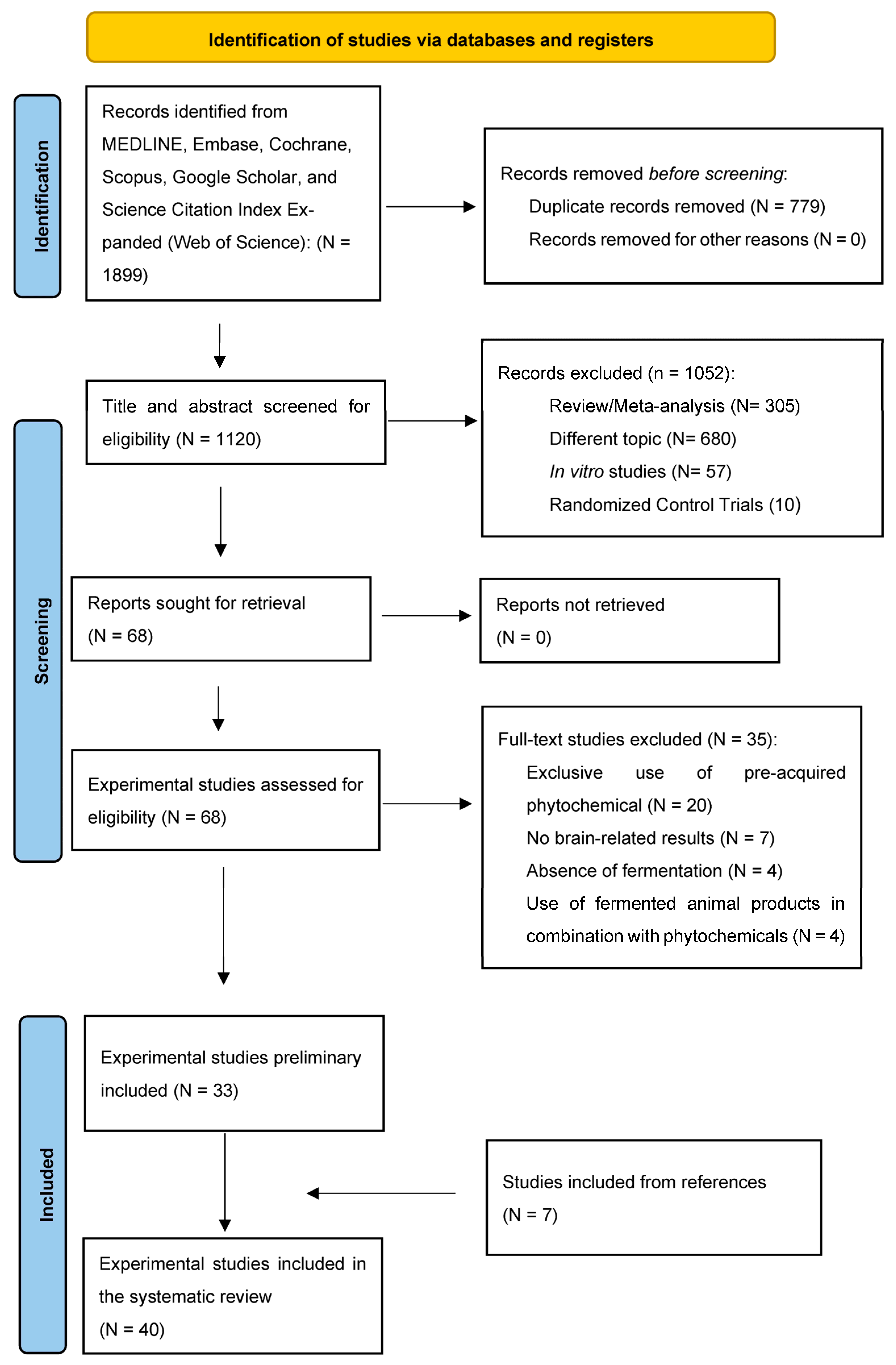
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
- Report: author, year, and source of publication.
- Experimental design and features: number of animals included, sampling mechanism, treatment assignment mechanism, length.
- Animal model: species, with/without genetic modification, age, gender, weight.
- Intervention: type, duration, dose, timing, and mode of delivery.
- Main Outcomes: phytochemical analysis, cognitive function, Amyloid-β deposition, AChE activity, Ach levels, oxidative stress status, neuroinflammation.
- Secondary Outcomes: hypertension, hyperglycemia, hyperlipidemia.
3. Results and Discussion
3.1. Soy Isoflavones
3.2. Ginsenosides
3.3. Kimchi Phytochemicals
3.4. Alcoholic Beverages Phytochemicals
3.5. Tea Phytochemicals
3.6. Phytochemicals from Traditional Medicines
3.7. Fermented Functional Food Phytochemicals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 20 January 2023).
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.; Wu, Y.; Prina, M. World Alzheimer Report 2015. In The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Alzheimer’s Disease International. Available online: https://www.alzint.org/about/dementia-facts-figures/ (accessed on 20 January 2023).
- NASSP. Alzheimer’s Study Group, A National Alzheimer’s Strategic Plan: The Report of the Alzheimer’s Study Group. Alzheimer’s Study Group, A National Alzheimer’s Strategic Plan. Available online: https://www.alz.org/documents/national/report_asg_alzplan.pdf (accessed on 20 January 2023).
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 2006, CD005593. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer’s disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef] [PubMed]
- Biogen. Available online: https://investors.biogen.com/news-releases/news-release-details/update-phase-4-confirmatory-study-aduhelmr (accessed on 20 January 2023).
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process. Food Chem. 2018, 250, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Seo, S.-H.; Ahn, J.-K.; Kim, S.-H. Effect of processing, fermentation, and aging treatment to content and profile of phenolic compounds in soybean seed, soy curd and soy paste. Food Chem. 2011, 127, 960–967. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Alrahmany, R.; Avis, T.J.; Tsopmo, A. Treatment of oat bran with carbohydrases increases soluble phenolic acid content and influences antioxidant and antimicrobial activities. Food Res. Int. 2013, 52, 568–574. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Budaraju, S.; Mallikarjunan, K.; Annor, G.; Schoenfuss, T.; Raun, R. Effect of pre-treatments on the antioxidant potential of phenolic extracts from barley malt rootlets. Food Chem. 2018, 266, 31–37. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Enhancing phenolic content in carrots by pulsed electric fields during post-treatment time: Effects on cell viability and quality attributes. Innov. Food Sci. Emerg. Technol. 2020, 59, 102252. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Peinado, J.; López de Lerma, N.; Peralbo-Molina, A.; Priego-Capote, F.; de Castro, C.; McDonagh, B. Sunlight exposure increases the phenolic content in postharvested white grapes. An evaluation of their antioxidant activity in Saccharomyces cerevisiae. J. Func. Foods 2013, 5, 1566–1575. [Google Scholar] [CrossRef]
- El Khalifa, A.O.; El Tinay, A.H. Effect of fermentation on protein fractions and tannin content of low- and high-tannin cultivars of sorghum. Food Chem. 1994, 49, 265–269. [Google Scholar] [CrossRef]
- Li, X.; Bao, X.; Wang, R. Experimental models of Alzheimer’s disease for deciphering the pathogenesis and therapeutic screening. Int. J. Mol. Med. 2016, 37, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- PRISMA Statement. Available online: http://www.prisma-statement.org/PRISMAStatement/FlowDiagram (accessed on 20 January 2023).
