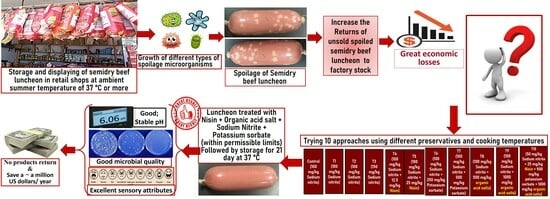
Nisin and Organic Acid Salts Improved the Microbial Quality, Extended the Shelf Life, and Maintained the Sensory Attributes of Semidry Beef Luncheon Marketed at Adverse (35–40 °C) Ambient Summer Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Preservatives and Their Suppliers
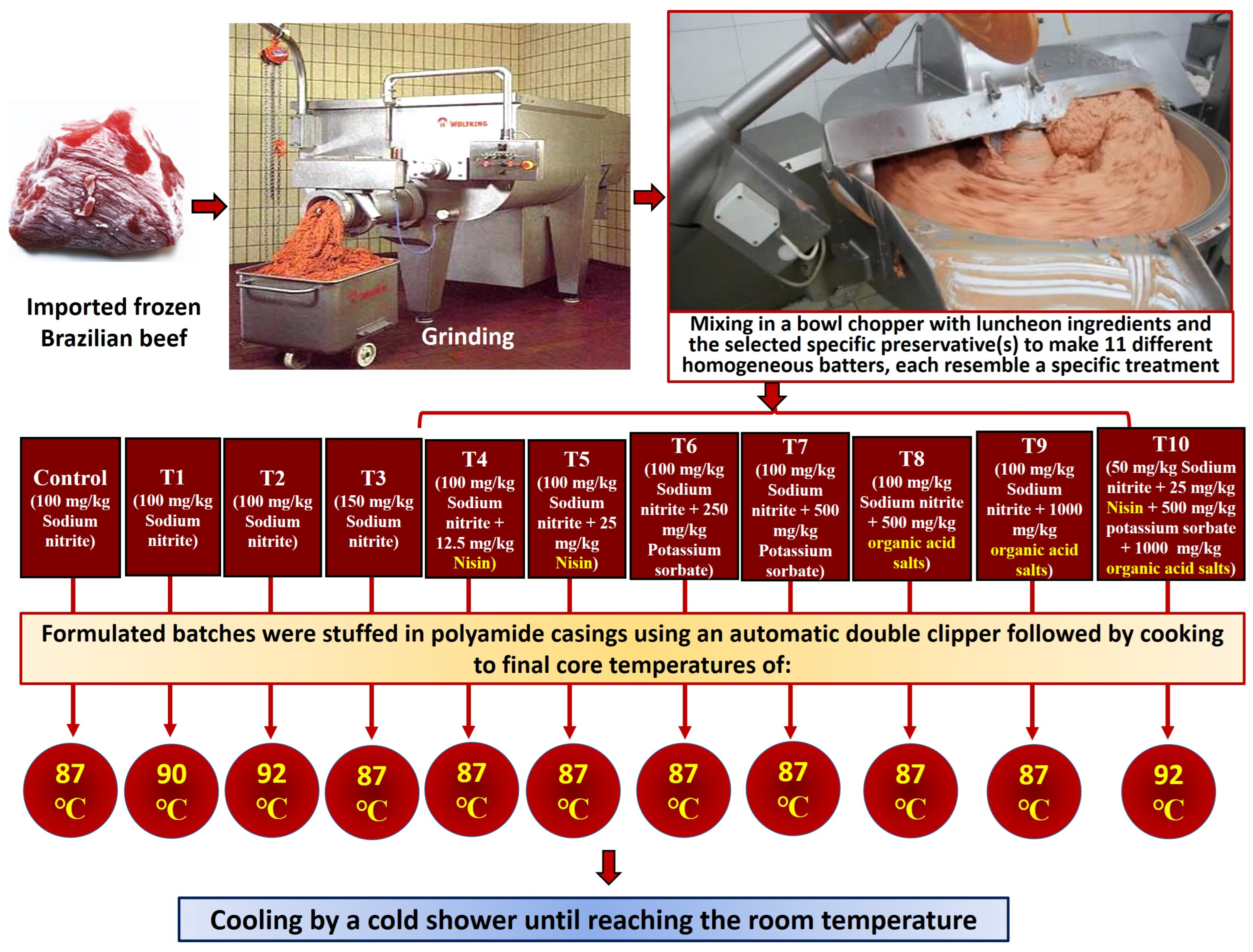
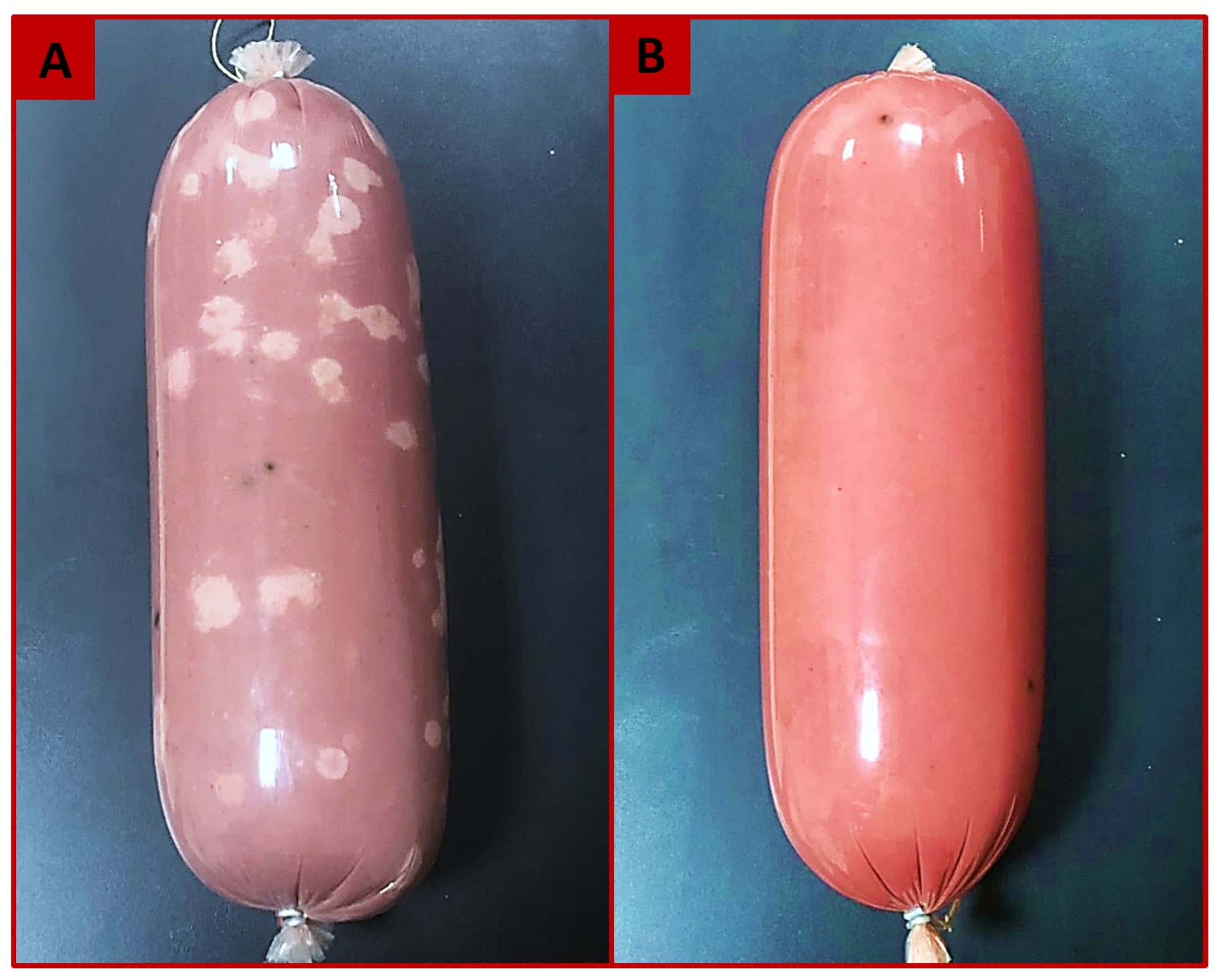
2.2. Manufacturing of Semidry Luncheon
2.3. Microbiological Examination
2.3.1. Sample Preparation for Microbiological Examinations
2.3.2. Aerobic Plate Counts (APCs)
2.3.3. Lactic Acid Bacteria Count (LABC)
2.3.4. Anaerobic Plate Count (ANPC)
2.3.5. Mold and Yeast Counts (MYC)
2.4. Measurement of pH
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Applying Different Preservatives and Cooking to Different Final Core Temperatures (FCT) on the Different Microbial Categories of Semidry Beef Luncheon during Storage at 37 °C
3.1.1. Aerobic Plate Counts (APCs)
3.1.2. Anaerobic Plate Counts (ANPC)
3.1.3. Lactic Acid Bacterial Count (LABC)
3.1.4. Mold and Yeast Count (MYC)
3.2. Effect of Applying Different Preservatives and Cooking to Different Final Core Temperatures (FCT) on the pH of Semidry Beef Luncheon during Storage at 37 °C for 21 Days
3.3. Effect of Applying Different Preservatives and Cooking to Different Final Core Temperatures (FCT) on the Sensory Attributes of Semidry Beef Luncheon during Storage at 37 °C for 21 Days
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]
- Lipinski, B. Why does animal-based food loss and waste matter? Anim. Front. 2020, 10, 48–52. [Google Scholar] [CrossRef]
- ICS: 67.120.10; 1114 Egyptian Standard Specification for Luncheon Meat. Egyptian Organization Standardization (EOS): Cairo Governatore, Egypt, 2005.
