Effects of Tea Polyphenol Palmitate Existing in the Oil Phase on the Stability of Myofibrillar Protein O/W Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MP
2.3. Preparation of MP-Stabilized Emulsions
2.4. Droplet Surface Average Size (d3,2)
2.5. ζ-Potential Measurements
2.6. Measurement of EAI and ESI
2.7. Visual Appearance
2.8. Stability Analysis with a Multiple Light Scatterer
2.9. Viscosity
2.10. Data Analysis
3. Results
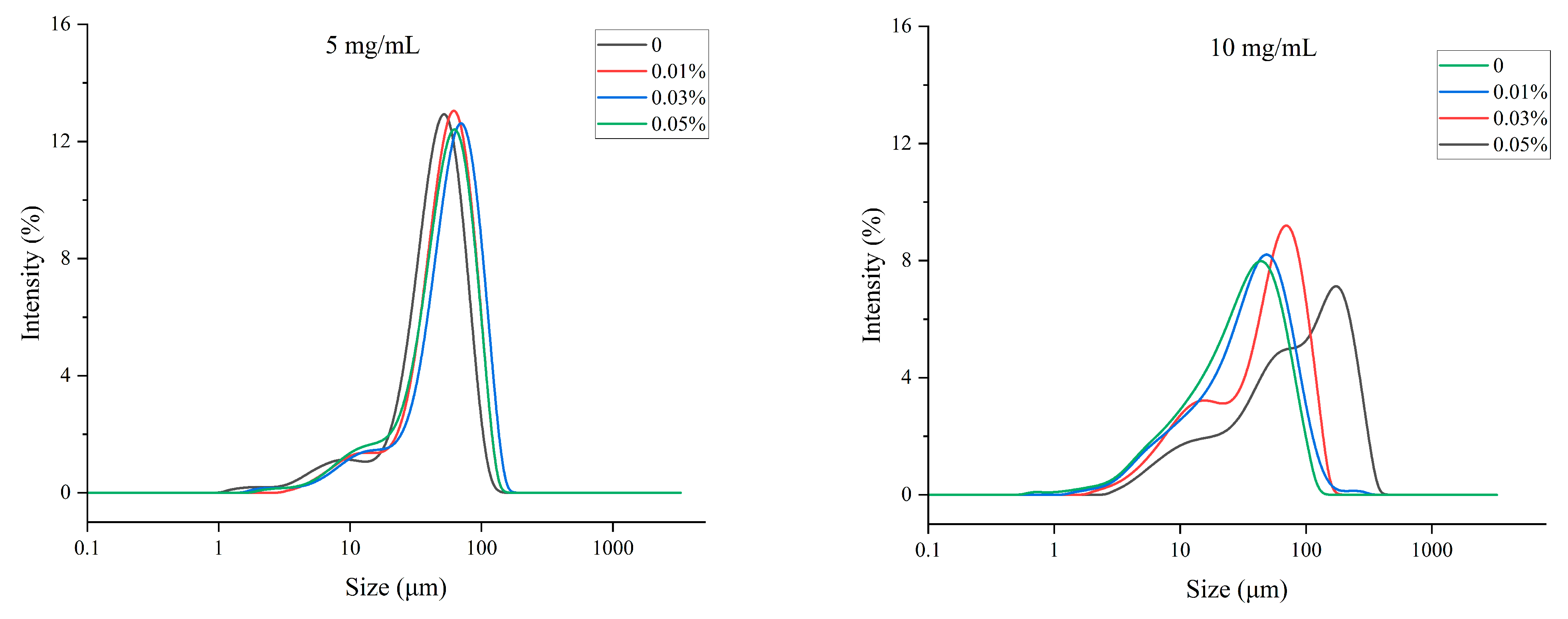
3.1. Effect of TPP on the Droplet Size Distribution of MP O/W Emulsion
3.2. Effect of TPP on the ζ-Potential of MP
3.3. Effect of TPP on the EAI and ESI of MP
3.4. Effect of TPP on the Appearance of MP O/W Emulsion
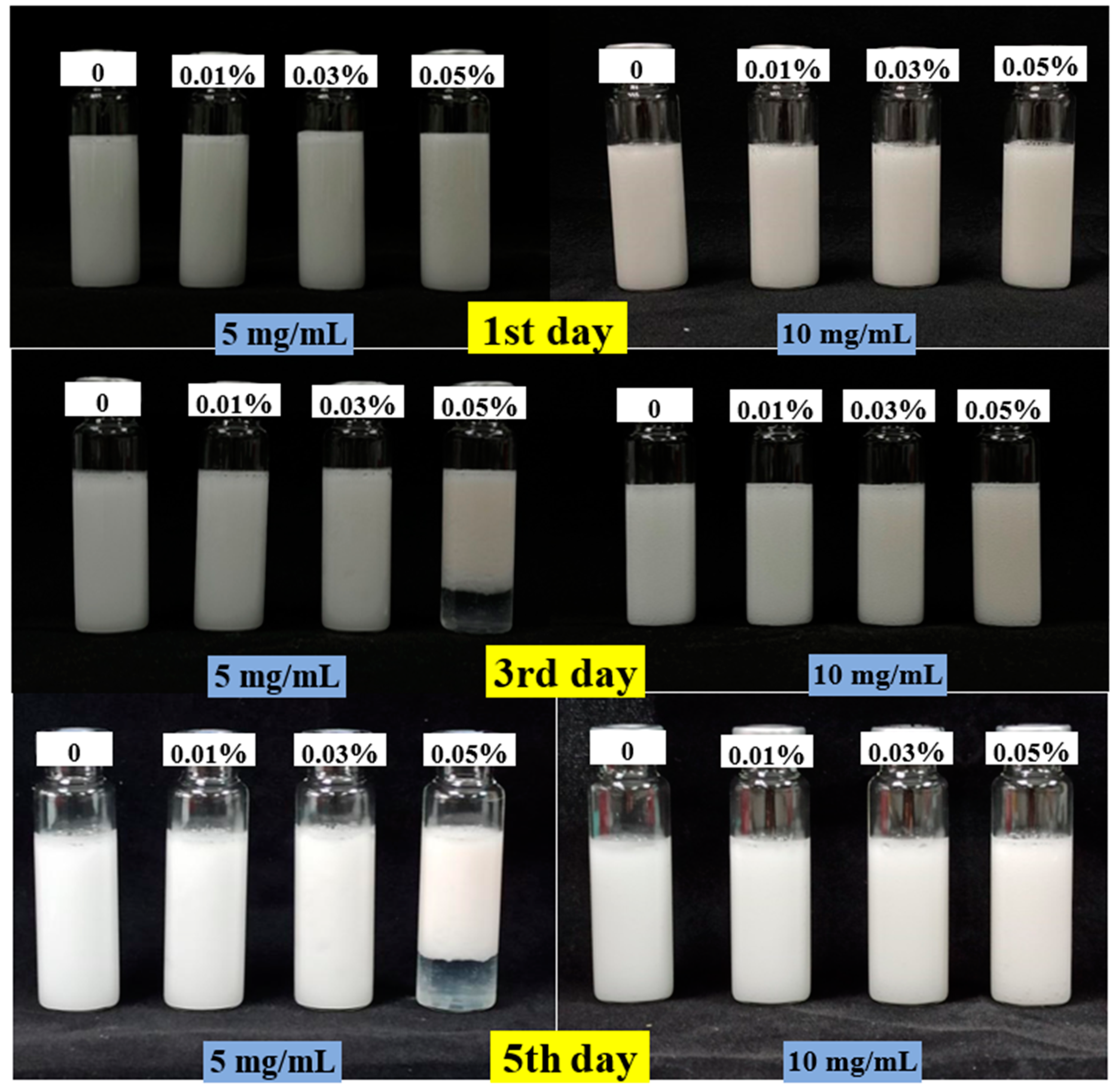
3.4.1. Visual Appearance
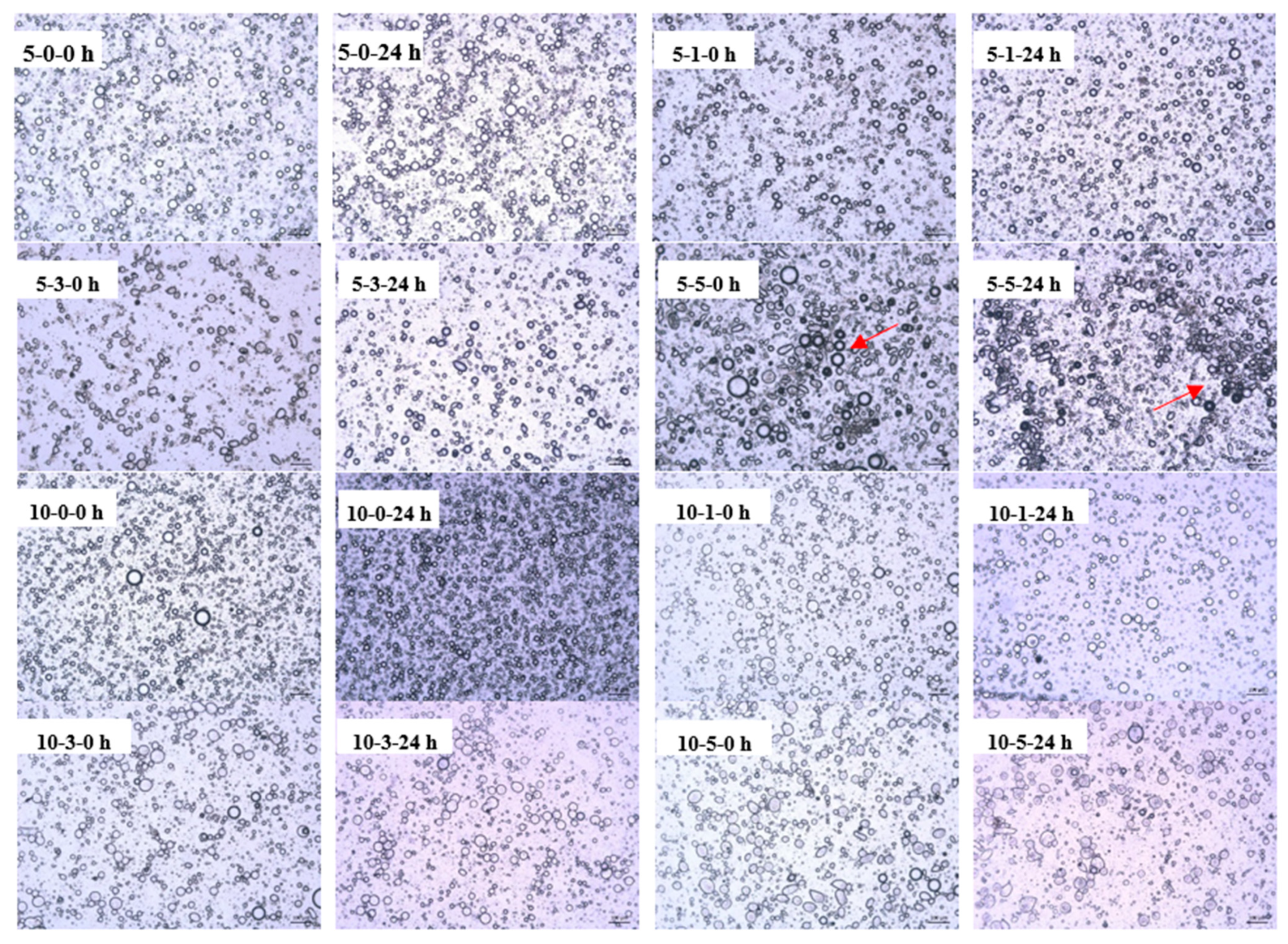
3.4.2. Optical Micrographs
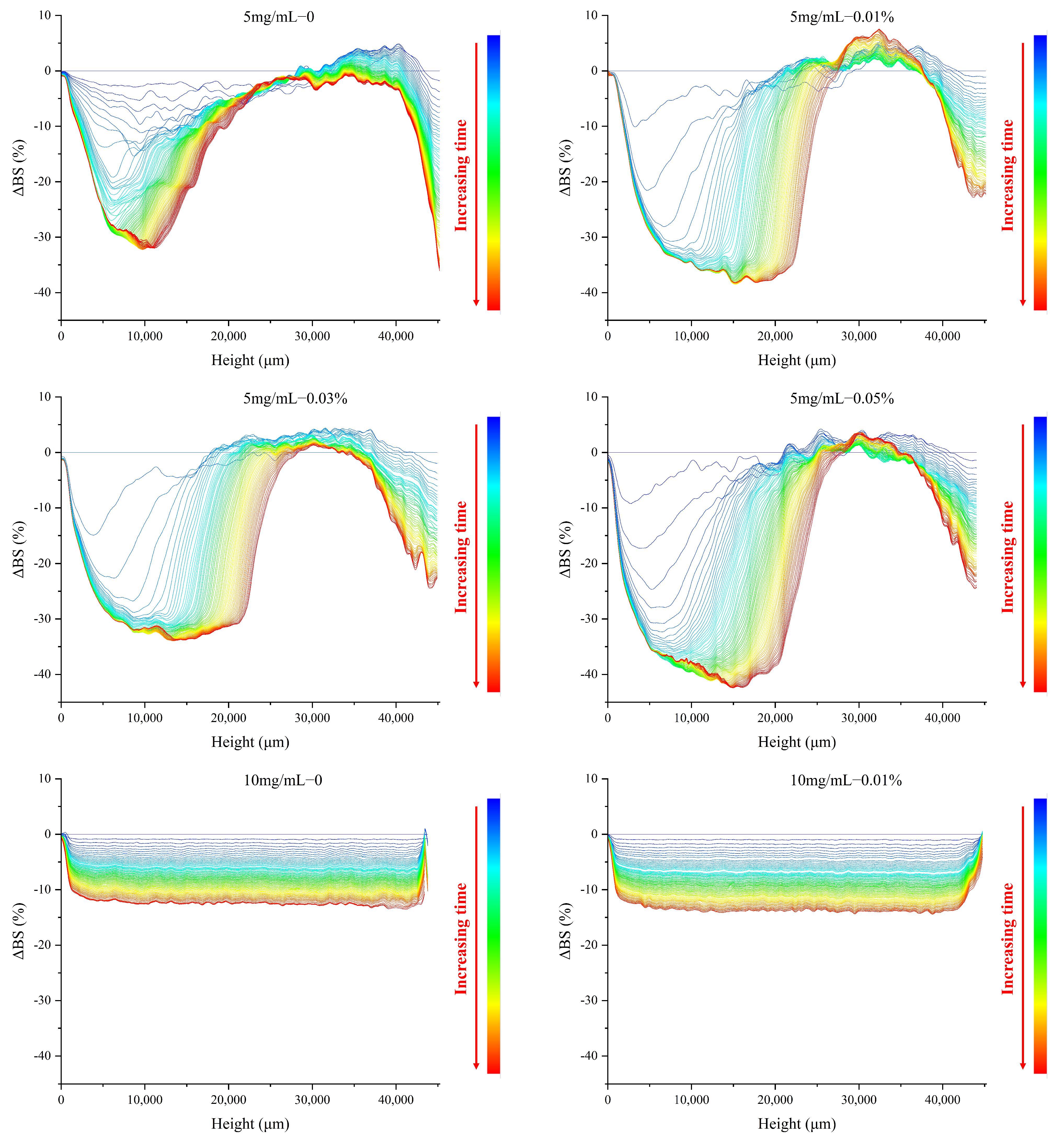
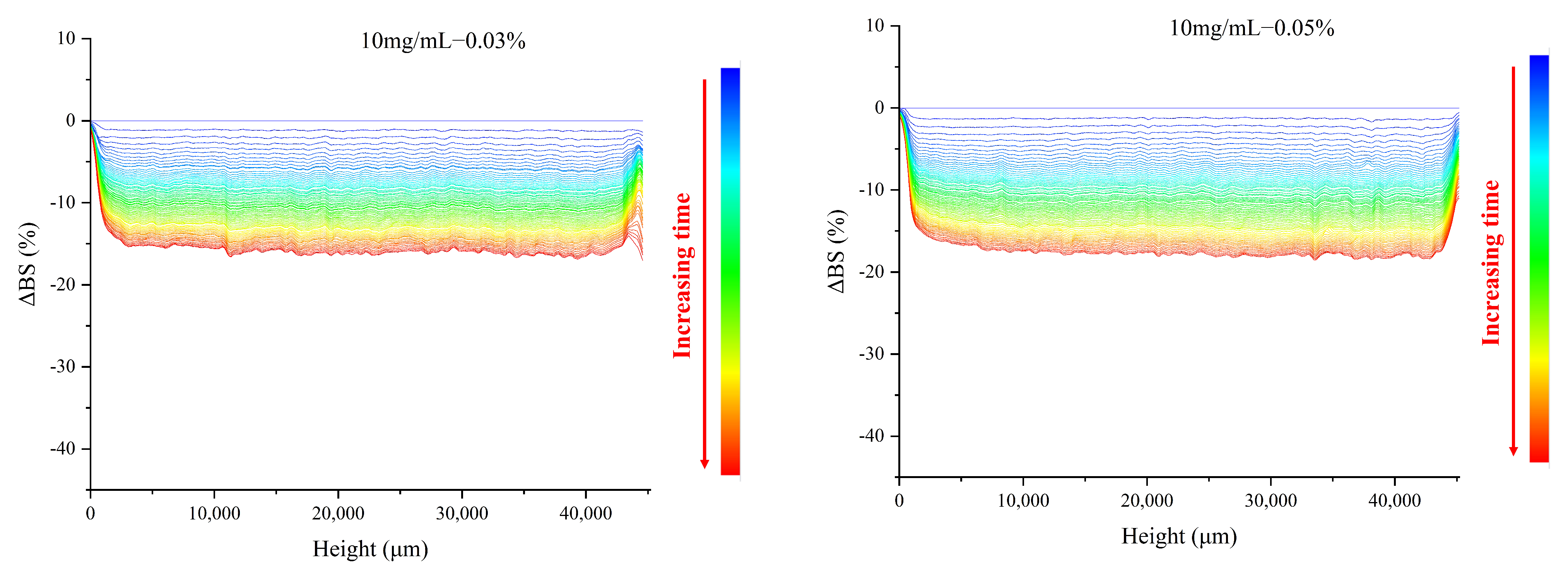
3.5. Effect of TPP on the Backscattered Light Properties of MP O/W Emulsion
3.6. Effect of TPP on the Viscosity of MP Solution and O/W Emulsion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Lu, T.; Liu, X.-Y.; Zhao, M.-T.; Yin, F.-W.; Rakariyatham, K.; Zhou, D.-Y. Improving the oxidative stability and lengthening the shelf life of DHA algae oil with composite antioxidants. Food Chem. 2020, 313, 126139. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zheng, Z.; Liu, C.; Wang, Y.; Li, J.; Liu, Y. Identification and quantification of synergetic antioxidants and their application in sunflower oil. LWT 2020, 118, 108726. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Yang, Z.; Du, F.; Li, Z.; Wu, C.; Fang, A.; Xu, X.; Zhou, G. Molecular dynamics simulation exploration of the interaction between curcumin and myosin combined with the results of spectroscopy techniques. Food Hydrocoll. 