- Yoo, D.H.; Kim, D.H. Lactobacillus pentosus var. plantarum C29 increases the protective effect of soybean against scopolamine-induced memory impairment in mice. Int. J. Food Sci. Nutr. 2015, 66, 912–918. [Google Scholar] [CrossRef]
- Lee, H.J.; Hwang, Y.H.; Kim, D.H. Lactobacillus plantarum C29-Fermented Soybean (DW2009) Alleviates Memory Impairment in 5XFAD Transgenic Mice by Regulating Microglia Activation and Gut Microbiota Composition. Mol. Nutr. Food Res. 2018, 62, e1800359. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, S.M.; Ko, D.B.; Jeong, J.J.; Hwang, Y.H.; Kim, D.H. Soyasapogenol B and Genistein Attenuate Lipopolysaccharide-Induced Memory Impairment in Mice by the Modulation of NF-κB-Mediated BDNF Expression. J. Agric. Food Chem. 2017, 65, 6877–6885. [Google Scholar] [CrossRef]
- Liu, T.H.; Chiou, J.; Tsai, T.Y. Effects of Lactobacillus plantarum TWK10-Fermented Soymilk on Deoxycorticosterone Acetate-Salt-Induced Hypertension and Associated Dementia in Rats. Nutrients 2016, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Kim, J.E.; Kwak, M.H.; Koh, E.K.; Song, S.H.; Sung, J.E.; Kim, D.S.; Hong, J.; Hwang, D.Y. Neuroprotective effects of fermented soybean products (Cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on trimethyltin-induced cognitive defects mice. Nutr. Neurosci. 2016, 19, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.E.; Kwak, M.H.; Go, J.; Son, H.J.; Kim, D.S.; Hwang, D.Y. In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway. Lab. Anim. Res. 2013, 29, 113–126. [Google Scholar] [CrossRef]
- Yang, H.J.; Kwon, D.Y.; Kim, H.J.; Kim, M.J.; Jung, D.Y.; Kang, H.J.; Kim, D.S.; Kang, S.; Moon, N.R.; Shin, B.K.; et al. Fermenting soybeans with Bacillus licheniformis potentiates their capacity to improve cognitive function and glucose homeostaisis in diabetic rats with experimental Alzheimer’s type dementia. Eur. J. Nutr. 2015, 54, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Chung, Y.S.; Kwak, C.S.; Kwon, Y.H. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef]
- Chan, Y.; Lee, I.; Wang, M.; Yeh, W.; Liang, B. Tempeh Attenuates Cognitive deficit, Antioxidant Imbalance, and Amyloid β of Senescence-Accelerated Mice by Modulating Nrf2 Expression via MAPK Pathway. J. Funct. Foods 2018, 50, 112–119. [Google Scholar] [CrossRef]
- Ayuningtyas, A.; Murbawani, E.A.; Nuryanto, N. The effect of tempeh intake on spatial memory in prediabetic rats. Nutr. Food Sci. 2019, 49, 592–599. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Pathak, S.; Kumar, V.; Panda, B.P. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology 2018, 26, 105–118. [Google Scholar] [CrossRef]
- Nagao, M.; Yamano, S.; Imagawa, N.; Igami, K.; Miyazaki, T.; Ito, H.; Watanabe, T.; Kubota, K.; Katsurabayashi, S.; Iwasaki, K. Effect of Lactobacillus paracasei A221-fermented ginseng on impaired spatial memory in a rat model with cerebral ischemia and β-amyloid injection. Tradit. Kampo Med. 2019, 6, 96–104. [Google Scholar] [CrossRef]
- An, K.S.; Choi, Y.O.; Lee, S.M.; Ryu, H.Y.; Kang, S.J.; Yeon, Y.; Kim, Y.R.; Lee, J.G.; Kim, C.J.; Lee, Y.J.; et al. Ginsenosides Rg5 and Rk1 Enriched Cultured Wild Ginseng Root Extract Bioconversion of Pediococcus pentosaceus HLJG0702: Effect on Scopolamine-Induced Memory Dysfunction in Mice. Nutrients 2019, 11, 1120. [Google Scholar] [CrossRef]
- Kim, C.J.; Ryu, H.Y.; Lee, S.; Lee, H.J.; Chun, Y.S.; Kim, J.K.; Yu, C.Y.; Ghimire, B.K.; Lee, J.G. Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice. Molecules 2021, 26, 3001. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Noh, J.S.; Cho, E.J.; Song, Y.O. Bioactive Compounds of Kimchi Inhibit Apoptosis by Attenuating Endoplasmic Reticulum Stress in the Brain of Amyloid β-Injected Mice. J. Agric. Food Chem. 2018, 66, 4883–4890. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, M.J.; Song, Y.O. Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients 2018, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimer’s Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Cecarini, V.; Gogoi, O.; Bonfili, L.; Veneruso, I.; Pacinelli, G.; De Carlo, S.; Benvenuti, F.; D’Argenio, V.; Angeletti, M.; Cannella, N.; et al. Modulation of Gut Microbiota and Neuroprotective Effect of a Yeast-Enriched Beer. Nutrients 2022, 14, 2380. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Ijomone, O.M.; Sanni, O.; Aschner, M.; Islam, M.S. Type 2 diabetes induced oxidative brain injury involves altered cerebellar neuronal integrity and elemental distribution, and exacerbated Nrf2 expression: Therapeutic potential of raffia palm (Raphia hookeri) wine. Metab. Brain Dis. 2019, 34, 1385–1399. [Google Scholar] [CrossRef]
- Mathiyazahan, D.B.; Thenmozhi, A.J.; Manivasagam, T. Protective Effect of Black Tea Extract against Aluminium Chloride-Induced Alzheimer’s Disease in Rats: A Behavioural, Biochemical and Molecular Approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Oh, Y.C.; Pak, M.E.; Li, W.; Go, Y.; Lee, J.J. Pu’er tea water extract protects against cognitive impairment in a mouse model of lipopolysaccharide-induced neuroinflamma-tion. Phytomedicine 2020, 79, 153338. [Google Scholar] [CrossRef]
- Lee, C.L.; Kuo, T.F.; Wang, J.J.; Pan, T.M. Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J. Neurosci. Res. 2007, 85, 3171–3182. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Kuo, T.F.; Wu, C.L.; Wang, J.J.; Pan, T.M. Red mold rice promotes neuroprotective sAPPalpha secretion instead of Alzheimer’s risk factors and amyloid beta expression in hyperlipidemic Aβ40-infused rats. J. Agric. Food Chem. 2010, 58, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Lee, H.; Park, H.; Cho, W.K.; Ma, J.Y. Fermented Sipjeondaebo-tang Alleviates Memory Deficits and Loss of Hippocampal Neurogenesis in Scopolamine-induced Amnesia in Mice. Sci. Rep. 2016, 6, 22405. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Yang, H.S.; Jo, J.H.; Lee, S.C.; Park, T.Y.; Choi, B.S.; Seo, K.S.; Huh, C.K. Anti-Amnesic Effect of Fermented Ganoderma lucidum Water Extracts by Lactic Acid Bacteria on Scopolamine-Induced Memory Impairment in Rats. Prev. Nutr. Food Sci. 2015, 20, 126–132. [Google Scholar] [CrossRef]