- Kalschne, D.L.; Womer, R.; Mattana, A.; Sarmento, C.M.P.; Colla, L.M.; Colla, E. Characterization of the spoilage lactic acid bacteria in “sliced vacuum-packed cooked ham”. Braz. J. Microbiol. 2015, 46, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Työppönen (Née Erkkilä), S.; Markkula, A.; Petäjä, E.; Suihko, M.-L.; Mattila-Sandholm, T. Survival of Listeria monocytogenes in North European type dry sausages fermented by bioprotective meat starter cultures. Food Control 2003, 14, 181–185. [Google Scholar] [CrossRef]
- Govari, M.; Pexara, A. Nitrates and nitrites in meat products. J. Hellenic Vet. Med. Soc. 2018, 66, 127. [Google Scholar] [CrossRef]
- Dikeman, M.; Devine, C. Encyclopedia of Meat Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-384734-8. [Google Scholar]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- Greer, F.R.; Shannon, M.; the Committee on Nutrition; the Committee on Environmental Health. Infant Methemoglobinemia: The role of dietary nitrate in food and water. Pediatrics 2005, 116, 784–786. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Code of Federal Regulations (CFR). 2016. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.12 (accessed on 10 June 2016).
- De Arauz, L.J.; Jozala, A.F.; Mazzola, P.G.; Vessoni Penna, T.C. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Hampikyan, H.; Ugur, M. The Effect of nisin on L. monocytogenes in Turkish fermented sausages (Sucuks). Meat Sci. 2007, 76, 327–332. [Google Scholar] [CrossRef]
- Krivorotova, T.; Cirkovas, A.; Maciulyte, S.; Staneviciene, R.; Budriene, S.; Serviene, E.; Sereikaite, J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll. 2016, 54, 49–56. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Use of Nisin in Processed Meat Products: Final Assessment Report, Application A565. 2007. Available online: https://www.foodstandards.gov.au/code/applications/documents/A565_FAR_Nisin_FINAL.pdf (accessed on 19 March 2023).
- ISO 4833-2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria-Colony-Count Technique at 30 °C. International Organization for Standardization: Geneva, Switzerland, 2021.
- Mauerhofer, L.-M.; Pappenreiter, P.; Paulik, C.; Seifert, A.H.; Bernacchi, S.; Rittmann, S.K.-M.R. Methods for quantification of growth and productivity in anaerobic microbiology and biotechnology. Folia Microbiol. 2019, 64, 321–360. [Google Scholar] [CrossRef] [PubMed]
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. International Organization for Standardization: Geneva, Switzerland, 2018.
- American Meat Science Association. Research Guidelines for Cookery, Sensory evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; American Meat Science Association: Champaign, IL, USA, 2015; pp. 1–104. Available online: https://meatscience.org/docs/default-source/publications-resources/amsa-sensory-and-tenderness-evaluation-guidelines/research-guide/2015-amsa-sensory-guidelines-1-0.pdf?sfvrsn=6 (accessed on 7 September 2023).
- Egypt’s National Food Safety Authority Technical Regulations of Microbiological Criteria for Food: Heat-Treated Processed Comminated Meat, Poultry Products, National official gazette, The Arab Republic of Egypt, The Presidency of the Republic, Issued No. 1, 2021; 8.3.2. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Egypt%27s%20National%20Food%20Safety%20Authority%20Issues%20New%20Decision%20Regulating%20Microbiological%20Contaminants%20%20_Cairo_Egypt_07-10-2021.pdf (accessed on 15 September 2023).
