Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Experiment Based on RSM
2.2. Experiment Design
2.3. Raw Materials
2.4. Processing of Pretreated Cassava Flour
2.5. MC Analysis
2.6. Color Measurement
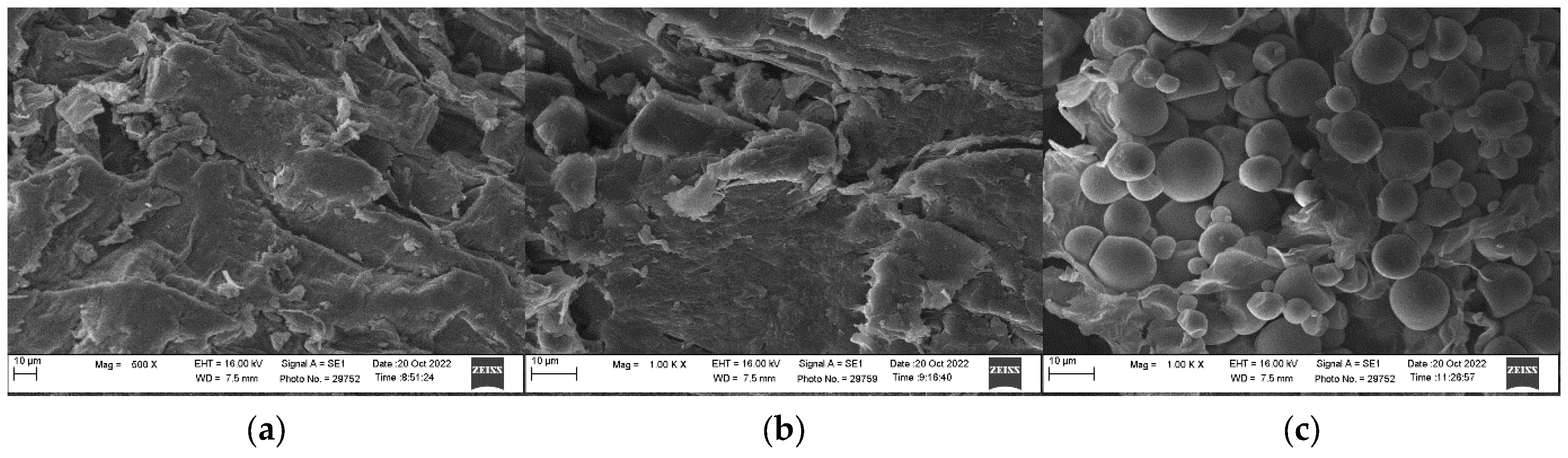
2.7. Microstructure Analysis
3. Results and Discussion
3.1. Statistical Analysis of MC
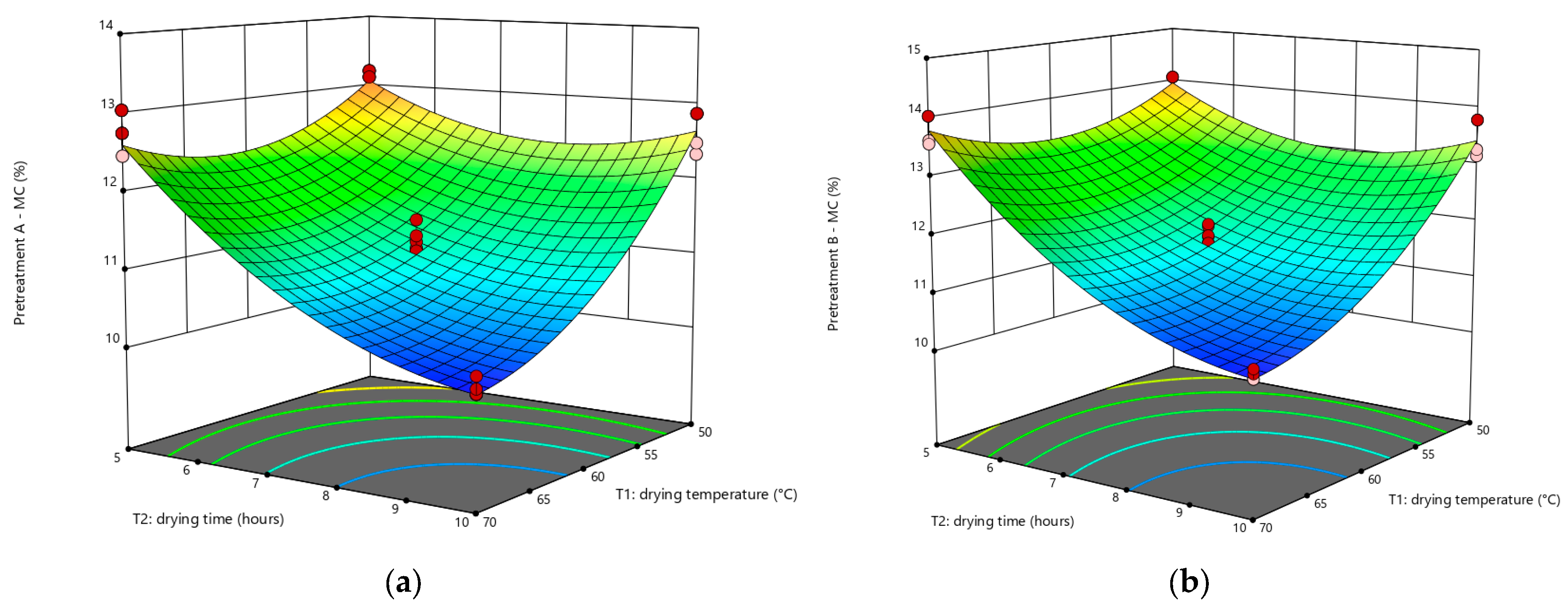
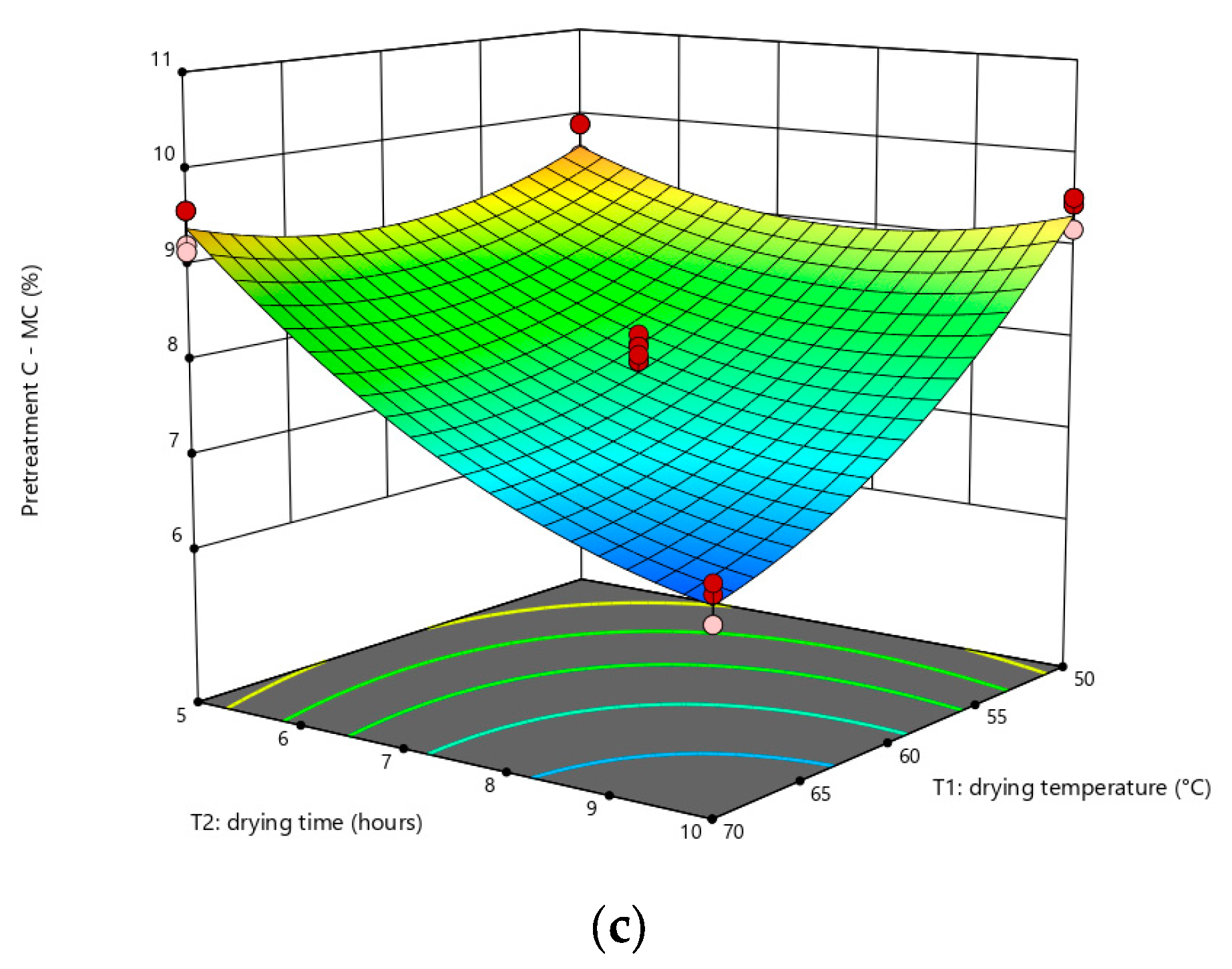
3.2. Effect of Factors on MC
3.3. Statistical Analysis of WI
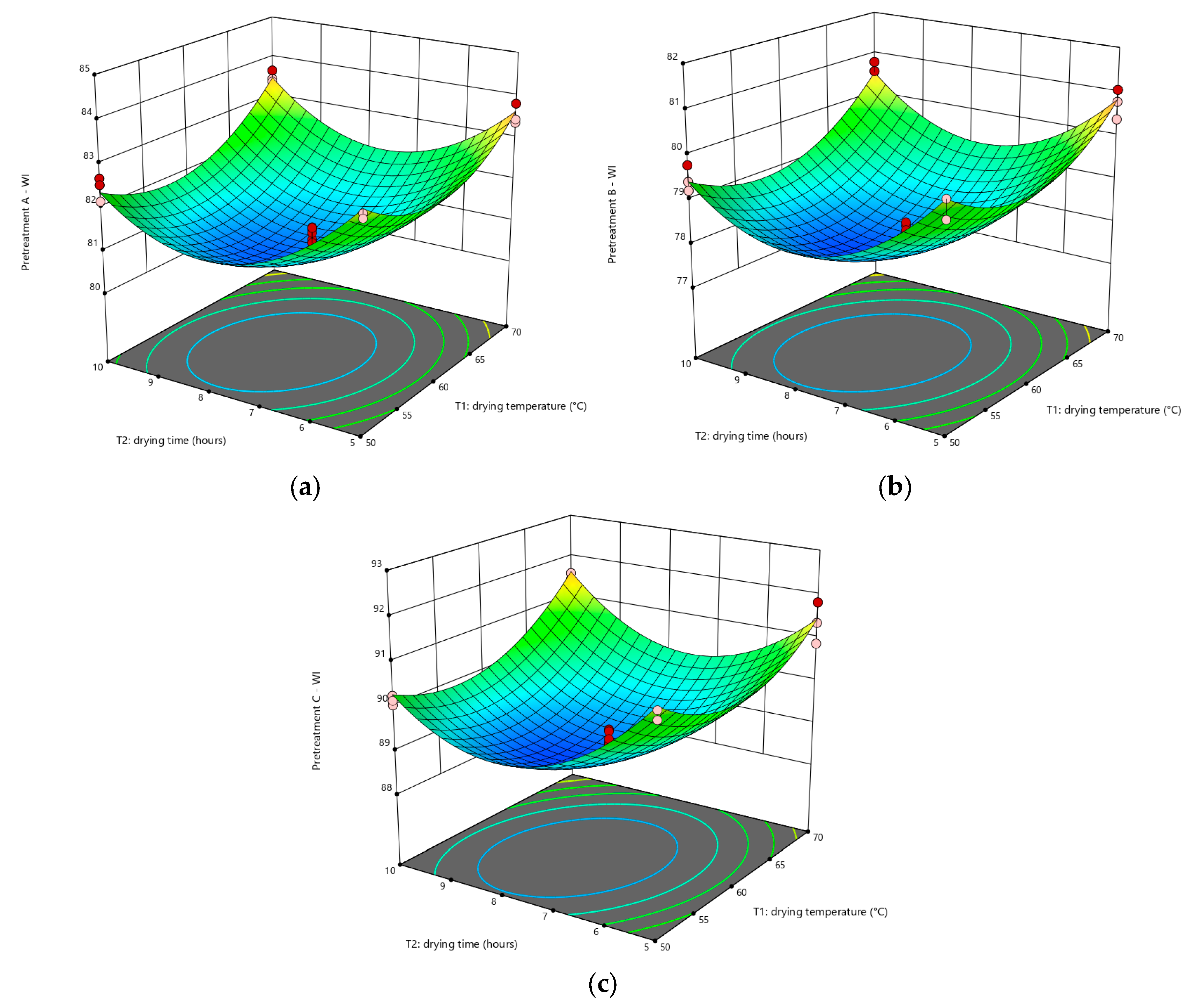
3.4. Effect of Factors on WI
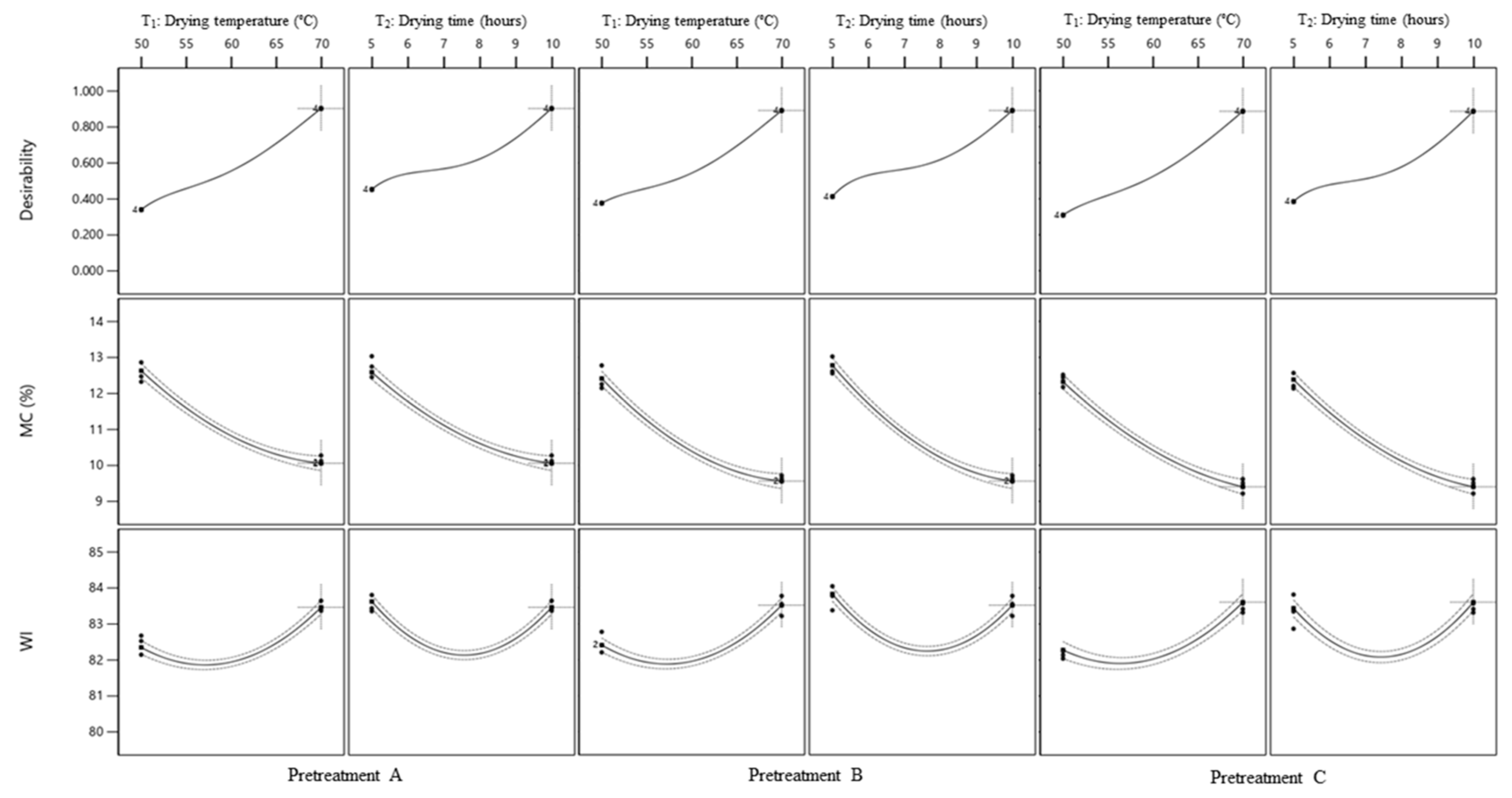
3.5. Optimization of MC and WI
3.6. Experimental Verification
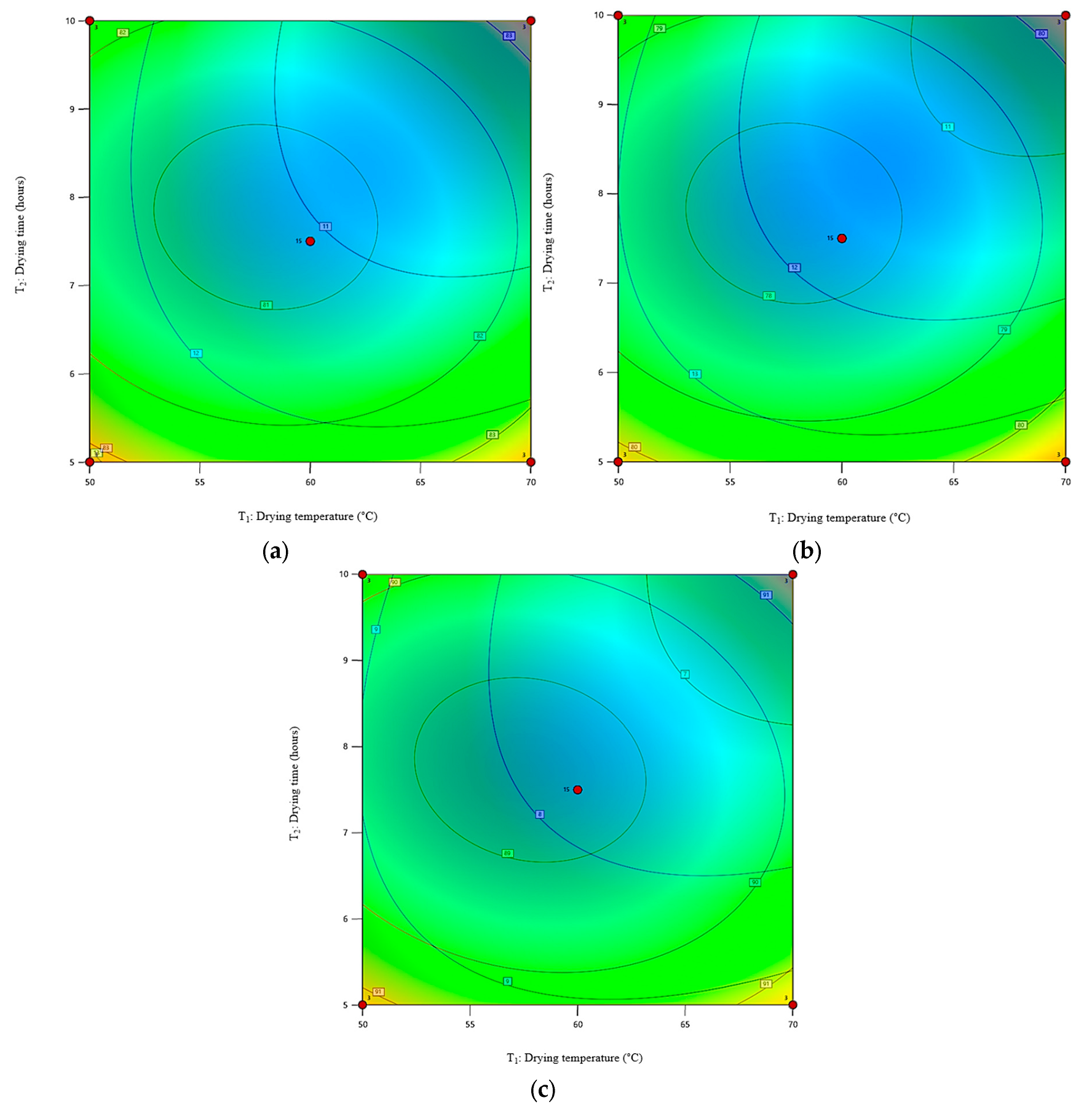
3.7. Contour Plots Superimposition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DTP. Laporan Tahunan Tahun 2020; Direktorat Jenderal Tanaman Pangan: Daerah Khusus Ibukota Jakarta, Indonesia, 2020. [Google Scholar]
- FAOSTAT. Crops and Livestock Products. In Statistics Division Food and Agriculture Organization of the United Nations; FAOSTAT: Rome, Italy, 2020; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 October 2022).