- Weon, J.B.; Lee, J.; Eom, M.R.; Jung, Y.S.; Ma, C.J. Cognitive enhancing effect of the fermented Gumiganghwal-tang on scopolamine-induced memory impairment in mice. Nutr. Neurosci. 2016, 19, 125–130. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Lee, C.L. Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation 2021, 7, 39. [Google Scholar] [CrossRef]
- He, X.; Zou, Y.; Yoon, W.B.; Park, S.J.; Park, D.S.; Ahn, J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef]
- Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Ko, H.J.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. Cognitive-Enhancing Effect of Steamed and Fermented Codonopsis lanceolata: A Behavioral and Biochemical Study. Evid. Based Complement. Altern. Med. 2014, 2014, 319436. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Jeong, D.-Y.; Jeong, S.-Y.; Kim, M.J. Black carrots fermented with Lactobacillus plantarum or Aspergillus oryzae prevent cognitive dysfunction by improving hippocampal insulin signalling in amyloid-β infused rats. J. Funct. Foods 2016, 25, 354–366. [Google Scholar] [CrossRef]
- Hong, S.M.; Soe, K.H.; Lee, T.H.; Kim, I.S.; Lee, Y.M.; Lim, B.O. Cognitive Improving Effects by Highbush Blueberry (Vaccinium crymbosum L.) Vinegar on Scopolamine-Induced Amnesia Mice Model. J. Agric. Food Chem. 2018, 66, 99–107. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, J.E.; Lee, S.; Cho, E.J.; Kim, H.Y. Effects of the fermented Zizyphus jujuba in the amyloid β25-35-induced Alzheimer’s disease mouse model. Nutr. Res. Pract. 2021, 15, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Huh, E.; Lim, S.; Kim, H.G.; Ha, S.K.; Park, H.Y.; Huh, Y.; Oh, M.S. Ginger fermented with Schizosaccharomyces pombe alleviates memory impairment via protecting hippocampal neuronal cells in amyloid beta1–42 plaque injected mice. Food Funct. 2018, 9, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.; Yuan, H.; Huang, S.; Park, S. Mitigation of Memory Impairment with Fermented Fucoidan and λ-Carrageenan Supplementation through Modulating the Gut Microbiota and Their Metagenome Function in Hippocampal Amyloid-β Infused Rats. Cells 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Kanouchi, H.; Kakimoto, T.; Nakano, H.; Suzuki, M.; Nakai, Y.; Shiozaki, K.; Akikoka, K.; Otomaru, K.; Nagano, M.; Matsumoto, M. The Brewed Rice Vinegar Kurozu Increases HSPA1A Expression and Ameliorates Cognitive Dysfunction in Aged P8 Mice. PLoS ONE 2016, 11, e0150796. [Google Scholar] [CrossRef] [PubMed]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by β-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Eun, C.S.; Lim, J.S.; Lee, J.; Lee, S.P.; Yang, S.A. The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement Altern. Med. 2017, 17, 367. [Google Scholar] [CrossRef]
- Saleh, S.R.; Masry, A.M.; Ghareeb, D.A.; Newairy, A.A.; Sheta, E.; Maher, A.M. Trichoderma reesei fungal degradation boosted the potentiality of date pit extract in fighting scopolamine-induced neurotoxicity in male rats. Sci. Rep. 2021, 11, 14872. [Google Scholar] [CrossRef]
- Pan, M.; Li, Z.; Yeung, V.; Xu, R.J. Dietary supplementation of soy germ phytoestrogens or estradiol improves spatial memory performance and increases gene expression of BDNF, TrkB receptor and synaptic factors in ovariectomized rats. Nutr. Metab. 2010, 7, 75. [Google Scholar] [CrossRef]
- Murphy, P.A.; Hu, J.; Barua, K.; Hauck, C.C. Group B saponins in soy products in the U.S. Department of Agriculture–Iowa State University isoflavone database and their comparison with isoflavone contents. J. Agric. Food Chem. 2008, 56, 8534–8540. [Google Scholar] [CrossRef]
- File, S.E.; Jarrett, N.; Fluck, E.; Duffy, R.; Casey, K.; Wiseman, H. Eating soya improves human memory. Psychopharmacology 2001, 157, 430–436. [Google Scholar] [CrossRef]
- Lee, I.A.; Park, Y.J.; Yeo, H.K.; Han, M.J.; Kim, D.H. Soyasaponin I attenuates TNBS-Induced colitis in mice by inhibiting NF-κB pathway. J. Agric. Food Chem. 2010, 58, 10929–10934. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Yoo, D.H.; Woo, J.Y.; Jeong, J.J.; Yang, J.H.; Kim, D.H. Soyasaponins Ab and Bb prevent scopolamine-induced memory impairment in mice without the inhibition of acetylcholinesterase. J. Agric. Food Chem. 2014, 62, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhen, H.; Jin, Y.; Xu, L.; Jiang, X.; Sun, S.; Li, C.; Xu, H. Absorption and plasma disposition of genistin differ from those of genistein in healthy women. J. Agric. Food Chem. 2012, 60, 1428–1436. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Han, M.-J.; Han, S.-J.; Kim, D.-H. Metabolism of soyasaponin I by human intestinal microflora and its estrogenic and cytotoxic effects. Biomol. Ther. 2009, 17, 430–437. [Google Scholar] [CrossRef]
- Choo, M.K.; Park, E.K.; Yoon, H.K.; Kim, D.H. Antithrombotic and antiallergic activities of daidzein, a metabolite of puerarin and daidzin produced by human intestinal microflora. Biol. Pharm. Bull. 2002, 25, 1328–1332. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging 2015, 10, 549–560. [Google Scholar] [CrossRef]
- Tang, J.; Pei, Y.; Zhou, G. When aging-onset diabetes is coming across with Alzheimer disease, comparable pathogenesis and therapy. Exp. Gerontol. 2013, 48, 744–750. [Google Scholar] [CrossRef]
- Correia, S.C.; Santos, R.X.; Perry, G.; Zhu, X.; Moreira, P.I.; Smith, M.A. Insulin-resistant brain state: The culprit in sporadic Alzheimer’s disease? Ageing Res. Rev. 2011, 10, 264–273. [Google Scholar] [CrossRef]