2020, 101, 105455. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, C.; Lu, T.; Ding, X.Y.; Zhao, M.T.; Zhang, M.; Liu, H.L.; Song, L.; Zhou, D.Y. Effects of gallic acid alkyl esters and their combinations with other antioxidants on oxidative stability of DHA algae oil. Food Res. Int. 2021, 143, 110280. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wang, J.-H.; Xie, H.-K.; Wang, Y.-F.; Zhao, Q.; Zhou, D.-Y.; Shahidi, F. Improving oxidative stability of flaxseed oil with a mixture of antioxidants. J. Food Process. Preserv. 2020, 44, e14355. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wu, Z.X.; Xie, H.K.; Li, A.; Wang, Y.F.; Song, L.; Zhou, D.Y.; Wang, T. Improving the oxidative stability of flaxseed oil with composite antioxidants comprising gallic acid alkyl ester with appropriate chain length. LWT 2021, 138, 110763. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Kurozawa, L.E.; Hubinger, M.D. High internal phase emulsions (HIPE) using pea protein and different polysaccharides as stabilizers. Food Hydrocoll. 2020, 105, 105775. [Google Scholar] [CrossRef]
- Li, Y.; Gong, S.; Guan, X.; Jiang, H.; Tao, S.; Yang, C.; Ngai, T. One-Step Preparation of All-Natural Pickering Double Emulsions Stabilized by Oppositely Charged Biopolymer Particles. Adv. Mater. Interfaces 2021, 8, 2101568. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Stabilization of vitamin E-enriched mini-emulsions: Influence of organic and aqueous phase compositions. Coll. Surf. A Physicochem. Eng. Asp. 2014, 449, 65–73. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- Fernández Sosa, E.I.; Chaves, M.G.; Henao Ossa, J.S.; Quiroga, A.V.; Avanza, M.V. Protein isolates from Cajanus cajan L. as surfactant for o:w emulsions: pH and ionic strength influence on protein structure and emulsion stability. Food Biosci. 2021, 42, 101159. [Google Scholar] [CrossRef]