- Maieves, H.A.; Oliveira, D.C.; De Bernardo, C.; Müller, C.M.D.O.; Amante, E.R. Microscopy and texture of raw and cooked cassava (manihot esculenta crantz) roots. J. Texture Stud. 2012, 43, 164–173. [Google Scholar] [CrossRef]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 2009, 8, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Cazumbá da Silva, Í.R.; de Cardoso, R.C.V.; Góes, J.Â.W.; Druzian, J.I.; Vidal Júnior, P.O.; de Andrade, A.C.B. Food safety in cassava “flour houses” of Copioba Valley, Bahia, Brazil: Diagnosis and contribution to geographical indication. Food Control 2017, 72, 97–104. [Google Scholar] [CrossRef]
- Salcedo, A. Insights into the Physiological, Biochemical and Molecular Basis of Postharvest Deterioration in Cassava (Manihot esculenta) Roots. Am. J. Exp. Agric. 2011, 1, 414–431. [Google Scholar] [CrossRef]
- Sowmyapriya, S. Assessment of Biochemical Changes during Postharvest Physiological Deterioration in Cassava Tubers. Int. J. Pure Appl. Biosci. 2017, 5, 732–739. [Google Scholar] [CrossRef]
- Akubor, P.I. Effect of ascorbic acid and citric acid treatments on the functional and sensory properties of yam flour. Int. J. Agric. Policy Res. 2013, 1, 103–108. [Google Scholar]
- Buckman, E.; Plahar, W.; Oduro, I.; Carey, E. Effects of Sodium Metabisulphite and Blanching Pretreatments on the Quality Characteristics of Yam Bean (Pachyrhizus erosus) Flour. Br. J. Appl. Sci. Technol. 2015, 6, 138–144. [Google Scholar] [CrossRef]