- Zhong, Y.; Miao, Y.; Jia, W.P.; Yan, H.; Wang, B.Y.; Jin, J. Hyperinsulinemia, insulin resistance and cognitive decline in older cohort. Biomed. Environ. Sci. 2020, 25, 8–14. [Google Scholar]
- Zhou, J.B.; Tang, X.; Han, M.; Yang, J.; Simó, R. Impact of Antidiabetic Agents on Dementia Risk: A Bayesian Network Meta-Analysis. Metabolism 2020, 109, 154265. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants ex-tracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Lacković, Z.; Salković, M. Streptozotocin and alloxan produce alterations in rat brain monoamines independently of pancreatic beta cells destruction. Life Sci. 1990, 46, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ceretta, L.B.; Réus, G.Z.; Abelaira, H.M.; Ribeiro, K.F.; Zappellini, G.; Felisbino, F.F.; Steckert, A.V.; Dal-Pizzol, F.; Quevedo, J. Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats. Exp. Diabetes Res. 2012, 2012, 302682. [Google Scholar] [CrossRef]
- Cao, Z.H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef]
- Hsu, R.L.; Lee, K.T.; Wang, J.H.; Lee, L.Y.; Chen, R.P. Amyloid degrading ability of nattokinase from Bacillus subtilis natto. J. Agric. Food Chem. 2009, 57, 503–508. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K, an emerging nutrient in brain function. BioFactors. 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Alisi, L.; Cao, R.; De Angelis, C.; Cafolla, A.; Caramia, F.; Cartocci, G.; Librando, A.; Fiorelli, M. The Relationships between Vitamin K and Cognition: A Review of Current Evidence. Front. Neurol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Sim, J.Y.; Heo, J.H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Chen, M.H.; Wang, H.E.; Lu, C.L.; Wang, Y.P.; Zhang, B. Inflammatory Bowel Disease and Neurodegenerative Diseases. Gut Liver. 2023, 17, gnl220523. [Google Scholar] [CrossRef]
- Woo, M.; Kim, M.; Noh, J.S.; Song, Y.O. Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress. J. Funct. Foods 2017, 32, 218–225. [Google Scholar] [CrossRef]
- Alcohol.org. Available online: https://alcohol.org/guides/the-alcohol-industry-in-data/ (accessed on 2 March 2023).
- Hilal, Y. Morphology, manufacturing, types, composition and medicinal properties of Tea (Camellia sinensis). J. Basic Appl. Plant Sci. 2017, 1, 107. [Google Scholar]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C.M. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef]
- Techome, K. Effect of tea processing methods on biochemical composition and sensory quality of black tea (Camellia sinensis (L.) O. Kuntze): A review. J. Hortic. For. 2019, 11, 84–95. [Google Scholar]
- Chen, W.; Feng, Y.; Molnar, I.A.; Chen, F. Nature and nurture: Confluence of pathway de-terminism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef]
- Wang, A.; Xiao, C.; Zheng, J.; Ye, C.; Dai, Z.; Wu, Q.; Liu, J.; Strappe, P.; Zhou, Z. Terpenoids of Ganoderma lucidum reverse cognitive impairment through attenuating neurodegener-ation via suppression of PI3K/AKT/mTOR expression in vivo model. J. Funct. Foods 2020, 73, 104142. [Google Scholar] [CrossRef]
- Yun, B.R.; Weon, J.B.; Lee, J.; Eom, M.R.; Ma, C.J. Neuroprotective effect of the fermented Gumiganghwal-tang. J. Biosci. Bioeng. 2014, 118, 235–238. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chen, Y.P. Clinical experience of Professor Chen about Cordyceps cicadae. Chin. J. Inform. Tradit. Chin. Med. 2000, 7, 71. [Google Scholar]
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7–12. [Google Scholar] [CrossRef]
- Ogbodo, J.O.; Agbo, C.P.; Njoku, U.O.; Ogugofor, M.O.; Egba, S.I.; Ihim, S.A.; Echezona, A.C.; Brendan, K.C.; Upaganlawar, A.B.; Upasani, C.D. Alzheimer’s Disease: Pathogenesis and Therapeutic Interventions. Curr Aging Sci. 2022, 15, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef] [PubMed]
- Auxtero, M.D.; Chalante, S.; Abade, M.R.; Jorge, R.; Fernandes, A.I. Potential Herb-Drug Interactions in the Management of Age-Related Cognitive Dysfunction. Pharmaceutics 2021, 13, 124. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits-Review Article. Foods 2019, 8, 424. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Stoner, G.D. Ginger: Is it ready for prime time? Cancer Prev. Res. 2013, 6, 257–262. [Google Scholar] [CrossRef]
- Yoo, H.J.; You, D.J.; Lee, K.W. Characterization and immunomodulatory effects of high molecular weight fucoidan fraction from the Sporophyll of Undaria pinnatifida in cyclophosphamide-induced immunosuppressed mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef]
- Nishidai, S.; Nakamura, Y.; Torikai, K.; Yamamoto, M.; Ishihara, N.; Mori, H.; Ohigashi, H. Kurosu, a traditional vinegar produced from unpolished rice, suppresses lipid peroxidation in vitro and in mouse skin. Biosci. Biotechnol. Biochem. 2000, 64, 1909–1914. [Google Scholar] [CrossRef]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.M.; Zeb, A.; Mahmood, M.; Abbas, S.R.; Ahmad, Z.; Iqbal, N. Phytochemical analysis and biological activities of ethanolic extract of Curcuma longa rhizome. Braz. J. Biol. 2021, 81, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Gul, B.; Khan, S.; Ahmad, I. Extraction of phytochemicals from date palm (Phoenix dactylifera L.) seeds by enzymatic hydrolysis. J. Food Process Preserv. 2022, 46, e17007. [Google Scholar] [CrossRef]
| Reference | Fermented Food | Phytochemical Analysis | Comparison between Fermented and Unfermented Products | Experimental Design | Main Findings |
|---|---|---|---|---|---|
| [24] | Fermented defatted soybean with Lactobacillus pentosus C29 | The contents of main constituents in soybeans fermented (2) and nonfermented (1). Content (mg/g). Genistin: 203.6 ± 27(1); 6.6 ± 2.0(2) Genistein: 8.6 ± 0.8(1); 45.9 ± 15.4(2) Daidzin: 185.1 ± 19.1(1); 18.6 ± 3.3(2) Daidzein: 3.5 ± 2.7(1); 34 ± 10.3(2) Soyasaponin Ab: 918.5 ± 106.4(1); 190.6 ± 48.4(2) Soyasaponin I: 1037.2 ± 55.2 (1) 767.5 ± 37.9(2) Soyasapogenol A: 160.9 ± 67.1 (2) Soyasapogenol B: 91.2 ± 41.6 (2) | Yes | Scopolamine-induced memory impairment (intraperitoneal injection 1 mg/kg b.w., 30 min after administration of test agents) | Inhibition of AChE activity. Increased BDNF expression. Improved cognitive function. |