- Kalschne, D.L.; Geitenes, S.; Veit, M.R.; Sarmento, C.M.P.; Colla, E. Growth inhibition of lactic acid bacteria in ham by nisin: A model approach. Meat Sci. 2014, 98, 744–752. [Google Scholar] [CrossRef]
- Khorsandi, A.; Eskandari, M.H.; Aminlari, M.; Shekarforoush, S.S.; Golmakani, M.T. Shelf-life extension of vacuum packed emulsion-type sausage using combination of natural antimicrobials. Food Control 2019, 104, 139–146. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, H.W.; Lee, J.Y.; Ahn, D.U.; Kim, C.-J.; Paik, H.-D. Antimicrobial effect of nisin against Bacillus cereus in beef jerky during storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 272–276. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Colak, H.; Hampikyan, H.; Bingol, E.B.; Aksu, H. The effect of nisin and bovine lactoferrin on the microbiological quality of Turkish-style meatball (Tekirdağ Köfte). J. Food Saf. 2008, 28, 355–375. [Google Scholar] [CrossRef]
- Pawar, D.D.; Malik, S.V.S.; Bhilegaonkar, K.N.; Barbuddhe, S.B. Effect of nisin and its combination with sodium chloride on the survival of Listeria monocytogenes added to raw buffalo meat mince. Meat Sci. 2000, 56, 215–219. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Anjaneyulu, A.S.R.; Kondaiah, N. Development of shelf stable pork sausages using hurdle technology and their quality at ambient temperature (37 ± 1 °C) storage. Meat Sci. 2008, 79, 1–12. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.-W.; Paik, H.-D. Application of natural preservatives for meat and meat products against food-borne pathogens and spoilage bacteria: A review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Makni, I.; Attia, H. Thermal Diffusivities and influence of cooking time on textural, microbiological and sensory characteristics of Turkey meat prepared products. Food Bioprod. Process. 2009, 87, 327–333. [Google Scholar] [CrossRef]
- Feng, C.-H.; Sun, D.-W.; García Martín, J.F.; Zhang, Z.-H. Effects of different cooling methods on shelf-life of cooked jumbo plain sausages. LWT—Food Sci Technol. 2013, 54, 426–433. [Google Scholar] [CrossRef]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro, C.; Haros, C.M.; Pérez-Álvarez, J.A. Chia (Salvia Hispanica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Sci. 2019, 156, 139–145. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Effect of orange dietary fibre, oregano essential oil and packaging conditions on shelf-life of bologna sausages. Food Control 2010, 21, 436–443. [Google Scholar] [CrossRef]
- Laranja, D.C.; Malheiros, P.D.S.; Tondo, E.C. Effective use of nisin to control lactic acid bacterial spoilage in sliced cooked ham. J. Food Process Preserv. 2019, 43, e14132. [Google Scholar] [CrossRef]
- Kemp, J.D.; Langlois, B.E.; Fox, J.D. Effects of vacuum packaging and potassium sorbate on yield, yeast and mold growth, and quality of dry-cured hams. J. Food Sci. 1981, 46, 1015–1017. [Google Scholar] [CrossRef]
- Ozturk, I. Antifungal activity of propolis, thyme essential oil and hydrosol on natural mycobiota of sucuk, a Turkish fermented sausage: Monitoring of their effects on microbiological, color and aroma properties: Antifungal activity of propolis and essential oil. J. Food Process. Preserv. 2015, 39, 1148–1158. [Google Scholar] [CrossRef]