- Ngoma, K.; Mashau, M.E.; Silungwe, H. Physicochemical and Functional Properties of Chemically Pretreated Ndou Sweet Potato Flour. Int. J. Food Sci. 2019, 2019, 4158213. [Google Scholar] [CrossRef]
- Ulfa, Z.; Julianti, E.; Nurminah, M. Effect of pre-treatment in the production of purple-fleshed sweet potato flour on cookies quality. IOP Conf. Ser Earth Environ. Sci. 2019, 260, 012095. [Google Scholar] [CrossRef]
- Coelho, D.G.; de Andrade, M.T.; de Mélo Neto, D.F.; Ferreira-Silva, S.L.; do Simões, A.N. Escurecimento em mandioca de mesa minimamente processada com uso de antioxidantes e revestimento de amido. Rev. Caatinga 2017, 30, 503–512. [Google Scholar] [CrossRef]
- Coelho, D.G.; Fonseca, K.S.; de Mélo Neto, D.F.; de Andrade, M.T.; Junior, L.F.C.; Ferreira-Silva, S.L.; Simões, A.D.N. Association of preharvest management with oxidative protection and enzymatic browning in minimally processed cassava. J. Food Biochem. 2019, 43, e12840. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; Wiley and Sons Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Pornpraipech, P.; Khusakul, M.; Singklin, R.; Sarabhorn, P.; Areeprasert, C. Effect of temperature and shape on drying performance of cassava chips. Agric. Nat. Resour. 2017, 51, 402–409. [Google Scholar] [CrossRef]