| [25] | Soybean fermented with Lactobacillus plantarum C29 | No | Yes | 5XFAD transgenic mice | Decreased amyloid-β, β/γ—secretases, caspase-3 expression, and NF-κB activation. Decreased neuron apoptosis and microglia activation. Increased BDNF expression. Improved cognitive function. |
| [26] | Defatted soybean fermented with Lactobacillus plantarum C29 | No | Yes | Intraperitoneal injection of LPS (8 μg/kg b.w./day for 10 days) | Increased CREB phosphorylation and BDNF expression. Inhibited NF-κB activation. Improved cognitive function. |
| [27] | Soymilk fermented with Lactobacillus plantarum TWK10 | No | Yes | Deoxycorticosterone acetate (subcutaneously 20 mg/kg b.w. 2 times per week over a period of 90 days) | Decreased blood pressure and AChE activity. Improved oxidative stress status. Improved cognitive function. |
| [28] | Cheonggukjang obtained by soybean fermentation with Lactobacillus sakei 383 and Bacillus subtilis MC31 | Cheonggukjang analysis Total Flavonoids:17.5 mg/g Total polyphenolic content: 37.2 mg/g. Daidzein 0.086 mg/g Genistein 0.030 mg/g | No | Trimethyltin chloride (i.p. 2.5 m/kg b.w., single dose) | Decreased AChE activity and MDA levels. Increase in NGF concentration and activation of the NGF signaling pathway. Increase in SOD activity. Improved cognitive function. |
| [29] | Chungkookjang | No | No | Tg2576 transgenic mice | Increased NGF levels. No effects on cognitive function. |
| [30] | Chungkookjang obtained by soybean fermentation with Bacillus lichenifomis | Non-fermented, cooked soybeans (1), Traditionally fermented Chungkookjang (2), Chungkookjang obtained by fermentation with Bacillus lichenifomis (3) Proline 1.4 ± 1.9 (1) 0.7 ± 1.3 (2) 25.0 ± 5.7 (3). Adenine: 67.9 ± 33.1 (1) 147.0 ± 15.0 (2) 96.6 ± 30.4 (3). Tyrosine: 71.3 ± 16.5 (1) 222.3 ± 38.2 (2) 66.4 ± 8.2 (3). Leucine/isoleucine 121.9 ± 28.2 (1) 188.3 ± 47.9 (2) 502.5 ± 249.6 (3). Phenylalanine: 271.1 ± 90.0 (1) 1429.5 ± 229.9 (2) 2127.2 ± 635.9 (3). Ser-Pro: 0.0 ± 0.0 (1) 0.0 ± 0.0 (2) 153.7 ± 64.2 (3). Val-Glu: 0.0 ± 0.0 (1) 0.0 ± 0.0 (2) 75.0 ± 47.4 (3). Val-Leu: 0.0 ± 0.0 (1) 22.1 ± 9.3 (2) 167.5 ± 13.7 (3). Glu-Phe: 1739.3 ± 106.6 (1) 58.5 ± 13.9 (2) 365.3 ± 22.8 (3). Daidzin: 545.6 ± 95.8 (1) 100.2 ± 17.1 (2) 79.1 ± 7.6 (3). Genistin: 460.1 ± 162.7 (1) 179.1 ± 21.0 (2) 106.1 ± 11.3 (3). Acetylgenistin: 328.8 ± 78.0 (1) 3.4 ± 3.4 (2) 13.8 ± 2.6 (3). Daidzein: 191.6 ± 126.8 (1) 1837.0 ± 111.2 (2) 1074.6 ± 120.2 (3). Genistein: 181.2 ± 113.8 (1) 1633.5 ± 132.6 (2) 891.5 ± 113.0 (3). B soyasaponin Bb’: 37.5 ± 20.1 (1) 53.0 ± 13.3 (2) 26.3 ± 16.0 (3). E soyasaponin: 51.4 ± 40.2 (1) 133.3 ± 50.8 (2) 602.7 ± 190.5 (3). DDMP Soyasaponin βg: 2021 ± 1245 (1) 210 ± 252 (2) 1185 ± 750 (3). | Yes | Hippocampal infusion of Aβ25–35 (3.6 nmol/day for 14 days) and 90 % pancreatectomy | Decreased Amyloidβ accumulation. Improved insulin signaling in the hippocampus. Restored β-cell mass. Improved cognitive function. |
| [31] | Doenjang | No | Yes | Hyperlipidemic diet (45.2 kcal% fat and 1% cholesterol) for 11 weeks | Decreased neuron cell loss in the hippocampus, oxidative metabolites, Tau hyperphosphorylation. Reduced mRNA expression of oxidative stress and neuroinflammation-related genes. Improved cognitive function. |
| [32] | Tempeh | No | No | Senescence-accelerated SAMP8 mice | Improved oxidative stress markers in the cortex, hippocampus, and striatum. Increased nuclear factor erythroid 2-related factor 2 (Nrf2) levels. Reduced Amyloidβ levels. Improved cognitive function. |
| [33] | Tempeh | No | No | Alloxan-induced pre-diabetes (single i.p. 120 mg/kg b.w.) | No significant improvement in blood glucose or cognitive function. |
| [34] | Solid-state soybean fermentation with Bacillus subtilis MTCC 2616 | Units expressed per mg fermented soybean powder. Daidzin 50.31 ± 0.4 µg, Genistin 49 ± 0.3 µg, and Glycitin 23.53 ± 0.6 µg. Nattokinase activity 353 ± 2.3 FU g−1. | No | I.c.v. colchicine (15 µg/5 µL, single dose) | Increase in AChE activity. Reduced hippocampal activity of GSH, CAT, and SOD. Decreased lipid peroxidation and carbonyl protein levels. Improved cognitive function. |
| [35] | Ginseng fermented with Lactobacillus paracasei A221 | Components of ginseng according to fermentation status. Content (%). Fermented ginseng: 0.0 (Rb1); 0.1 (Rb2); 0.5 (Rc); 0.1 (Rd); 0.2 (Rg1); 0.9 (Compound K). Non-Fermented ginseng: 2.0 (Rb1); 1.7 (Rb2); 2.2 (Rc); 1.3(Rd); 0.4 (Rg1); 0 (Compound K). | Yes | I.c.v. infusion of Aβ1-42 (600 pmol/20 µL per day for 7 days) and bilateral electrocauterization of the vertebral arteries | Ameliorated hippocampal neuron loss. Increased Iba-1 and caspase-3 levels. Improved cognitive function. |
| [36] | Wild ginseng root fermented with Pediococcus pentosaceus | Content of ginsenosides in fermented products Rg5 and Rk1: 21.48 and 18.71 mg/g, respectively. | No | Scopolamine-induced memory dysfunction (i.p. 1 mg/kg b.w. 30 min after administration of test agents) | Decreased AChE activity. Increased ACh level. Improved cognitive function. |
| [37] | Ginseng fermented with Pediococcus pentosaceus | Ginsenoside Contents (mg/g). Cultured wild ginseng root: (Rb1) 51.53 ± 1.34; (Rc) 38.16 ± 1.10; (Rb2) 34.36 ± 1.26; (Rb3) 8.10 ± 0.52; (Rd) 55.90± 0.85; (Rg3) N.D.; (Rk1) N.D.; (Rg5) N.D. Total: 188.06± 4.98. Fermented ginseng root: (Rb1) 9.26 ±0.28; (Rc) 4.93± 0.41: (Rb2) 6.36 ± 0.40; (Rb3) 2.83 ± 0.35; (Rd) 11.26 ± 0.56; (Rg3) 44.26 ± 1.02; (Rk1) 15.93 ± 0.32; (Rg5) 23.10 ± 0.59. Total: 117.96 ± 3.38 | No | Male mice: scopolamine-induced memory deficit (i.p. 1 mg/kg b.w. 30 min after administration of test agents). Female mice: D-galactose-induced aging (s.c. 100 mg/kg b.w.) and ovariectomy | Decreased AChE activity, increased Ach level in female mice groups. Decreased MDA levels and increased CAT activity in female groups. Improved cognitive function. |