- Guo, X.; Gao, F.; Zhang, Y.; Peng, Z.; Jamali, M.A. Effect of l-histidine and l-lysine on the properties of oil-in-water emulsions stabilized by porcine myofibrillar proteins at low/high ionic strength. LWT 2021, 141, 110883. [Google Scholar] [CrossRef]
- Parajuli, S.; Dorris, A.L.; Middleton, C.; Rodriguez, A.; Haver, M.O.; Hammer, N.I.; Ureña-Benavides, E. Surface and Interfacial Interactions in Dodecane/Brine Pickering Emulsions Stabilized by the Combination of Cellulose Nanocrystals and Emulsifiers. Langmuir 2019, 35, 12061–12070. [Google Scholar] [CrossRef] [PubMed]
- Tamm, F.; Drusch, S. Impact of enzymatic hydrolysis on the interfacial rheology of whey protein/pectin interfacial layers at the oil/water-interface. Food Hydrocoll. 2017, 63, 8–18. [Google Scholar] [CrossRef]
- Rühs, P.A.; Scheuble, N.; Windhab, E.J.; Mezzenga, R.; Fischer, P. Simultaneous Control of pH and Ionic Strength during Interfacial Rheology of β-Lactoglobulin Fibrils Adsorbed at Liquid/Liquid Interfaces. Langmuir 2012, 28, 12536–12543. [Google Scholar] [CrossRef] [PubMed]
- Bergfreund, J.; Diener, M.; Geue, T.; Nussbaum, N.; Kummer, N.; Bertsch, P.; Nystrom, G.; Fischer, P. Globular protein assembly and network formation at fluid interfaces: Effect of oil. Soft Matter 2021, 17, 1692–1700. [Google Scholar] [CrossRef]
- Cai, R.; Yang, Z.; Li, Z.; Wang, P.; Han, M.; Xu, X. Nano Filling Effect of Nonmeat Protein Emulsion on the Rheological Property of Myofibrillar Protein Gel. Foods 2022, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Wu, C.; Cai, R.; Xu, X.; Zhou, G.; Wu, T.; Zhang, Y. Inhibition of Heat-Induced Flocculation of Myosin-Based Emulsions through Steric Repulsion by Conformational Adaptation-Enhanced Interfacial Protein with an Alkaline pH-Shifting-Driven Method. Langmuir 2018, 34, 8848–8856. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhao, X.; Li, R.; Bassey, A.; Bai, Y.; Ye, K.; Deng, S.; Zhou, G. Synergistic effects of polysaccharide addition-ultrasound treatment on the emulsified properties of low-salt myofibrillar protein. Food Hydrocoll. 2022, 123, 107143. [Google Scholar] [CrossRef]
- Shen, X.; Fang, T.; Gao, F.; Guo, M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017, 63, 668–676. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, J.; Li, Z.; Qi, Y.; Wang, P.; Xu, X. Robustness of protein: Using pH shifting and low speed shearing to partially recover conformation and dispersibility of myosin from pale, soft, exudative (PSE)-like chicken breast. LWT 2021, 138, 110786. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Y.; Du, X.; Xing, X.; Zhu, G. Stability of pH-responsive Pickering emulsion stabilized by carboxymethyl starch/xanthan gum combinations. Food Hydrocoll. 2020, 109, 106093. [Google Scholar] [CrossRef]
- Genccelep, H.; Saricaoglu, F.T.; Anil, M.; Agar, B.; Turhan, S. The effect of starch modification and concentration on steady-state and dynamic rheology of meat emulsions. Food Hydrocoll. 2015, 48, 135–148. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Z.; Zhang, Y.; Lu, T.; Zhang, X.; Qi, Y.; Wang, P.; Xu, X. Innovative Characterization Based on Stress Relaxation and Creep to Reveal the Tenderizing Effect of Ultrasound on Wooden Breast. Foods 2021, 10, 195. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, L.; Fu, Y.; Dai, H.; Zhang, Y. Fabrication and characterization of myofibrillar microgel particles as novel Pickering stabilizers: Effect of particle size and wettability on emulsifying capacity. LWT 2021, 151, 112002. [Google Scholar] [CrossRef]
