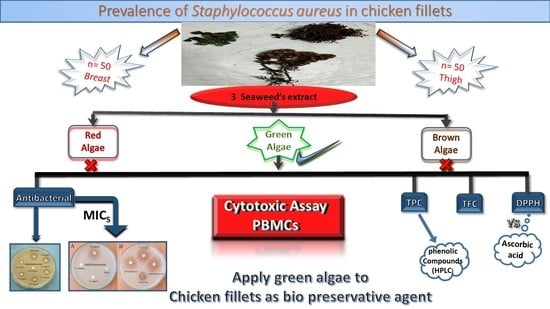
Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Chicken Fillet and Determination of S. aureus
2.2. Bacterial Strain
2.3. Algal Materials and Extraction
2.4. Antibacterial Activity
2.4.1. Assessment of the Antibacterial Activity of Lyophilized Seaweed Extracts
2.4.2. Evaluation of the Minimum Inhibitory Concentrations (MICs) of Lyophilized HO Extract
2.5. Phytochemical Analysis of the Lyophilized HO Algal Extract
2.5.1. Assessment of the Radical Scavenging Capacity by the DPPH Assay
2.5.2. Total Phenolic and Total Flavonoid Contents of Lyophilized HO Algal Extract
2.6. HPLC Evaluation of Phenolic Compounds Profiles of the Lyophilized HO Algal Extract
2.7. Safety and Cytotoxicity Assay of Lyophilized HO Algal Extract
2.8. Assessment of the Antibacterial Effect of Lyophilized HO Algal Extract against S. aureus Experimentally Inoculated into Chicken Fillet
2.9. Assessment of the Acceptability of Chicken Fillet Fortified with the Lyophilized HO Algal Extract
2.10. Statistical Analysis
3. Results and Discussion
3.1. Prevalence of S. aureus in Chicken Fillet
3.2. Antibacterial Activity of Lyophilized Seaweed Extracts
3.3. Minimum Inhibitory Concentrations (MICs) of Lyophilized HO Extract