- Barmpalia, I.M.; Koutsoumanis, K.P.; Geornaras, I.; Belk, K.E.; Scanga, J.A.; Kendall, P.A.; Smith, G.C.; Sofos, J.N. Effect of antimicrobials as ingredients of pork bologna for Listeria monocytogenes control during storage at 4 or 10 °C. Food Microbiol. 2005, 22, 205–211. [Google Scholar] [CrossRef]
- Chongsrimsirisakhol, O.; Pirak, T. Total polyphenol content and antioxidant properties of cold brew coffee extracts as affected by ultrasound treatment and their application in low fat pork sausage. Int. J. Food Prop. 2022, 25, 813–826. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Yaghoubi, M.; Khaneghah, A.M.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Combined effects of ε-polylysine and ε-polylysine nanoparticles with plant extracts on the shelf life and quality characteristics of nitrite-free frankfurter-type sausages. Meat Sci. 2021, 172, 108318. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Gurunathan, K. Bio-preservative effect of blends of essential oils: Natural anti-oxidant and anti-microbial agents for the shelf life enhancement of emulsion based chicken sausages. J. Food Sci. Technol. 2020, 57, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Herrero, A.; Tahmouzi, S.; Razavi, S.; Triki, M.; Rodríguez-Salas, L.; Samcová, K.; Jiménez-Colmenero, F. Properties of reformulated hot dog sausage without added nitrites during chilled storage. Food Sci. Technol. Int. 2016, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pando, G.; Cofrades, S.; Ruiz-Capillas, C.; Solas, M.T.; Triki, M.; Jiménez-Colmenero, F. Low-fat frankfurters formulated with a healthier lipid combination as functional ingredient: Microstructure, lipid oxidation, nitrite content, microbiological changes and biogenic amine formation. Meat Sci. 2011, 89, 65–71. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.-Z.; Atuna, R.A.; Salifu, R.; Nubalanaan, B.-A.; Amagloh, F.K.; Han, S.-Y. Physicochemical, oxidative stability and sensory properties of frankfurter-type sausage as influenced by the addition of carrot (Daucus carota) Paste. Foods 2021, 10, 3032. [Google Scholar] [CrossRef]
- Lima, T.L.S.; Costa, G.F.D.; Cruz, G.R.B.D.; Araújo, Í.B.D.S.; Ribeiro, N.L.; Ferreira, V.C.D.S.; Silva, F.A.P.D.; Beltrão Filho, E.M. Effect of storage time on colorimetric, physicochemical, and lipid oxidation parameters in sheep meat sausages with pre-emulsified linseed oil. Food Sci. Technol. 2022, 42, e24721. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Makino, Y. Simultaneous assessment of various quality attributes and shelf life of packaged bratwurst using hyperspectral imaging. Meat Sci. 2018, 146, 26–33. [Google Scholar] [CrossRef]
- Ozaki, M.M.; Santos, M.D.; Ribeiro, W.O.; Azambuja Ferreira, N.C.D.; Picone, C.S.F.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R. Radish powder and oregano essential oil as nitrite substitutes in fermented cooked sausages. Food Res. Int. 2021, 140, 109855. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Choi, H.M.; Kim, S.Y.; Lee, J.R.; Kim, H.J.; Jo, C.; Jung, S. Influence of Perilla Frutescens Var. Acuta water extract on the shelf life and physicochemical qualities of cooked beef patties. Korean J. Food Sci. Anim. Resour. 2015, 35, 389–397. [Google Scholar] [CrossRef]