- Binks, B.P.; Whitby, C.P. Silica Particle-Stabilized Emulsions of Silicone Oil and Water: Aspects of Emulsification. Langmuir 2004, 20, 1130–1137. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.-W.; Tey, B.-T.; Chan, E.-S. Effects of environmental factors on the physical stability of pickering-emulsions stabilized by chitosan particles. Food Hydrocoll. 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Li, L.; Cai, R.; Wang, P.; Xu, X.; Zhou, G.; Sun, J. Manipulating interfacial behavior and emulsifying properties of myosin through alkali-heat treatment. Food Hydrocoll. 2018, 85, 69–74. [Google Scholar] [CrossRef]
- Du, F.; Qi, Y.; Huang, H.; Wang, P.; Xu, X.; Yang, Z. Stabilization of O/W emulsions via interfacial protein concentrating induced by thermodynamic incompatibility between sarcoplasmic proteins and xanthan gum. Food Hydrocoll. 2022, 124, 107242. [Google Scholar] [CrossRef]
- Ma, H.; Forssell, P.; Partanen, R.; Buchert, J.; Boer, H. Charge Modifications to Improve the Emulsifying Properties of Whey Protein Isolate. J. Agricult. Food Chem. 2011, 59, 13246–13253. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Bovay, C.; Rouvet, M.; Shojaei-Rami, S.; Kolodziejczyk, E. Whey Protein Soluble Aggregates from Heating with NaCl: Physicochemical, Interfacial, and Foaming Properties. Langmuir 2007, 23, 4155–4166. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhong, Q. Glycation of Whey Protein to Provide Steric Hindrance against Thermal Aggregation. J. Agricult. Food Chem. 2012, 60, 9754–9762. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yan, H.-L.; Tang, C.-H. Wet media planetary ball milling remarkably improves functional and cholesterol-binding properties of okara. Food Hydrocoll. 2021, 111, 106386. [Google Scholar] [CrossRef]