- Omolola, A.O.; Kapila, P.F.; Anyasi, T.A.; Jideani, A.I.O.; Mchau, G.A. Optimization of Color and Thermal Properties of Sweet Cassava (Manihot esculenta Crantz Var. UVLNR 0005) Flour Using Response Surface Methodology. Asian J. Agric. Res. 2017, 11, 57–65. [Google Scholar] [CrossRef]
- Udoro, E.O.; Anyasi, T.A.; Jideani, A.I.O. Interactive Effects of Chemical Pretreatment and Drying on the Physicochemical Properties of Cassava Flour Using Response Surface Methodology. Int. J. Food Sci. 2020, 2020, 7234372. [Google Scholar] [CrossRef]
- Savas, E. The Modelling of Convective Drying Variables’ Effects on the Functional Properties of Sliced Sweet Potatoes. Foods 2022, 11, 741. [Google Scholar] [CrossRef]
- Senevirathna, S.S.J.; Ramli, N.S.; Azman, E.M.; Juhari, N.H.; Karim, R. Optimization of the drum drying parameters and citric acid level to produce purple sweet potato (Ipomoea batatas L.) powder using response surface methodology. Foods 2021, 10, 1378. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef]
- BLP. Processing of Cassava Flour and Tapioca. Badan Litbang Pertanian, Agroinovasi. Published. 2011. Available online: http://www.litbang.pertanian.go.id/ (accessed on 15 October 2022).