- Baldin, J.C.; Munekata, P.E.S.; Michelin, E.C.; Polizer, Y.J.; Silva, P.M.; Canan, T.M.; Pires, M.A.; Godoy, S.H.S.; Fávaro-Trindade, C.S.; Lima, C.G.; et al. Effect of microencapsulated Jabuticaba (Myrciaria cauliflora) extract on quality and storage stability of mortadella sausage. Food Res. Int. 2018, 108, 551–557. [Google Scholar] [CrossRef] [PubMed]
| * Treatment | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |
| Control | 1.56 ab;w ± 0.05 | 3.25 b;x ± 0.15 | 6.23 c;y ± 0.25 | 8.55 e;z ± 0.26 |
| T1 | 1.15 ab;w ± 0.18 | 3.15 b;x ± 0.16 | 4.66 bc;y ± 0.23 | 6.45 bc;z ± 0.11 |
| T2 | 1.03 a;w ± 0.19 | 2.68 b;x ± 0.17 | 3.65 a;y ± 0.15 | 5.33 b;z ± 0.13 |
| T3 | 1.55 ab;w ± 0.15 | 3.15 b;x ± 0.18 | 4.35 ab;y ± 0.26 | 6.65 c;z ± 0.24 |
| T4 | 1.66 ab;x ± 0.17 | 3.68 c;y ± 0.16 | 4.13 ab;y ± 0.19 | 5.16 b;z ± 0.13 |
| T5 | 1.65 ab;w ± 0.13 | 3.35 b;x ± 0.19 | 4.35 ab;y ± 0.18 | 5.03 b;z ± 0.12 |
| T6 | 1.34 ab;w ± 0.17 | 3.76 c;x ± 0.07 | 5.34 cd;y ± 0.21 | 7.34 cd;z ± 0.19 |
| T7 | 1.69 ab;w ± 0.16 | 3.19 b;x ± 0.18 | 5.29 d;y ± 0.16 | 7.69 d;z ± 0.09 |
| T8 | 1.88 b;w ± 0.12 | 3.36 b;x ± 0.12 | 5.86 cd;y ± 0.17 | 6.88 c;z ± 0.19 |
| T9 | 1.76 b;w ± 0.12 | 3.19 b;x ± 0.09 | 4.66 b;y ± 0.13 | 5.65 b;z ± 0.17 |
| T10 | 1.22 ab;x ± 0.11 | 2.16 a;y ± 0.19 | 3.66 a;z ± 0.16 | 4.23 a;z ± 0.11 |
| * Treatment | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |
| Control | 1.52 a;x ± 0.19 | 3.88 c;y ± 0.35 | 4.24 e;y ± 0.26 | 5.35 c;z ± 0.31 |
| T1 | 1.45 a;x ± 0.21 | 1.86 a;xy ± 0.11 | 2.19 ab;y ± 0.13 | 3.58 b;z ± 0.19 |
| T2 | 1.22 a;y ± 0.12 | 1.58 a;y ± 0.23 | 2.28 ab;z ± 0.02 | 2.76 ab;z ± 0.15 |
| T3 | 1.31 a;y ± 0.19 | 1.46 a;y ± 0.16 | 2.38 abc;z ± 0.23 | 2.54 ab;z ± 0.23 |
| T4 | 1.51 a;x ± 0.16 | 1.88 a;x ± 0.22 | 2.58 bcd;y ± 0.11 | 3.42 ab;z ± 0.15 |
| T5 | 1.15 a;x ± 0.19 | 1.65 a;x ± 0.15 | 2.57 bcd;y ± 0.26 | 3.51 b;z ± 0.21 |
| T6 | 1.53 a;x ± 0.19 | 2.89 b;y ± 0.24 | 3.36 de;y ± 0.18 | 4.55 bc;z ± 0.19 |
| T7 | 1.34 a;w ± 0.14 | 2.12 ab;x ± 0.14 | 3.28 cd;y ± 0.21 | 4.28 bc;z ± 0.18 |
| T8 | 1.59 a;x ± 0.13 | 2.85 b;y ± 0.17 | 3.39 de;y ± 0.17 | 4.88 bc;z ± 0.12 |
| T9 | 1.36 a;x ± 0.17 | 1.54 a;x ± 0.31 | 2.37 ab;y ± 0.13 | 3.64 b;z ± 0.11 |
| T10 | 1.23 a;z ± 0.12 | 1.34 a;z ± 0.16 | 1.48 a;z ± 0.26 | 1.81 a;z ± 0.12 |
| * Treatment | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |
| Control | 1.62 a;x ± 0.09 | 2.26 a;x ± 0.18 | 5.24 c;y ± 0.24 | 7.35 e;z ± 0.16 |
| T1 | 1.55 a;x ± 0.27 | 2.19 a;x ± 0.16 | 3.29 ab;y ± 0.13 | 5.28 bc;z ± 0.19 |
| T2 | 1.12 a;x ± 0.36 | 1.55 a;xy ± 0.25 | 2.27 a;y ± 0.25 | 4.76 b;z ± 0.23 |
| T3 | 1.24 a;w ± 0.29 | 2.26 a;x ± 0.32 | 3.57 b;y ± 0.16 | 5.84 c;z ± 0.13 |
| T4 | 1.56 a;y ± 0.13 | 2.38 a;y ± 0.26 | 3.98 b;z ± 0.39 | 4.37 ab;z ± 0.11 |
| T5 | 1.35 a;w ± 0.19 | 2.35 a;x ± 0.16 | 3.27 ab;y ± 0.16 | 4.28 ab;z ± 0.22 |