- Yust, M.d.M.; Pedroche, J.; Millán-Linares, M.d.C.; Alcaide-Hidalgo, J.M.; Millán, F. Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chem. 2010, 122, 1212–1217. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of canola and flaxseed protein isolates produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2991–2998. [Google Scholar] [CrossRef]
- Guo, Q.; Mu, T.H. Emulsifying properties of sweet potato protein: Effect of protein concentration and oil volume fraction. Food Hydrocoll. 2011, 25, 98–106. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, Y.; Gao, J.; Deng, Q.; Wan, C.; Li, B.; Zhou, B. Desalted duck egg white nanogels combined with κ-carrageenan as stabilisers for food-grade Pickering emulsion. Int. J. Food Sci. Technol. 2021, 57, 2819–2829. [Google Scholar] [CrossRef]
- Wu, C.S.; Guo, J.H.; Lin, M.J. Stability Evaluation of pH-Adjusted Goat Milk for Developing Ricotta Cheese with a Mixture of Cow Cheese Whey and Goat Milk. Foods 2020, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.; Degner, B.; McClements, D.J. Reduced calorie emulsion-based foods: Protein microparticles and dietary fiber as fat replacers. Food Res. Int. 2014, 64, 664–676. [Google Scholar] [CrossRef]
- Sarıçoban, C.; Yılmaz, M.T.; Karakaya, M.; Tiske, S.S. The effect of different levels of sunflower head pith addition on the properties of model system emulsions prepared from fresh and frozen beef. Meat Sci. 2010, 84, 186–195. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, T.K.; Jung, S.; Kim, Y.B.; Choi, Y.