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Torbica, A.; Hadnadev, M.; Dapčević, H.T. Rice and buckwheat flour characterisation and its relation to cookie quality. Food Res. Int. 2012, 48, 277–283. [Google Scholar] [CrossRef]
- Birch, G.G.; Blakebrough, N.; Parker, K.J. Enzymes and Food Processing; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- State, O. Modeling of hot-air drying of pretreated cassava chips. Agric. Eng. Int. CIGR J. 2010, 12, 34–41. [Google Scholar]
- Xiao, H.W.; Yao, X.D.; Lin, H.; Yang, W.X.; Meng, J.S.; Gao, Z.J. Effect of SSB (Superheated Steam Blanching) time and drying temperature on hot air impingement drying kinetics and quality attributes of yam slices. J. Food Process. Eng. 2012, 35, 370–390. [Google Scholar] [CrossRef]
- Fernandez, C.; Alvarez, M.D.; Canet, W. The effect of low-temperature blanching on the quality of fresh and frozen/thawed mashed potatoes. Int. J. Food Sci. Technol. 2006, 41, 577–595. [Google Scholar] [CrossRef]
- Ai, Z.; Xie, Y.; Li, X.; Lei, D.; Ambrose, K.; Liu, Y. Revealing color change and drying mechanisms of pulsed vacuum steamed Cistanche deserticola through bioactive components, microstructural and starch gelatinization properties. Food Res. Int. 2022, 162, 112079. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, J.; Li, X.; Wang, Y.; Miao, J.; Mao, X.; Zhao, C.; Gao, W. Effect of blanching and drying temperatures on starch-related physicochemical properties, bioactive components and antioxidant activities of yam flours. LWT 2017, 82, 303–310. [Google Scholar] [CrossRef]
- Tacer-Caba, Z.; Nilufer-Erdil, D.; Boyacioglu, M.H.; Ng, P.K.W. Evaluating the effects of amylose and Concord grape extract powder substitution on physicochemical properties of wheat flour extrudates produced at different temperatures. Food Chem. 2014, 157, 476–484. [Google Scholar] [CrossRef]
- Rayas-Duarte, P.; Majewska, K.; Doetkott, C. Effect of extrusion process parameters on the quality of buckwheat flour mixes. Cereal Chem. 1998, 75, 338–345. [Google Scholar] [CrossRef]
- Dhanalakshmi, K.; Bhattacharya, S. Agglomeration of turmeric powder and its effect on physico-chemical and microstructural characteristics. J. Food Eng. 2014, 120, 124–134. [Google Scholar] [CrossRef]
- Kuttigounder, D.; Lingamallu, J.R.; Bhattacharya, S. Turmeric Powder and Starch: Selected Physical, Physicochemical, and Microstructural Properties. J. Food Sci. 2011, 76, C1284–C1291. [Google Scholar] [CrossRef]
- Morrot, G.; Brochet, F.; Dubourdieu, D. The color of odors. Brain Lang. 2001, 79, 309–320. [Google Scholar] [CrossRef]
- Zellner, D.A.; Durlach, P. Effect of color on expected and experienced refreshment, intensity, and liking of beverages. Am. J. Psychol. 2003, 116, 633–647. [Google Scholar] [CrossRef]
- Jimoh, K.; Olurin, T.O.; Aina, J.O. Effect of drying methods on the rheological characteristics and colour of yam flours. African J. Biotechnol. 2009, 8, 2325–2328. [Google Scholar]
- Omolola, A.O.; Jideani, A.I.O.; Kapila, P.F.; Jideani, V.A. Optimization of microwave drying conditions of two banana varieties using response surface methodology. Food Sci. Technol. 2015, 35, 438–444. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Kunwisawa, J.; Soponronnarit, S. Determination of effective moisture diffusivity and assessment of quality attributes of banana slices during drying. LWT 2011, 44, 1502–1510. [Google Scholar] [CrossRef]
- Akintunde, B.; Tunde-Akintunde, T. Effect of drying method and variety on quality of cassava starch extracts. Afr. J. Food Agric. Nutr. Dev. 2013, 13, 8351–8367. [Google Scholar] [CrossRef]
- Anyasi, T.A.; Jideani, A.I.O.; McHau, G.R.A. Effect of organic acid pretreatment on some physical, functional and antioxidant properties of flour obtained from three unripe banana cultivars. Food Chem. 2015, 172, 515–522. [Google Scholar] [CrossRef]