3.4. DPPH Radical Scavenging Capacity
3.5. TPC and TFC of Lyophilized HO Extract
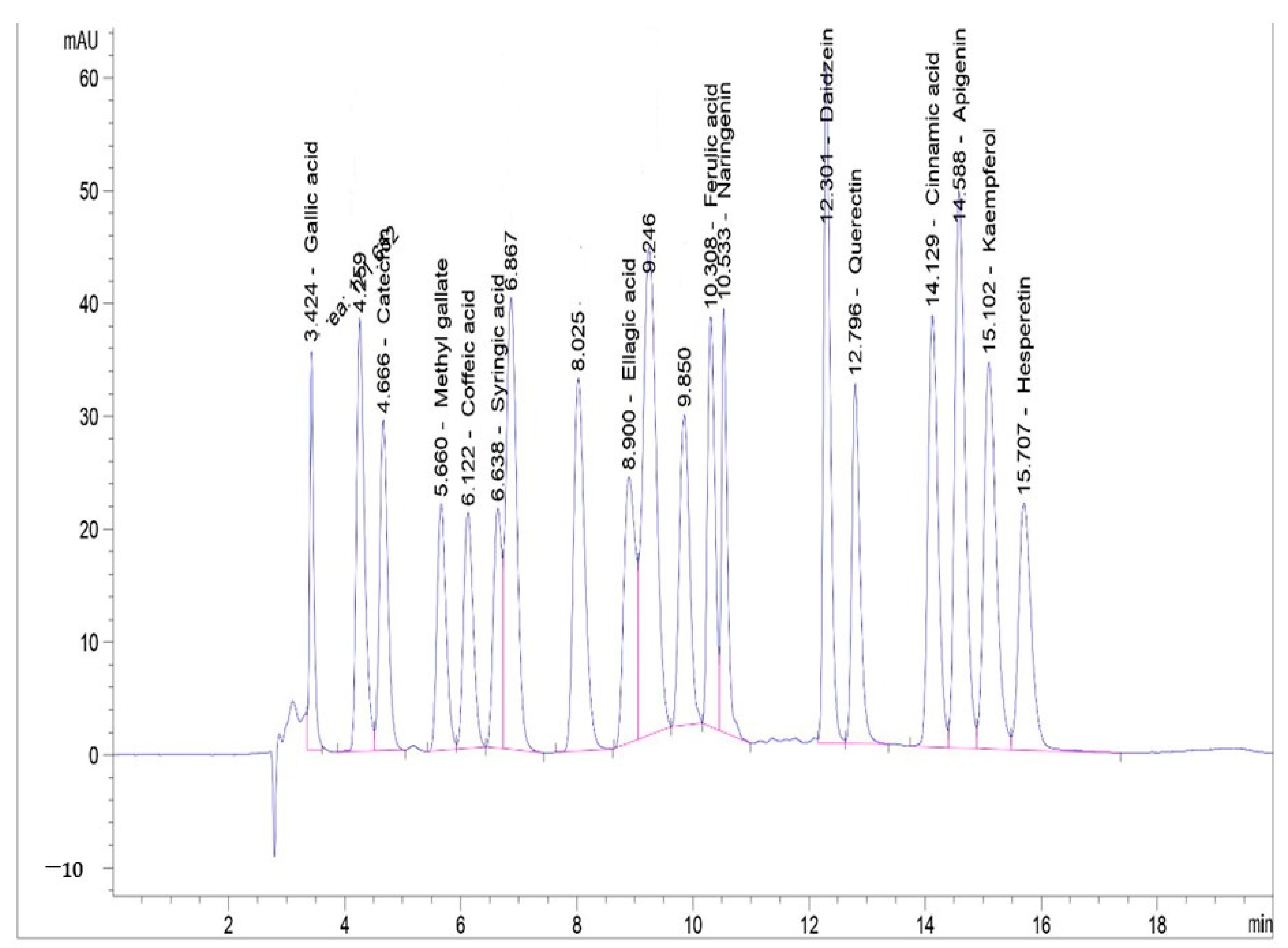
3.6. Phenolic Profile of Lyophilized HO Algal Extract by HPLC
3.7. Safety and Cytotoxicity Assay of Lyophilized HO Algal Extract
3.8. Chicken Fillets Challenge Study
3.9. Acceptability of Chicken Fillet Fortified with Lyophilized HO Algal Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Yoon, Y. Etiological agents Implicated in foodborne illness worldwide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Farm to Fork Strategy for a Fair, Healthy and Environmentally Friendly Food System. 2020. Available online: https//ec.europa.eu/food/horizontal-topics/farm-fork-strategyEn (accessed on 14 June 2022).
- WHO. Draft WHO Global Strategy for Food Safety 2022–2030, towards Stronger Food Safety Systems and Global Cooperation, Department of Nutrition and Food Safety Prepared by WHO Secretariat. 2021. Available online: https://www.who.int/publications/m/item/draft-who-global-strategy-for-food-safety-2022-2030 (accessed on 5 August 2022).
- PIANZ. Industry Information. 2021. Available online: http://www.pianz.org.nz/industry-information/industry-statistics/meat-consumption/chicken-remains-new-zealands-favourite-meat (accessed on 21 July 2022).