-S. Quality of reduced-fat meat emulsion: Effect of pre-emulsified duck skin and hydrocolloids. J. Food Sci. Technol. 2021, 58, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Pengyu, Z.; Yang, Y.; Ling, Z.; Jian, G.; Bing, W.; Xin, B.; Dehui, Y.; Linlin, L.; Congyu, L.; Na, Z. The effect of trehalose on the thermodynamic stability and emulsification of soybean 11S globulin in the molten globule state. Food Hydrocoll. 2021, 118, 106811. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta Protein Struct. 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Pang, B.; Wang, S.; Chen, W.; Hassan, M.; Lu, H. Effects of flow behavior index and consistency coefficient on hydrodynamics of power-law fluids and particles in fluidized beds. Powder Technol. 2020, 366, 249–260. [Google Scholar] [CrossRef]
- Verma, K.; Tarafdar, A.; Mishra, V.; Dilbaghi, N.; Kondepudi, K.K.; Badgujar, P.C. Nanoencapsulated curcumin emulsion utilizing milk cream as a potential vehicle by microfluidization: Bioaccessibility, cytotoxicity and physico-functional properties. Food Res. Int. 2021, 148, 110611. [Google Scholar] [CrossRef]



| d3,2 (μm) | 0 | 0.01% | 0.03% | 0.05% |
|---|---|---|---|---|
| 5 mg/mL | 25.25 ± 0.14 Ac | 34.5 ± 1.90 Aa | 34.82 ± 1.13 Aa | 31.62 ± 0.58 Ab |
| 10 mg/mL | 14.12 ± 0.80 Ba | 16.55 ± 1.56 Bb | 17.03 ± 0.84 Bb | 21.68 ± 0.96 Bc |
| ζ-Potential (mV) | 0 | 0.01% | 0.03% | 0.05% |
|---|---|---|---|---|
| 5 mg/mL | −20.75 ± 0.7 Ab | −19.96 ± 0.16 Ab | −19.98 ± 0.17 Ab | −18.62 ± 0.7 Aa |
| 10 mg/mL | −21.97 ± 0.23 Bd | −20.84 ± 0.13 Bc | −20.00 ± 0.36 Ab | −18.99 ± 0.13 Aa |
| Treatment | 0% | 0.01% | 0.03% | 0.05% | |
|---|---|---|---|---|---|
| 5 mg/mL | EAI (m2/g) | 1.67 ± 0.095 Aa | 0.83 ± 0.087 Ab | 0.75 ± 0.048 Ab | 0.49 ± 0.059 Ac |