- Quayson, E.T.; Ayernor, G.S.; Johnson, P.N.T.; Ocloo, F.C.K. Effects of two pre-treatments, blanching and soaking, as processing modulation on non-enzymatic browning developments in three yam cultivars from Ghana. Heliyon 2021, 7, e07224. [Google Scholar] [CrossRef]
- Sanful, R.E.; Oduro, I.; Ellis, W.O. Effects of pre-treatment and drying methods on the pasting characteristics, amylose content and colour of Aerial Yam (Dioscorea bulbifera) flour. Int. J. Food Sci. Nutr. 2017, 2, 23–28. [Google Scholar]
- Adedeji, O.; Jegede, D.; Abdulsalam, K.; Umeohia, U.; Ajayi, O.; Iboyi, J. Effect of Processing Treatments on the Proximate, Functional and Sensory Properties of Soy-Sorghum-Roselle Complementary Food. Br. J. Appl. Sci. Technol. 2015, 6, 635–643. [Google Scholar] [CrossRef]
- Makinde, F.M.; Ladipo, A.T. Physico-chemical and microbial quality of sorghum-based complementary food enriched with soybean (Glycine max) and sesame (Sesamum indicum). J. Food Technol. 2012, 10, 46–49. [Google Scholar] [CrossRef]
- Thacker, B.H.; Doebling, S.W.; Hemez, F.M.; Anderson, M.C.; Pepin, J.E.; Rodriguez, E. Concepts of Model Verification and Validation; Los Alamos National Lab.: Los Alamos, NM, USA, 2002. [Google Scholar]
- Onitilo, M.O.; Sanni, L.O.; Oyewole, O.B.; Maziya-Dixon, B. Physicochemical and functional properties of sour starches from different cassava varieties. Int. J. Food Prop. 2007, 10, 607–620. [Google Scholar] [CrossRef]
- Jampala, P.; Tadikamalla, S.; Preethi, M.; Ramanujam, S.; Uppuluri, K.B. Concurrent production of cellulase and xylanase from Trichoderma reesei NCIM 1186: Enhancement of production by desirability-based multi-objective method. 3 Biotech 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]

| Factor | Unit | Notation | Level | ||||
|---|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | 1 | 1.414 | |||
| Temperature | °C | T1 | 45.8579 | 50 | 60 | 70 | 74.1421 |
| Time | Hours | T2 | 3.9644 | 5 | 7.5 | 10 | 11.0355 |
| Sample | Coded Factor | Uncoded Factor | ||||
|---|---|---|---|---|---|---|
| Pretreatment A | Pretreatment B | Pretreatment C | T1 | T2 | T1 | T2 |
| A1 | B1 | C1 | −1 | −1 | 50 | 5 |
| A2 | B2 | C2 | −1 | −1 | 50 | 5 |
| A3 | B3 | C3 | −1 | −1 | 50 | 5 |
| A4 | B4 | C4 | 1 | −1 | 70 | 5 |
| A5 | B5 | C5 | 1 | −1 | 70 | 5 |
| A6 | B6 | C6 | 1 | −1 | 70 | 5 |
| A7 | B7 | C7 | −1 | 1 | 50 | 10 |
| A8 | B8 | C8 | −1 | 1 | 50 | 10 |
| A9 | B9 | C9 | −1 | 1 | 50 | 10 |
| A10 | B10 | C10 | 1 | 1 | 70 | 10 |
| A11 | B11 | C11 | 1 | 1 | 70 | 10 |
| A12 | B12 | C12 | 1 | 1 | 70 | 10 |
| A13 | B13 | C13 | −1.414 | 0 | 45.8579 | 7.5 |
| A14 | B14 | C14 | −1.414 | 0 | 45.8579 | 7.5 |
| A15 | B15 | C15 | −1.414 | 0 | 45.8579 | 7.5 |
| A16 | B16 | C16 | 1.414 | 0 | 74.1421 | 7.5 |
| A17 | B17 | C17 | 1.414 | 0 | 74.1421 | 7.5 |
| A18 | B18 | C18 | 1.414 | 0 | 74.1421 | 7.5 |
| A19 | B19 | C19 | 0 | −1.414 | 60 | 3.9644 |
| A20 | B20 | C20 | 0 | −1.414 | 60 | 3.9644 |
| A21 | B21 | C21 | 0 | −1.414 | 60 | 3.9644 |
| A22 | B22 | C22 | 0 | 1.414 | 60 | 11.0355 |
| A23 | B23 | C23 | 0 | 1.414 | 60 | 11.0355 |
| A24 | B24 | C24 | 0 | 1.414 | 60 | 11.0355 |
| A25 | B25 | C25 | 0 | 0 | 60 | 7.5 |
| A26 | B26 | C26 | 0 | 0 | 60 | 7.5 |
| A27 | B27 | C27 | 0 | 0 | 60 | 7.5 |
| A28 | B28 | C28 | 0 | 0 | 60 | 7.5 |
| A29 | B29 | C29 | 0 | 0 | 60 | 7.5 |
| A30 | B30 | C30 | 0 | 0 | 60 | 7.5 |
| A31 | B31 | C31 | 0 | 0 | 60 | 7.5 |
| A32 | B32 | C32 | 0 | 0 | 60 | 7.5 |
| A33 | B33 | C33 | 0 | 0 | 60 | 7.5 |
| A34 | B34 | C34 | 0 | 0 | 60 | 7.5 |
| A35 | B35 | C35 | 0 | 0 | 60 | 7.5 |
| A36 | B36 | C36 | 0 | 0 | 60 | 7.5 |
| A37 | B37 | C37 | 0 | 0 | 60 | 7.5 |
| A38 | B38 | C38 | 0 | 0 | 60 | 7.5 |
| A39 | B39 | C39 | 0 | 0 | 60 | 7.5 |
| Sample | Response | Sample | Response | Sample | Response | |||
|---|---|---|---|---|---|---|---|---|