- Qian, C.; Castaneda-Gulla, K.; Sattlegger, E.; Mutukumira, A.N. Enterotoxigenicity and genetic relatedness of Staphylococcus aureus in a commercial poultry plant and poultry farm. Int. J. Food Microbiol. 2022, 363, 109454. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.R.; Board, R.G. The Microbiology of Meat and Poultry, 1st ed.; Thomson Science: Exmouth, UK, 1998. [Google Scholar]
- Massawe, H.F.; Mdegela, R.H.; Kurwijila, L.R. Antibiotic resistance of Staphylococcus aureus isolates from milk produced by smallholder dairy farmers in Mbeya Region, Tanzania. Int. J. One Health 2019, 5, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Dehkordi, F.S.; Gandomi, H.; Basti, A.A.; Misaghi, A.; Rahimi, E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob. Resist. Infect. Control 2017, 6, 104. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofiber/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Rajauria, G.; O’doherty, J.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Bhuvana, P.; Sangeetha, P.; Anuradha, V.; Syed Ali, M. Spectral characterization of bioactive compounds from microalgae. N. oculata C. vulgaris. Biocatal. Agric. Biotechnol. 2019, 19, 101094. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Pestana, J.M.; Puerta, B.; Santos, H. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020, 99, 2519–2532. [Google Scholar] [CrossRef]
- Banach, J.; Burg, S.; Fels-Klerx, H. Food safety during seaweed cultivation at offshore wind farms: An exploratory study in the North Sea. Mar. Policy. 2020, 120, 104082. [Google Scholar] [CrossRef]
- El-Khawas, K.M.; Hendy, B.A.S. Assessment and improvement of hygienic status of chicken fillet from slaughterhouses using organic acids from natural sources. Assiut Vet. Med. J. 2015, 61, 8–17. [Google Scholar]
- Eldin, R.M.B.; Talaat, D.; Elbaba, A.H.; Ibrahim, M.S. Antibacterial activity y of some plant extracts on different bacteria in chicken fillet. Eur. J. Pharm. Med. Res. 2020, 7, 84–95. [Google Scholar]
- Salem, W.M.; Galal, H.; Nasr El-deen, F. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, M.; Alalawy, A.I.; Almutairi, F.M.; Al-Duais, M.A.; Wang, J.; Salama, E.S. Identification and characterization of marine seaweeds for biocompounds production. Environ. Technol. Innov. 2021, 24, 101848. [Google Scholar] [CrossRef]
- Weese, J.S.; Anderson, M.E.C.; Lowe, A.; Penno, R.; Da Costa, T.M.; Button, L.; Goth, K.C. Screening of the equine intestinal microflora for potential probiotic organisms. Equine Vet. J. 2004, 36, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.G.; Zeitoun, A.M. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef] [PubMed]
- Kadaikunnan, S.; Rejiniemon, T.; Khaled, J.M.; Alharbi, N.S.; Mothana, R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens Ann. Clin. Microbiol. Antimicrob. 2015, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of Aflatoxin B1 and Ochratoxin A Using Salvia farinacea and Azadirachta indica Water Extract and Application in Meat Products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef]
- Hamad, G.; Ombarak, R.A.; Eskander, M.; Mehany, T.; Anees, F.R.; Elfayoumy, R.A.; Omar, S.A.; Lorenzo, J.M.; Abou-Alella, S.A.-E. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT 2022, 163, 113603. [Google Scholar] [CrossRef]