| [38] | Traditional fermented kimchi | Conducted previously by authors cited in this present article. The content of active compounds per 1 kg of Kimchi: ascorbic acid, HDMPPA, quercitrin, and quercetin were 0.28, 0.04, 0.03, 0.02, and 0.27 g, respectively. Total phenolic contents of Kimchi were 15.75 ± 3.91 mg of GAE/g extract. | Yes | Singular i.c.v. administration of Aβ25-35 (5 nmol/5 μL) | Decrease in BACE, APP, and phosphorylated Tau protein expression level. Decreased protein expression of ER stress markers, proapoptotic molecules, and CHOP. Increased protein expression of anti-apoptotic molecules. Decreased oxidative stress markers. |
| [39] | Traditional fermented kimchi | Conducted previously by authors cited in this present article. The content of active compounds per 1 kg of Kimchi: ascorbic acid, HDMPPA, quercitrin, and quercetin were 0.28, 0.04, 0.03, 0.02, and 0.27 g, respectively. Total phenolic contents of Kimchi were 15.75 ± 3.91 mg of GAE/g extract. | Yes | Singular i.c.v. administration of Aβ25-35 (5 nmol/5 μL) | Decreased levels of oxidative stress markers. Increased protein expression level of antioxidant enzymes. Decreased protein expression levels of inflammation-related enzymes. Improved cognitive function. |
| [40] | Red wine Cabernet Sauvignon | Compounds identified in the Cabernet Sauvignon (mg/L). Gallic acid: 8.1. Protocatechuic acid: 0.9. Caffeic acid derivative: 8.5. p-Coumaric acid derivative: 2.4. Gallotannin: 4.9. Catechin: 7.3. Caffeic acid: 6.6. Syringic acid: 4.5. p-Coumaric acid: 3.6. Flavonoid glycoside: 5.8. Flavonoid: 5.1. Resveratrol: 0.2. Ferulic acid derivative: 1.2. Flavonoid aglycone: 2.1 | Yes | Tg2576 mice | Decreased Aβ peptide generation. Increased non-amyloidogenic processing of amyloid precursor protein. Improved cognitive function. |
| [41] | Muscadine wine | Constituent polyphenolic components in Muscadine wine: Gallic acid, Procyanidin, p-Courmaric acid, Ellagitannin, Cinnaminic acid derivative, Resveratrol, Ellagic acid, Flavonoid, Delphindin, Cyanidin, Petunidin, Peonidin, Malvidin. No quantitative data available. | No | Tg2576 mice | Reduced levels of soluble high molecule weight oligomeric Aβ species in the hippocampus and cerebral cortex. Improved cognitive function. |
| [42] | Wine polyphenolic extract (100 mg/L gallic acid equivalents) | Quantification (μg/mg of freeze-dried PVPP-white wine extract). Gallic acid: 83.06 ± 5.46 3,4-Dihydroxybenzoic acid: 3.19 ± 1.04 2-S-Glutathionyl caftaric acid (GRP): 5.57 ± 0.19 trans-Caftaric acid: 351.75 ± 20.53 Catechin: 17.76 ± 7.71 Hydroxycinnamic acid: 5.59 ± 2.50 Coutaric acid: 28.24 ± 0.37 Chlorogenic acid: 42.40 ± 0.50 Caffeic acid: 10.55 ± 0.82 Catechin derivative: 23.76 ± 9.13 Hydroxycinnamic acid: 9.51 ± 0.15 Resveratrol derivative: 0.47 ± 0.09 Ferulic acid: 2.49 ± 0.20 Resveratrol: 0.73 ± 0.28 Proanthocyanidin: 9.12 ± 0.27 Proanthocyanidin (oligomer of catechin): 280.63 ± 13.66 Ethyl caffeic: 2.97 ± 0.10 Ferulic acid derivative: 2.67 ± 0.05 Total phenolic compounds 880.38 ± 58.68 µg GAE/ mL Hydroxybenzoic acids: 86.25 ± 4.44 Hydroxycinnamic acids: 461.73 ± 24.41 Catechins plus Proanthocyanidins: 331.27 ± 26.06 | No | 3xTg-AD mice | Increased brain accumulation of hydroxybenzoic acid derivatives and catechins. Modulation of oxidative stress markers. Decreased levels of Aβ1-42 and Aβ1-40 in the brain. |
| [43] | Beer enriched with Saccharomyces cerevisiae | No | Yes | 3xTg-AD mice | Decreased Aβ1-42 in the hippocampus and prefrontal cortex. Reduced pro-inflammatory molecules. Increased concentration of anti-inflammatory molecules. Improved cognitive function. |
| [44] | Raffia Palm (Raphia hookeri) wine | No | No | High fructose diet (10% fructose solution) for 2 weeks followed by Streptozotocin-induced type 2 diabetes (single i.p. 40 mg/kg b.w.) | Improved neuronal integrity and reduced heavy metal burden in the brain. Oxidative stress modulation. Decreased AChE activity. |
| [45] | Black tea (Camellia sinensis) | Compositional analysis of black tea extract. Total polyphenols: 442.17 (mg/100 g gallic acid equivalent) Theaflavin: 2.16 (%) Thearubigins: 19.31 (%) Total catechins: 2.04 (%) Caffeine: 1.81 (%) Theanine: 4.1 (mg/100 mL) | No | Chronic AlCl3 administration (i.p. 100 mg/kg b.w./day for 60 days) | Diminished expressions of APP, Aβ1–42, β and γ secretases. Ameliorated protein expression changes in apoptotic indices. Significantly ameliorated oxidative stress by diminishing the lipid peroxidative products and enhancing antioxidant indices. Improved cognitive function. |
| [46] | Pu’er tea (Camellia sinensis) | Catechin and Epicatechin | No | LPS-induced neuroinflammation (400 µg/kg b.w. for 1 week) | Inhibited the expression of amyloid genesis proteins. Inhibited production of inflammatory proteins. Decreased activation of inflammatory pathways. Decreased expression of inflammatory mediator mRNAs in hippocampal tissue. Improved cognitive function. |
| [47] | Monascus-fermented red mold rice | No | No | I.c.v. infusion of Aβ40 (total of 4.9–5.5 nmol/234 µL) for 28 days | Potently reversed increases of AChE activity, ROS, and lipid peroxidation. Decreased total antioxidant status and SOD activity in the brain. Improved cognitive function. |
| [48] | Monascus-fermented red mold rice | No | No | I.c.v. infusion of Aβ40 (total of 4.9–5.5 nmol/234 µL) for 28 days and hyperlipidemic diet (4.85 kcal/g) | Downregulated Aβ40 formation and deposition by suppressing the cholesterol-raised β-secretase activity and apolipoprotein E expression. Mediated proteolytic process of APP toward neuroprotective sAPPR secretion in the hippocampus. Improved cognitive function. |