| MC (%) | WI | MC (%) | WI | MC (%) | WI | |||
| A1 | 13.21 | 83.17 | B1 | 13.58 | 80.28 | C1 | 9.51 | 90.98 |
| A2 | 13.12 | 83.53 | B2 | 13.78 | 79.86 | C2 | 9.26 | 91.45 |
| A3 | 12.82 | 83.06 | B3 | 14.07 | 80.59 | C3 | 9.88 | 91.18 |
| A4 | 12.46 | 83.81 | B4 | 14.03 | 80.78 | C4 | 9.57 | 91.35 |
| A5 | 12.75 | 83.36 | B5 | 13.62 | 81.05 | C5 | 9.21 | 91.82 |
| A6 | 13.04 | 83.44 | B6 | 13.56 | 80.38 | C6 | 9.14 | 90.87 |
| A7 | 12.33 | 82.68 | B7 | 13.15 | 79.41 | C7 | 9.18 | 90.04 |
| A8 | 12.48 | 82.53 | B8 | 13.78 | 79.78 | C8 | 9.45 | 90.25 |
| A9 | 12.87 | 82.15 | B9 | 13.26 | 79.21 | C9 | 9.52 | 90.14 |
| A10 | 10.07 | 83.44 | B10 | 10.64 | 80.55 | C10 | 6.22 | 91.42 |
| A11 | 10.13 | 83.65 | B11 | 10.72 | 80.22 | C11 | 6.51 | 91.57 |
| A12 | 10.28 | 83.37 | B12 | 10.56 | 80.78 | C12 | 6.62 | 91.32 |
| A13 | 13.23 | 82.13 | B13 | 14.07 | 79.24 | C13 | 9.58 | 90.22 |
| A14 | 13.27 | 82.17 | B14 | 13.94 | 79.18 | C14 | 10.02 | 90.18 |
| A15 | 13.39 | 82.15 | B15 | 14.12 | 79.28 | C15 | 9.65 | 90.13 |
| A16 | 10.84 | 83.29 | B16 | 11.62 | 80.57 | C16 | 7.23 | 90.82 |
| A17 | 11.13 | 83.36 | B17 | 11.86 | 80.45 | C17 | 7.61 | 91.46 |
| A18 | 10.72 | 83.29 | B18 | 11.82 | 80.45 | C18 | 7.78 | 91.62 |
| A19 | 13.32 | 84.05 | B19 | 14.35 | 81.13 | C19 | 9.88 | 92.25 |
| A20 | 12.88 | 84.05 | B20 | 14.42 | 81.27 | C20 | 10.13 | 91.72 |
| A21 | 12.86 | 84.14 | B21 | 14.56 | 81.15 | C21 | 9.96 | 92.07 |
| A22 | 10.82 | 83.07 | B22 | 11.42 | 80.22 | C22 | 7.34 | 91.58 |
| A23 | 11.12 | 83.18 | B23 | 11.74 | 80.21 | C23 | 7.52 | 91.62 |
| A24 | 11.07 | 83.24 | B24 | 11.66 | 80.24 | C24 | 7.65 | 91.46 |
| A25 | 11.15 | 80.56 | B25 | 11.58 | 78.16 | C25 | 7.45 | 88.87 |
| A26 | 11.12 | 80.83 | B26 | 11.85 | 77.58 | C26 | 7.45 | 89.17 |
| A27 | 11.18 | 80.62 | B27 | 11.75 | 77.95 | C27 | 8.06 | 88.72 |
| A28 | 11.55 | 80.91 | B28 | 11.52 | 77.92 | C28 | 7.73 | 88.56 |
| A29 | 11.07 | 81.14 | B29 | 12.03 | 77.72 | C29 | 7.65 | 88.58 |
| A30 | 11.12 | 80.71 | B30 | 11.72 | 77.67 | C30 | 7.58 | 88.61 |
| A31 | 11.26 | 80.48 | B31 | 11.73 | 77.71 | C31 | 8.16 | 89.15 |
| A32 | 10.83 | 81.06 | B32 | 11.52 | 77.76 | C32 | 7.86 | 88.68 |
| A33 | 11.34 | 81.20 | B33 | 12.07 | 77.74 | C33 | 7.72 | 88.70 |
| A34 | 10.83 | 80.61 | B34 | 11.54 | 77.62 | C34 | 7.61 | 88.64 |
| A35 | 10.84 | 80.76 | B35 | 11.07 | 77.67 | C35 | 8.03 | 88.61 |
| A36 | 10.87 | 80.82 | B36 | 11.87 | 77.81 | C36 | 7.54 | 88.82 |
| A37 | 10.91 | 81.18 | B37 | 11.74 | 77.80 | C37 | 7.56 | 89.12 |
| A38 | 11.12 | 80.59 | B38 | 11.63 | 78.24 | C38 | 7.87 | 88.68 |
| A39 | 11.14 | 80.61 | B39 | 11.56 | 78.06 | C39 | 7.94 | 88.94 |
| Source | Notation | Sum of Squares | Mean Square | Coefficient | Standard Error | p | R2 | R2 (adj) |
|---|---|---|---|---|---|---|---|---|
| Pretreatment A | ||||||||
| Constant | 11.0887 | 0.0560 | 0.000 | 0.9624 | 0.9567 | |||
| Temperature | T1 | 13.93 | 13.93 | −0.7618 | 0.0443 | 0.000 | ||
| Time | T2 | 13.20 | 13.20 | −0.7415 | 0.0443 | 0.000 | ||
| Temperature∗time | T1*T2 | 3.31 | 3.31 | −0.5250 | 0.0626 | 0.000 | ||
| Temperature∗temperature | T1*T1 | 5.71 | 5.71 | 0.5230 | 0.0475 | 0.000 | ||
| Time∗time | T2*T2 | 4.82 | 4.82 | 0.4805 | 0.0475 | 0.000 | ||
| Lack of fit | 0.2347 | 0.0782 | 0.172 | |||||
| Error | 1.32 | 0.0440 | ||||||
| Total | 41.30 | |||||||
| Pretreatment B | ||||||||
| Constant | 11.6787 | 0.0572 | 0.000 | 0.9713 | 0.9669 | |||
| Temperature | T1 | 13.72 | 13.72 | −0.7562 | 0.0453 | 0.000 | ||
| Time | T2 | 21.22 | 21.22 | −0.9402 | 0.0453 | 0.000 | ||
| Temperature∗time | T1*T2 | 5.40 | 5.40 | −0.6708 | 0.0640 | 0.000 | ||
| Temperature∗temperature | T1*T1 | 7.41 | 7.41 | 0.5959 | 0.0485 | 0.000 | ||
| Time∗time | T2*T2 | 8.98 | 8.98 | 0.6559 | 0.0485 | 0.000 | ||
| Lack of fit | 0.1800 | 0.0600 | 0.310 | |||||
| Error | 1.44 | 0.0481 | ||||||
| Total | 56.47 | |||||||
| Pretreatment C | ||||||||