- El Sohaimy, A.A.S.; El-Sheikh, H.M.; Refaay, M.T.; Zaytoun, A.M.M. Effect of harvesting in different ripening stages on olive (Olea europea) oil quality. Am. J. Food Technol. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Hamad, G.M.; Abu-serie, M.M.; Ali, S.H.; Hafez, E.E. Combination Probiotic Supernatants Reduce Growth and Aflatoxin Production by Aspergillus spp in Food Contamination. Am. J. Food Sci. Technol. 2018, 6, 57–67. [Google Scholar]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs-Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness; The Guilford Press: New York, USA, 2017. [Google Scholar]
- Morsy, M.K.; Elsabagh, R.; Trinetta, V. Evaluation of novel synergistic antimicrobial activity of nisin, lysozyme, EDTA nanoparticles, and/or ZnO nanoparticles to control foodborne pathogens on minced beef. Food Control 2018, 92, 249–254. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration Center for Food Safety & Applied Nutrition). Staphylococcus aureus toxin formation in hydrated batter mixes. In Fish and Fisheries Products Hazards and Controls Guidance; Food and Drug Administration Center for Food Safety & Applied Nutrition: Washington, DC, USA, 2001; pp. 201–208. [Google Scholar]
- Hamad, G.M.; Omar, S.A.; Mostafa, A.G.M.; Cacciotti, I.; Saleh, S.M.; Allam, M.G.; El-Nogoumy, B.; Abou-Alella, S.A.-E.; Mehany, T. Binding and removal of polycyclic aromatic hydrocarbons in cold smoked sausage and beef using probiotic strains. Food Res. Int. 2022, 161, 111793. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.Y.; Tanga, C.M.; Loon, J.J.A.; Dicke, M. Insects for sustainable animal feed: Inclusive business models involving smallholder. Curr. Opin. Environ. Sustain. 2019, 41, 23–30. [Google Scholar] [CrossRef]
- Elahi, U.; Ma, Y.B.; Wu, S.G.; Wang, J.; Zhang, H.J.; Qi, G.H. Growth performance, carcass characteristics, meat quality and serum profile of broiler chicks fed on housefly maggot meal as a replacement of soybean meal. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1075–1084. [Google Scholar] [CrossRef]
- Incili, G.K.; Çalıcıoglu, M. Change in scalding fluids by time in poultry slaughterhouse and its effect on microbiological quality of carcasses. J. Food Saf. 2015, 38, 12485. [Google Scholar] [CrossRef]
- Momtaz, H.; Dehkordi, F.S.; Rahimi, E.; Asgarifar, A.; Momeni, M. Virulence genes and antimicrobial resistance profiles of Staphylococcus aureus isolated from chicken meat in Isfahan province, Iran. J. Appl. Poult. Res. 2012, 22, 913–992. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441868/ (accessed on 11 June 2022).
- Alves, V.F.; Nino-Arias, F.C.; Pitondo-Silva, A.; Frazilio, D.D.; Oliveira Gonçalves, L.D.; Toubas, L.C.; Sapateiro Torres, I.M.; Oxaran, V.; Dittmann, K.K.; De Martinis, E.C.P. Molecular characterization of Staphylococcus aureus from some artisanal Brazilian dairies. Int. Dairy J. 2018, 85, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life. 2019, 21, 100346. [Google Scholar] [CrossRef]
- Ely, R.; Supriya, T.; Naik, C.G. Antimicrobial activity of marine organisms collected off the coast of South East India. J. Exp. Biol. Ecol. 2004, 309, 121–127. [Google Scholar] [CrossRef]