| [49] | Lactobacillus-fermented Sipjeondaebotang | No | Yes | Scopolamine-induced memory impairment (i.p. 1 mg/kg b.w./day for a total of 21 days) | Improved neurogenesis in the hippocampus. Decreased AChE activity and increased ACh levels. Improved oxidative stress status. Modulation of the cholinergic system and BDNF/CREB/Akt pathway. Improved cognitive function. |
| [50] | Ganoderma lucidum fermented with Lactobacillus sakei and Bifidobacterium bifidum | No | Yes | Scopolamine-induced memory impairment (i.p. 1 mg/kg b.w. for 5 days) | Decreased AChE activity. Improved cognitive function. Improved motor coordination. |
| [51] | Fermented Gumiganghwal-tang | No | No | Scopolamine-induced memory impairment (single s.c. 1 mg/kg b. w. 90 min after administration of test agent) | Decreased AChE activity. Improved cognitive function. |
| [52] | Whole submerged fermentation of Cordyceps cicada NTTU 868 with deep ocean water | No | Yes | I.c.v. infusion of 24.299 µg Aβ40 and 0.9 mg streptozotocin (continuous for 28 days) | Suppressed Aβ40, BACE, and expression of pro-inflammatory markers. Increased Mg2+ content in the cortex. Increased expression of sRAGE and inhibited release of inflammatory factors by microglia cells. Improved cognitive function. |
| [53] | Codonopsis lanceolata fermented with Lactobacillus rhamnosus and Bifidobacterium longum B6 | The total phenol content of C. lanceolata High-pressure extraction and L. rhamnosus fermentation: 8.45 mg GAE/g High-pressure extraction and B. logum fermentation: 8.25 mg GAE/g High-pressure extraction without fermentation: 7.38 mg GAE/g Conventional extraction without fermentation: 6.69 mg GAE/g Flavonoid content Fermented C. lanceolata extracts with B. logum (0.44 mg RE/g) and L. rhamnosus (0.45 mg RE/g) High-pressure extraction and B. logum fermentation contents of hydroxybenzaldehyde, cinnamic acid, and coumaric acid were 222.1, 202.0, and 178.6 μg/g, respectively. The amounts of cinnamic acid for the two fermented products were more than 6x higher than that of the non-fermented product. | Yes | Scopolamine-induced memory impairment (1 mg/kg b.w. 30 min after administration of test agents) | Inhibited α-glucosidase and tyrosinase activities. Improved cognitive function. |
| [54] | Codonopis lanceolata fermented with Bifidobacterium longum KACC 20587, Lactobacillus acidophilus KACC 12419, and Leuconostoc mesenteroides KACC 12312 | No | Yes | Scopolamine-induced memory deficit (s.c. 1 mg/kg b.w.) | Significant decrease in AChE activity. Decrease in pCREB/CREB ratio. Increased brain expression of BDNF. Improved cognitive function. |
| [55] | Black carrots fermented with Lactobacillus plantarum and Aspergillus oryzae | No | Yes | Hippocampal infusion of Aβ25–35 (3.6 nmol/day for 2 weeks) and type 2 diabetes (partial pancreatectomy + high fat diet) | Suppressed Aβ deposition in the hippocampus. Potentiated insulin signaling. Improved whole body and hepatic insulin resistance, first-phase insulin secretion, and insulin sensitivity in a hyperglycemic state. Improved cognitive function. |
| [56] | Highbush blueberry (Vaccinium corymbosum L.) vinegar obtained by fermentation with Saccharomyces cerevisiae KCCM 34709 and Acetobacter spp. KCCM 40085 | Content (mg/mL). Blueberry extract (1); Blueberry vinegar (2) L-ascorbic acid 1.73 ± 0.03 (1); 0.34 ± 0.03 (2) ellagic acid 0.66 ± 0.04 (1); 0.56 ± 0.04 (2) gallic acid 0.21 ± 0.01 (1); 0.25 ± 0.01 (2) D-catechin 0.41 ± 0.04 (1);1.74 ± 0.04 (2) vanillic acid 2.25 ± 0.15(1); 0.31 ± 0.04 (2) caffeic acid 2.02 ± 0.42 (1); 5.54 ± 0.52 (2) cyanidin chloride 26.34 ± 0.54 (1);28.54 ± 0.54 (2) epicatechin 20.24 ± 0.66 (1); 22.24 ± 0.56 (2) chlorogenic acid 2.43 ± 0.48 (1); 8.68 ± 0.35 (2) myricetin 2.68 ± 0.35 (1); 5.35 ± 0.31 (2) quinic acid 1.35 ± 0.31 (1);6.98 ± 0.34 (2) naringin 1.15 ± 0.43 (1); 6.25 ± 0.43 (2) kaempferol 1.35 ± 0.31 (1); 6.62 ± 0.38 (2) Data represent means ± SD (n = 3). | Yes | Scopolamine-induced memory impairment (i.p. 1 mg/kg b.w./day for 7 days) | Activation of BDNF/ CREB/ AKT signaling. Improved cognitive function. |
| [57] | Zizyphus jujuba fermented with Saccharomyces cerevisiae | No | Yes | I.c.v injection of Aβ25-35 (5 nM/5µL) for 5 consecutive days | Suppressed levels of MDA and NO in the liver, brain, and kidneys. Improved cognitive function. |
| [58] | Ginger fermented with Schizosaccharomyces pombe | No | Yes | Single i.c.v Aβ1–42 (1 mg mL−1 concentration, 3 µL in total) Scopolamine-induced amnesia (i.p. 1.1 mg kg/b.w. before behavioral test) | Inhibition of neuronal cell loss. Reinstated pre- and postsynaptic protein levels that were decreased by Aβ1–42 plaque-toxicity. Improved cognitive function. |
| [59] | Fucoidan and carrageenan fermented with Pseudoalteromonas carrageenovora and Luteolibacter algae | No | No | Aβ25-35 infusion in the CA1 region of the hippocampus for 3 weeks (0.005 mg/300 µL in total) | Potentiated hippocampal insulin signaling and increased the expression of CNTF and BDNF in the hippocampus. Increased insulin signaling. Increased serum acetate concentrations. Increased Akkermentia species in the gut microbiome. Improved cognitive function. |
| [60] | Kurozu vinegar and Kurozu moromi | No | No | Senescence accelerated P8 mice | Increased mRNA of expression anti-misfolding and aggregation proteins. Decreased Aβ deposition and plasma TBARS level. |
| [61] | Aged garlic | S-allylcysteine: 30.96 mg/g. Allicin: 32 µg/g. | No | Bilateral ventricular injection of Aβ1-42 (single dose of 1 µg/µL) | Reduced microglial activation. Reduced TNFα and IL-1 levels. Significantly improved short-term recognition memory. |