| Constant | 7.7473 | 0.0573 | 0.000 | 0.9648 | 0.9595 | |||
| Temperature | T1 | 14.89 | 14.89 | −0.7878 | 0.0453 | 0.000 | ||
| Time | T2 | 16.04 | 16.04 | −0.8175 | 0.0453 | 0.000 | ||
| Temperature∗time | T1*T2 | 5.43 | 5.43 | −0.6725 | 0.0640 | 0.000 | ||
| Temperature∗temperature | T1*T1 | 4.10 | 4.10 | 0.4430 | 0.0485 | 0.000 | ||
| Time∗time | T2*T2 | 5.09 | 5.09 | 0.4938 | 0.04854 | 0.000 | ||
| Lack of fit | 0.0955 | 0.0318 | 0.604 | |||||
| Error | 1.53 | 0.0509 | ||||||
| Total | 46.12 |
| Source | Notation | Sum of Squares | Mean Square | Coefficient | Standard Error | p | R2 | R2 (adj) |
|---|---|---|---|---|---|---|---|---|
| Pretreatment A | ||||||||
| Constant | 80.8053 | 0.0529 | 0.000 | 0.9774 | 0.9739 | |||
| Temperature | T1 | 3.29 | 3.29 | 0.3702 | 0.0418 | 0.000 | ||
| Time | T2 | 1.73 | 1.73 | −0.2683 | 0.0418 | 0.000 | ||
| Temperature∗time | T1*T2 | 0.4219 | 0.4219 | 0.1875 | 0.0592 | 0.003 | ||
| Temperature∗temperature | T1*T1 | 19.42 | 19.42 | 0.9646 | 0.0449 | 0.000 | ||
| Time∗time | T2*T2 | 41.47 | 41.47 | 1.4096 | 0.0449 | 0.000 | ||
| Lack of fit | 0.1154 | 0.0385 | 0.449 | |||||
| Error | 1.27 | 0.0424 | ||||||
| Total | 61.24 | |||||||
| Pretreatment B | ||||||||
| Constant | 77.8273 | 0.0547 | 0.000 | 0.9772 | 0.9737 | |||
| Temperature | T1 | 4.13 | 4.13 | 0.4150 | 0.0432 | 0.000 | ||
| Time | T2 | 2.08 | 2.08 | −0.2942 | 0.0432 | 0.000 | ||
| Temperature∗time | T1*T2 | 0.2324 | 0.2324 | 0.1392 | 0.0611 | 0.029 | ||
| Temperature∗temperature | T1*T1 | 21.15 | 21.15 | 1.0068 | 0.0463 | 0.000 | ||
| Time∗time | T2*T2 | 42.53 | 42.53 | 1.4276 | 0.0463 | 0.000 | ||
| Lack of fit | 0.0798 | 0.0266 | 0.639 | |||||
| Error | 1.40 | 0.0466 | ||||||
| Total | 64.75 | |||||||
| Pretreatment C | ||||||||
| Constant | 88.79 | 0.0656 | 0.000 | 0.9657 | 0.9605 | |||
| Temperature | T1 | 3.43 | 3.43 | 0.3782 | 0.0519 | 0.000 | ||
| Time | T2 | 0.9848 | 0.9848 | −0.2026 | 0.0519 | 0.004 | ||
| Temperature∗time | T1*T2 | 0.9919 | 0.9919 | 0.2875 | 0.0734 | 0.004 | ||
| Temperature∗temperature | T1*T1 | 17.55 | 17.55 | 0.9171 | 0.0556 | 0.000 | ||
| Time∗time | T2*T2 | 43.25 | 43.25 | 1.4396 | 0.0556 | 0.000 | ||
| Lack of fit | 0.3597 | 0.1199 | 0.130 | |||||
| Error | 1.77 | 0.0591 | ||||||
| Total | 62.08 |
| Sample | MC (%) | WI | ||||
|---|---|---|---|---|---|---|
| Predicted | Actual | Relative Deviation (%) | Predicted | Actual | Relative Deviation (%) | |
| Pretreatment A | ||||||
| AV1 | 10.06 | 10.12 | 0.59 | 83.47 | 83.62 | 0.18 |
| AV2 | 10.06 | 10.23 | 1.68 | 83.47 | 83.35 | 0.14 |
| AV3 | 10.06 | 10.28 | 2.16 | 83.47 | 83.43 | 0.05 |
| Mean | 1.48 | Mean | 0.12 | |||
| Pretreatment B | ||||||
| BV1 | 10.56 | 10.36 | 1.91 | 80.52 | 80.68 | 0.20 |
| BV2 | 10.56 | 10.71 | 1.68 | 80.52 | 80.38 | 0.17 |
| BV3 | 10.56 | 10.47 | 2.16 | 80.52 | 80.61 | 0.11 |
| Mean | 1.48 | Mean | 0.16 | |||
| Pretreatment C | ||||||
| CV1 | 6.41 | 6.37 | 0.63 | 91.61 | 91.42 | 0.21 |
| CV2 | 6.41 | 6.54 | 2.01 | 91.61 | 91.57 | 0.04 |
| CV3 | 6.41 | 6.49 | 1.24 | 91.61 | 91.83 | 0.24 |
| Mean | 1.29 | Mean | 0.16 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nainggolan, E.A.; Banout, J.; Urbanova, K. Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour. Foods 2023, 12, 2101. https://doi.org/10.3390/foods12112101
Nainggolan EA, Banout J, Urbanova K. Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour. Foods. 2023; 12(11):2101. https://doi.org/10.3390/foods12112101
Chicago/Turabian StyleNainggolan, Ellyas Alga, Jan Banout, and Klara Urbanova. 2023. "Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour" Foods 12, no. 11: 2101. https://doi.org/10.3390/foods12112101
APA StyleNainggolan, E. A., Banout, J., & Urbanova, K. (2023). Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour. Foods, 12(11), 2101. https://doi.org/10.3390/foods12112101