| T6 | 1.24 a;w ± 0.33 | 2.29 a;x ± 0.34 | 4.34 bc;y ± 0.21 | 6.97 d;z ± 0.19 |
| T7 | 1.61 a;x ± 0.27 | 2.39 a;x ± 0.44 | 4.28 bc;y ± 0.26 | 6.28 cd;z ± 0.09 |
| T8 | 1.38 a;x ± 0.39 | 2.25 a;x ± 0.47 | 4.39 bc;y ± 0.37 | 5.88 c;z ± 0.16 |
| T9 | 1.36 a;x ± 0.24 | 2.54 a;y ± 0.32 | 3.37 ab;y ± 0.23 | 4.64 ab;z ± 0.26 |
| T10 | 1.12 a;x ± 0.19 | 1.54 a;xy ± 0.06 | 2.28 a;y ± 0.16 | 3.88 a;z ± 0.22 |
| * Treatment | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |
| Control | 1.26 a;x ± 0.26 | 1.56 a;xy ± 0.26 | 2.32 a;y ± 0.12 | 3.75 c;z ± 0.16 |
| T1 | 1.23 a;y ± 0.13 | 1.63 a;y ± 0.16 | 2.56 a;z ± 0.29 | 3.21 c;z ± 0.19 |
| T2 | 1.26 a;x ± 0.16 | 1.53 a;x ± 0.29 | 2.55 a;y ± 0.17 | 3.36 c;z ± 0.14 |
| T3 | 1.24 a;y ± 0.14 | 1.86 a;yz ± 0.12 | 2.29 a;z ± 0.18 | 2.61 ab;z ± 0.11 |
| T4 | 1.34 a;y ± 0.26 | 1.66 a;y ± 0.23 | 2.36 a;z ± 0.22 | 2.97 b;z ± 0.11 |
| T5 | 1.56 a;y ± 0.26 | 1.89 a;y ± 0.13 | 2.25 a;yz ± 0.36 | 2.87 b;z ± 0.20 |
| T6 | 1.29 a;x ± 0.13 | 1.55 a;xy ± 0.12 | 2.17 a;yz ± 0.25 | 2.31 a;z ± 0.19 |
| T7 | 1.27 a;x ± 0.27 | 1.63 a;x ± 0.13 | 2.55 a;y ± 0.16 | 2.18 a;z ± 0.12 |
| T8 | 1.23 a;y ± 0.19 | 1.56 a;y ± 0.19 | 2.53 a;z ± 0.11 | 2.69 b;z ± 0.14 |
| T9 | 1.29 a;y ± 0.14 | 1.88 a;yz ± 0.37 | 2.46 a;z ± 0.13 | 2.71 b;z ± 0.16 |
| T10 | 1.13 a;z ± 0.19 | 1.23 a;z ± 0.54 | 1.56 a;z ± 0.22 | 1.86 a;z ± 0.34 |
| * Treatment | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |
| Control | 6.13 a;x ± 0.04 | 5.93 b;x ± 0.04 | 5.52 c;y ± 0.04 | 5.21 e;z ± 0.03 |
| T1 | 6.12 a;x ± 0.03 | 5.89 b;x ± 0.03 | 5.71 bc;y ± 0.03 | 5.33 bc;z ± 0.03 |
| T2 | 6.21 a;x ± 0.04 | 6.12 a;xy ± 0.02 | 5.79 ab;y ± 0.05 | 5.61 b;z ± 0.02 |
| T3 | 6.18 a;w ± 0.03 | 5.91 b;x ± 0.03 | 5.61 b;y ± 0.02 | 5.42 c;z ± 0.04 |
| T4 | 6.09 a;y ± 0.03 | 6.05 ab;y ± 0.04 | 5.89 ab;z ± 0.03 | 5.82 ab;z ± 0.03 |
| T5 | 6.11 a;w ± 0.04 | 6.10 a;x ± 0.05 | 5.91 ab;y ± 0.03 | 5.89 ab;z ± 0.03 |
| T6 | 6.13 a;w ± 0.04 | 5.89 b;x ± 0.02 | 5.59 bc;y ± 0.04 | 5.31 d;z ± 0.04 |
| T7 | 6.21 a;x ± 0.02 | 5.91 b;x ± 0.04 | 5.58 bc;y ± 0.03 | 5.32 cd;z ± 0.02 |
| T8 | 6.11 a;x ± 0.03 | 6.02 ab;x ± 0.04 | 5.71 bc;y ± 0.04 | 5.52 c;z ± 0.03 |
| T9 | 6.20 a;x ± 0.04 | 6.13 a;y ± 0.03 | 5.83 ab;y ± 0.03 | 5.72 ab;z ± 0.03 |
| T10 | 6.22 a;x ± 0.03 | 6.18 a;xy ± 0.03 | 6.11 a;y ± 0.02 | 6.12 a;z ± 0.04 |
| Storage Day | Sensory Attributes | * Treatments Applied | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | ||
| Day 0 | Color | 7.66 a | 7.73 a | 7.76 a | 7.64 a | 7.77 a | 7.69 a | 7.83 a | 7.78 a | 7.65 a | 7.76 a | 7.84 a |
| Odor | 7.74 a | 7.57 a | 7.45 a | 7.29 a | 7.55 a | 7.50 a | 7.65 a | 7.79 a | 7.61 a | 7.46 a | 7.57 a | |
| Taste | 7.66 a | 7.64 a | 7.78 a | 7.54 a | 7.80 a | 7.75 a | 7.63 a | 7.56 a | 7.64 a | 7.88 a | 7.82 a | |
| Texture | 7.23 a | 7.61 a | 7.75 a | 7.86 a | 7.39 a | 7.37 a | 7.41 a | 7.29 a | 7.57 a | 7.73 a | 7.88 a | |
| Overall acceptability | 7.75 a | 7.68 a | 7.75 a | 7.66 a | 7.73 a | 7.69 a | 7.80 a | 7.75 a | 7.66 a | 7.76 a | 7.84 a | |
| Day 7 | Color | 7.16 a | 7.23 a | 7.46 a | 7.14 a | 7.67 a | 7.59 a | 7.23 a | 7.28 a | 7.25 a | 7.56 a | 7.77 a |