| ESI (min) | 62.91 ± 2.52 Aa | 48.69 ± 3.96 Ab | 28.93 ± 7.09 Bc | 15.04 ± 1.71 Bd | |
| 10 mg/mL | EAI (m2/g) | 1.32 ± 0.17 Ba | 0.94 ± 0.09 Ab | 0.42 ± 0.07 Bc | 0.28 ± 0.01 Bc |
| ESI (min) | 28.68 ± 4.74 Bb | 31.74 ± 1.71 Bb | 57.68 ± 11.94 Aa | 53.31 ± 5.81 Aa |
| Treatment (CTPP, w/w) | 0 | 0.01% | 0.03% | 0.05% | |
|---|---|---|---|---|---|
| 5 mg/mL | K | 0.46 ± 0.042 Bb | 0.60 ± 0.088 Ba | 0.64 ± 0.10 Ba | 0.70 ± 0.042 Ba |
| n | 0.30 ± 0.012 Aa | 0.29 ± 0.024 Aab | 0.27 ± 0.041 Aab | 0.24 ± 0.01 Ab | |
| 10 mg/mL | K | 3.06 ± 0.098 Aa | 2.99 ± 0.081 Aab | 2.84 ± 0.057 Ab | 2.82 ± 0.11 Ab |
| n | 0.083 ± 0.027 Ba | 0.076 ± 0.0019 Bab | 0.10 ± 0.0073 Ba | 0.099 ± 0.0079 Ba | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, Z.; Li, Z.; Wu, L.; Shen, J.; Wang, J.; Wang, P. Effects of Tea Polyphenol Palmitate Existing in the Oil Phase on the Stability of Myofibrillar Protein O/W Emulsion. Foods 2022, 11, 1326. https://doi.org/10.3390/foods11091326
Li J, Yang Z, Li Z, Wu L, Shen J, Wang J, Wang P. Effects of Tea Polyphenol Palmitate Existing in the Oil Phase on the Stability of Myofibrillar Protein O/W Emulsion. Foods. 2022; 11(9):1326. https://doi.org/10.3390/foods11091326
Chicago/Turabian StyleLi, Jianchao, Zongyun Yang, Zhen Li, Ling Wu, Juan Shen, Jinhua Wang, and Peng Wang. 2022. "Effects of Tea Polyphenol Palmitate Existing in the Oil Phase on the Stability of Myofibrillar Protein O/W Emulsion" Foods 11, no. 9: 1326. https://doi.org/10.3390/foods11091326
APA StyleLi, J., Yang, Z., Li, Z., Wu, L., Shen, J., Wang, J., & Wang, P. (2022). Effects of Tea Polyphenol Palmitate Existing in the Oil Phase on the Stability of Myofibrillar Protein O/W Emulsion. Foods, 11(9), 1326. https://doi.org/10.3390/foods11091326