- Manivannan, K.; Karthikaidevi, G.; Anantharaman, P.; Balasubramanian, T. Antimicrobial potential of selected brown seaweeds from Vedalai coastal waters. Gulf Mannar. Asian Pac. Trop. Biomed. 2011, 1, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarudin, M.F.; Yasin, I.S.M.; Mazli, N.A.I.N.; Saadi, A.R.; Azizee, M.H.S.; Nooraini, M.A.; Saad, N.; Ferdous, U.T.; Fakhrulddin, I.M. Preliminary screening of antioxidant and cytotoxic potential of green seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Environmental impact on seaweed phenolic production and activity: An important step for compound exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Moubayed, N.M.S.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Sattarinezhad, E.; Fani, N.; Bordbar, A.; Hatami, P.; Abbasi Kajani, A.; Taki, M. Probing the physico-chemical, antioxidant and anticancer influence of β-lactoglobulin on dietary flavonoid daidzein. Inform. Med. Unlocked 2021, 25, 100643. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Shaghayegh Alavi, S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Hosein Farzaei, M.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 25–237. [Google Scholar]
- AL Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2019, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Kurhekar, J.V. Antimicrobial lead compounds from marine plants. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–274. [Google Scholar]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life. 2020, 23, 100439. [Google Scholar] [CrossRef]
- Kurt, O.; Özdal-Kurt, F.; Tuğlu, M.I.; Akçora, C.M. The cytotoxic, neurotoxic, apoptotic and antiproliferative activities of extracts of some marine algae on the MCF-7 cell line. Biotech. Histochem. 2014, 89, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Nazarudin, M.F.; Isha, A.; Mastuki, S.N.; Ain, N.M.; Mohd Ikhsan, N.F.; Abidin, A.Z.; Aliyu-Paiko, M.; Vilas-Boas, M. Chemical composition and evaluation of the a-glucosidase inhibitory and cytotoxic properties of marine algae Ulva intestinalis, Halimeda macroloba, and Sargassum ilicifolium. Evid. Based Complement. Altern. Med. 2020, 2020, 2753945. [Google Scholar] [CrossRef]
- Daghir, N.; Diab-El-Harake, M.; Kharroubi, S. Poultry production and its effects on food security in the mena region. J. Appl. Poult. Res. 2020, 30, 100110. [Google Scholar] [CrossRef]
- Klepacka, J.; Gujska, E.; Michalak, J. Phenolic compounds as cultivar- and variety-distinguishing factors in some plant products. Plant Foods Hum. Nutr. 2011, 66, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.; Mongal, D.; Mukherjee, G.; Sil, A.K. Edible Marine Algae: A Wellspring of Bioactive Agents Towards Sustainable Management of Human Welfare. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Chakraborthy, K.; Lipton, A.P.; Paulraj, R.; Vijayan, K.K. Antibacterial diterpernoids of Ulva fasciata Delile from South-western coast of Indian Peninsula. Food Chem. 2010, 119, 1399–1408. [Google Scholar] [CrossRef]
- Anand, K.V.; Mahalakshmi, D.; Muthamil Selvan, S.; Ravi, M.; Kannan, M.; Govindaraju, K.; Shalan, A.E. Biomass extract of green macroalga Halimeda opuntia assisted ZnO nanoparticles: Preparation, physico-chemical characterization, and antibacterial activity against Vibrio harveyi. Biomass Convers. Biorefin. 2022, 1–9. [Google Scholar] [CrossRef]
- Salvador, N.; Go´mez Garreta, A.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Grahl, S.; Palanisamy, M.; Strack, M.; Meier-Dinkel, L.; Toepfl, S.; Morlein, D. Towards more sustainable meat alternatives: How technical parameters affect the sensory properties of extrusion products derived from soy and algae. J. Clean. Prod. 2018, 198, 962–971. [Google Scholar] [CrossRef]
- Weinrich, R.; Gassler, B. Beyond classical van Westendorp: Assessing price sensitivity for variants of algae-based meat substitutes. J. Retail. Consum. Serv. 2021, 63, 102719. [Google Scholar] [CrossRef]