| Odor | 7.08 a | 7.18 a | 7.35 a | 7.09 a | 7.52 a | 7.62 a | 6.95 a | 6.89 a | 7.42 a | 7.56 a | 7.69 a | |
| Taste | 6.94 a | 7.14 a | 7.37 ab | 7.04 a | 7.48 ab | 7.71 b | 7.03 a | 7.16 a | 7.46 ab | 7.68 b | 7.88 b | |
| Texture | 6.68 a | 6.81 a | 6.95 a | 6.49 a | 7.53 b | 7.69 b | 6.54 a | 6.47 a | 7.07 a | 6.94 a | 7.92 b | |
| Overall acceptability | 6.75 a | 6.68 a | 6.75 a | 6.66 a | 7.63 b | 7.79 b | 6.80 a | 6.75 a | 6.86 a | 7.02 a | 7.95 b | |
| Day 14 | Color | 3.16 a | 4.23 b | 5.49 b | 4.14 b | 6.79 c | 7.66 d | 4.12 b | 4.28 b | 5.56 b | 6.25 b | 7.78 d |
| Odor | 3.28 a | 4.58 b | 5.87 c | 4.67 b | 7.12 d | 7.75 e | 4.66 b | 4.58 b | 5.63 c | 5.89 c | 7.92 e | |
| Taste | ND | 3.88 a | 4.88 b | 4.28 a | 6.85 d | 7.45 e | 4.18 a | 4.35 a | 5.57 c | 6.78 d | 7.75 e | |
| Texture | 2.18 a | 3.15 b | 4.63 d | 3.94 c | 7.52 f | 7.67 f | 4.44 cd | 4.12 c | 4.85 d | 5.71 e | 7.81 f | |
| Overall acceptability | 1.87 a | 3.45 b | 4.44 c | 3.58 b | 6.55 e | 7.53 f | 3.88 bc | 3.76 bc | 5.93 d | 5.85 d | 8.32 j | |
| Day 21 | Color | 1.58 a | 2.57 b | 3.82 c | 3.95 c | 6.54 f | 7.43 j | 2.37 b | 2.94 b | 4.65 d | 5.47 e | 7.85 j |
| Odor | 1.52 a | 1.74 a | 3.87 c | 2.38 b | 6.72 e | 7.65 f | 1.66 a | 1.72 a | 4.46 d | 4.85 d | 7.92 f | |
| Taste | ND | ND | 3.75 a | ND | 5.75 c | 6.65 d | ND | ND | 4.77 b | 5.83 c | 7.78 e | |
| Texture | 1.32 a | 1.65 a | 3.33 c | 2.34 b | 7.20 e | 7.69 ef | 2.48 b | 2.32 b | 3.35 c | 4.41 d | 7.85 f | |
| Overall acceptability | 1.18 a | 1.75 b | 3.24 d | 2.41 c | 5.85 g | 7.33 h | 2.43 c | 1.65 b | 4.63 e | 5.25 f | 8.12 i | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbanna, A.M.; Sabala, R.F.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Herman, V.; Sallam, K.I. Nisin and Organic Acid Salts Improved the Microbial Quality, Extended the Shelf Life, and Maintained the Sensory Attributes of Semidry Beef Luncheon Marketed at Adverse (35–40 °C) Ambient Summer Temperatures. Foods 2023, 12, 4283. https://doi.org/10.3390/foods12234283
Elbanna AM, Sabala RF, Abd-Elghany SM, Imre K, Morar A, Herman V, Sallam KI. Nisin and Organic Acid Salts Improved the Microbial Quality, Extended the Shelf Life, and Maintained the Sensory Attributes of Semidry Beef Luncheon Marketed at Adverse (35–40 °C) Ambient Summer Temperatures. Foods. 2023; 12(23):4283. https://doi.org/10.3390/foods12234283
Chicago/Turabian StyleElbanna, Ahmed Medhat, Rana Fahmi Sabala, Samir Mohammed Abd-Elghany, Kálmán Imre, Adriana Morar, Viorel Herman, and Khalid Ibrahim Sallam. 2023. "Nisin and Organic Acid Salts Improved the Microbial Quality, Extended the Shelf Life, and Maintained the Sensory Attributes of Semidry Beef Luncheon Marketed at Adverse (35–40 °C) Ambient Summer Temperatures" Foods 12, no. 23: 4283. https://doi.org/10.3390/foods12234283
APA StyleElbanna, A. M., Sabala, R. F., Abd-Elghany, S. M., Imre, K., Morar, A., Herman, V., & Sallam, K. I. (2023). Nisin and Organic Acid Salts Improved the Microbial Quality, Extended the Shelf Life, and Maintained the Sensory Attributes of Semidry Beef Luncheon Marketed at Adverse (35–40 °C) Ambient Summer Temperatures. Foods, 12(23), 4283. https://doi.org/10.3390/foods12234283