| [62] | Curcuma longa L. fermented with Lactobacillus plantarum K154 | The amounts of curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin in freeze-dried powder of fermented Cucurma longa were 10.37, 1.68, and 2.33 μg/mg, respectively. The total amount of curcuminoids was 1.44% (14.38 μg/mg). | No | Scopolamine-induced memory deficits (i.p. 1 mg/kg b.w.) | Regulation of CREB and BDNF expression. Improved cognitive function. |
| [63] | Date palm pits (Phoenix dactylifera) fermented with Trichoderma reesei | Fermented extract contained higher yield (12 g%), amounts of flavonoids (12.9 μg eq/mg extract) and phenolics (367.11 μg eq/mg) than the non-fermented extract, which contained 5.9 and 301.97 μg eq/mg of flavonoids and phenolics, respectively. Fungal degradation resulted in the appearance of 5 new compounds (Pyrogallol, 3-Hydroxytyrosol, Catechol, Cinnamic acid, and Myricetin) that were not present in the date pit extract. | Yes | Scopolamine-induced cognitive impairment (i.p. 2 mg/ kg b.w.) | Decreases in the levels of TBARS and NO in serum and brain. Increases in GSH level and GST, GPx, and SOD activities. Significant reductions in the activity and the expression level of AChE as well as the level of Aβ42. Significant decreases in the mRNA expression levels of Tau protein and inflammatory markers. Significantly restored the expression levels of ADAM17, BDNF, and CREB. Marked improvement of neuron morphology. |
| Study Reference | Selection Bias (Sequence Generation) | Selection Bias (Baseline Characteristics) | Selection Bias (Allocation Concealment) | Performance Bias (Random Housing) | Performance Bias (Blinding) | Detection Bias (Random Outcome Assessment) | Detection Bias (Blinding) | Attrition Bias (Incomplete Outcome Data) | Reporting Bias (Selective Outcome Reporting) |
|---|---|---|---|---|---|---|---|---|---|
| [24] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | HIGH | LOW |
| [25] | HIGH | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [26] | HIGH | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [27] | HIGH | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [28] | HIGH | UNCLEAR | UNCLEAR | LOW | HIGH | UNCLEAR | HIGH | HIGH | LOW |
| [29] | HIGH | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [30] | UNCLEAR | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [31] | UNCLEAR | UNCLEAR | UNCLEAR | LOW | HIGH | UNCLEAR | UNCLEAR | HIGH | LOW |
| [32] | UNCLEAR | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [33] | UNCLEAR | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [34] | HIGH | UNCLEAR | HIGH | LOW | HIGH | UNCLEAR | UNCLEAR | LOW | LOW |
| [35] | HIGH | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [36] | UNCLEAR | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [37] | HIGH | LOW | UNCLEAR | LOW | HIGH | UNCLEAR | UNCLEAR | LOW | LOW |
| [38] | HIGH | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [39] | HIGH | LOW | HIGH | LOW | UNCLEAR | UNCLEAR | HIGH | LOW | LOW |
| [40] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW |
| [41] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW |
| [42] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [43] | HIGH | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | LOW | LOW | LOW |
| [44] | HIGH | UNCLEAR | HIGH | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [45] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [46] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | HIGH | LOW |
| [47] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [48] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | HIGH |
| [49] | HIGH | LOW | HIGH | LOW | UNCLEAR | UNCLEAR | LOW | HIGH | LOW |
| [50] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [51] | HIGH | LOW | HIGH | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [52] | UNCLEAR | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [53] | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [54] | HIGH | LOW | UNCLEAR | LOW | UNCLEAR | HIGH | UNCLEAR | HIGH | LOW |
| [55] | UNCLEAR | LOW | HIGH | LOW | UNCLEAR | UNCLEAR | LOW | HIGH | LOW |
| [56] | UNCLEAR | UNCLEAR | LOW | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [57] | HIGH | UNCLEAR | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW |
| [58] | UNCLEAR | LOW | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW | UNCLEAR |
| [59] | HIGH | HIGH | UNCLEAR | LOW | UNCLEAR | UNCLEAR | LOW | HIGH | LOW |
| [60] | HIGH | HIGH | UNCLEAR | LOW | HIGH | UNCLEAR | UNCLEAR | HIGH | LOW |
| [61] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | HIGH | LOW | LOW |
| [62] | UNCLEAR | LOW | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | HIGH | LOW |
| [63] | UNCLEAR | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW | LOW | LOW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baciu, A.M.; Opris, R.V.; Filip, G.A.; Florea, A. Effects of Phytochemicals from Fermented Food Sources in Alzheimer’s Disease In Vivo Experimental Models: A Systematic Review. Foods 2023, 12, 2102. https://doi.org/10.3390/foods12112102
Baciu AM, Opris RV, Filip GA, Florea A. Effects of Phytochemicals from Fermented Food Sources in Alzheimer’s Disease In Vivo Experimental Models: A Systematic Review. Foods. 2023; 12(11):2102. https://doi.org/10.3390/foods12112102
Chicago/Turabian StyleBaciu, Alina Mihaela, Razvan Vlad Opris, Gabriela Adriana Filip, and Adrian Florea. 2023. "Effects of Phytochemicals from Fermented Food Sources in Alzheimer’s Disease In Vivo Experimental Models: A Systematic Review" Foods 12, no. 11: 2102. https://doi.org/10.3390/foods12112102
APA StyleBaciu, A. M., Opris, R. V., Filip, G. A., & Florea, A. (2023). Effects of Phytochemicals from Fermented Food Sources in Alzheimer’s Disease In Vivo Experimental Models: A Systematic Review. Foods, 12(11), 2102. https://doi.org/10.3390/foods12112102