| Extract/Material | Concentration/Volume | Inhibition Zone (mm) Against S. aureus |
|---|---|---|
| lyophilized HO extract (green algae) | 100 mg/mL | 43.16 ± 0.44 a |
| lyophilized TT extract (brown algae) | 100 mg/mL | NZ |
| lyophilized AF extract (red algae) | 100 mg/mL | NZ |
| Water | 20 µL | NZ |
| Tetracycline | 30 mg/mL | 15.26 ± 0.34 d |
| Chloramphenicol | 30 mg/mL | 28.17 ± 0.42 b |
| Sulphametmoxazole | 100 mg/mL | 23.33 ± 0.60 c |
| Minimum inhibitory concentrations (MICs) | ||
| Strain | lyophilized HO extract against S. aureus (mg/mL) | |
| S. aureus | Conc. (mg/mL) | Inhibition zone (mm) |
| 100 | 42.0 ± 0.28 | |
| 50 | 31.17 ± 0.43 | |
| 25 | 20.33 ± 0.72 | |
| 12.5 | 16.17 ± 0.44 | |
| 6.25 | 13.0 ± 0.26 | |
| 3.12 | 10.23 ± 0.57 | |
| 1.56 | ND | |
| Conc. (µg/mL) | Ascorbic Acid | Lyophilized HO Extract (Green Algae) | ||
|---|---|---|---|---|
| Inhibition (%) | IC50 (µg/mL) | Inhibition (%) | IC50 (µg/mL) | |
| 10 | 5.12 ± 0.005 b | 26.36 | 9.62 ± 0.006 a | 55.36 |
| 20 | 35.19 ± 0.006 a | 18.51 ± 0.008 b | ||
| 30 | 56.89 ± 0.007 a | 27.64 ± 0.007 b | ||
| 40 | 80.03 ± 0.035 a | 35.53 ± 0.003 b | ||
| 50 | 89.61 ± 0.003 a | 43.75 ± 0.006 b | ||
| 60 | 94.72 ± 0.004 a | 54.19 ± 0.005 b | ||
| 70 | 97.20 ± 0.005 a | 67.51 ± 0.004 b | ||
| 80 | 98.68 ± 0.003 a | 80.23 ± 0.007 b | ||
| 90 | 99.34 ± 0.004 a | 89.63 ± 0.006 b | ||
| 100 | 99.67 ± 0.002 a | 95.34 ± 0.011 b | ||
| Storage (Days) | CHF | CHF/HO 4% | CHF/HO 6% | CHF/ST | CHF/ST/HO 4% | CHF/ST/HO 6% |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 0.00 | 0.00 | 7.04 ± 0.022 Aa | 7.04 ± 0.022 Aa | 7.04 ± 0.022 Aa |
| 2nd | 0.00 | 0.00 | 0.00 | 7.18 ± 0.016 Ba | 6.71 ± 0.008 Bab | 5.78 ± 0.004 Bb |
| 4th | 0.00 | 0.00 | 0.00 | 7.32 ± 0.020 Ca | 4.38 ± 0.00 Cb | 2.61 ± 0.008 Cc |
| 6th | 0.00 | 0.00 | 0.00 | 7.40 ± 0.021 Da | 3.49 ± 0.014 Db | 0.00 Dc |
| 8th | 0.00 | 0.00 | 0.00 | 7.50 ± 0.007 Ea | 0.00 Eb | 0.00 Db |
| 10th | 0.00 | 0.00 | 0.00 | 7.72 ± 0.005 Fa | 0.00 Eb | 0.00 Db |
| Samples | Color | Odor | Taste | Texture | Overall Acceptance |
|---|---|---|---|---|---|
| CHF | 8.00 ± 0.13 B | 7.85 ± 0.29 C | 8.20 ± 0.25 B | 8.00 ± 0.27 B | 8.30 ± 0.15 B |
| CHF/HO 4% | 8.50 ± 0.15 A | 8.05 ± 0.16 B | 8.65 ± 0.15 A | 8.45 ± 0.17 A | 8.44 ± 0.14 A |
| CHF/HO 6% | 8.35 ± 0.19 A | 8.50 ± 0.13 A | 8.70 ± 0.11 A | 8.50 ± 0.14 A | 8.65 ± 0.11 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, G.; Amer, A.; Kirrella, G.; Mehany, T.; Elfayoumy, R.A.; Elsabagh, R.; Elghazaly, E.M.; Esatbeyoglu, T.; Taha, A.; Zeitoun, A. Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts. Foods 2023, 12, 20. https://doi.org/10.3390/foods12010020
Hamad G, Amer A, Kirrella G, Mehany T, Elfayoumy RA, Elsabagh R, Elghazaly EM, Esatbeyoglu T, Taha A, Zeitoun A. Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts. Foods. 2023; 12(1):20. https://doi.org/10.3390/foods12010020
Chicago/Turabian StyleHamad, Gamal, Amr Amer, Ghada Kirrella, Taha Mehany, Reham A. Elfayoumy, Rasha Elsabagh, Eman M. Elghazaly, Tuba Esatbeyoglu, Ahmed Taha, and Ahmed Zeitoun. 2023. "Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts" Foods 12, no. 1: 20. https://doi.org/10.3390/foods12010020
APA StyleHamad, G., Amer, A., Kirrella, G., Mehany, T., Elfayoumy, R. A., Elsabagh, R., Elghazaly, E. M., Esatbeyoglu, T., Taha, A., & Zeitoun, A. (2023). Evaluation of the Prevalence of Staphylococcus aureus in Chicken Fillets and Its Bio-Control Using Different Seaweed Extracts. Foods, 12(1), 20. https://doi.org/10.3390/foods12010020