The Impacts of Different Pea Protein Isolate Levels on Functional, Instrumental and Textural Quality Parameters of Duck Meat Batters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Chemicals
2.2. Dynamic Rheological Measurements
2.3. Cooking Yield and Water-Holding Capacity
2.4. Color Measurement
2.5. Texture Profile Analysis (TPA)
2.6. LF-NMR Relaxation Measurements
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
3. Results and Discussion
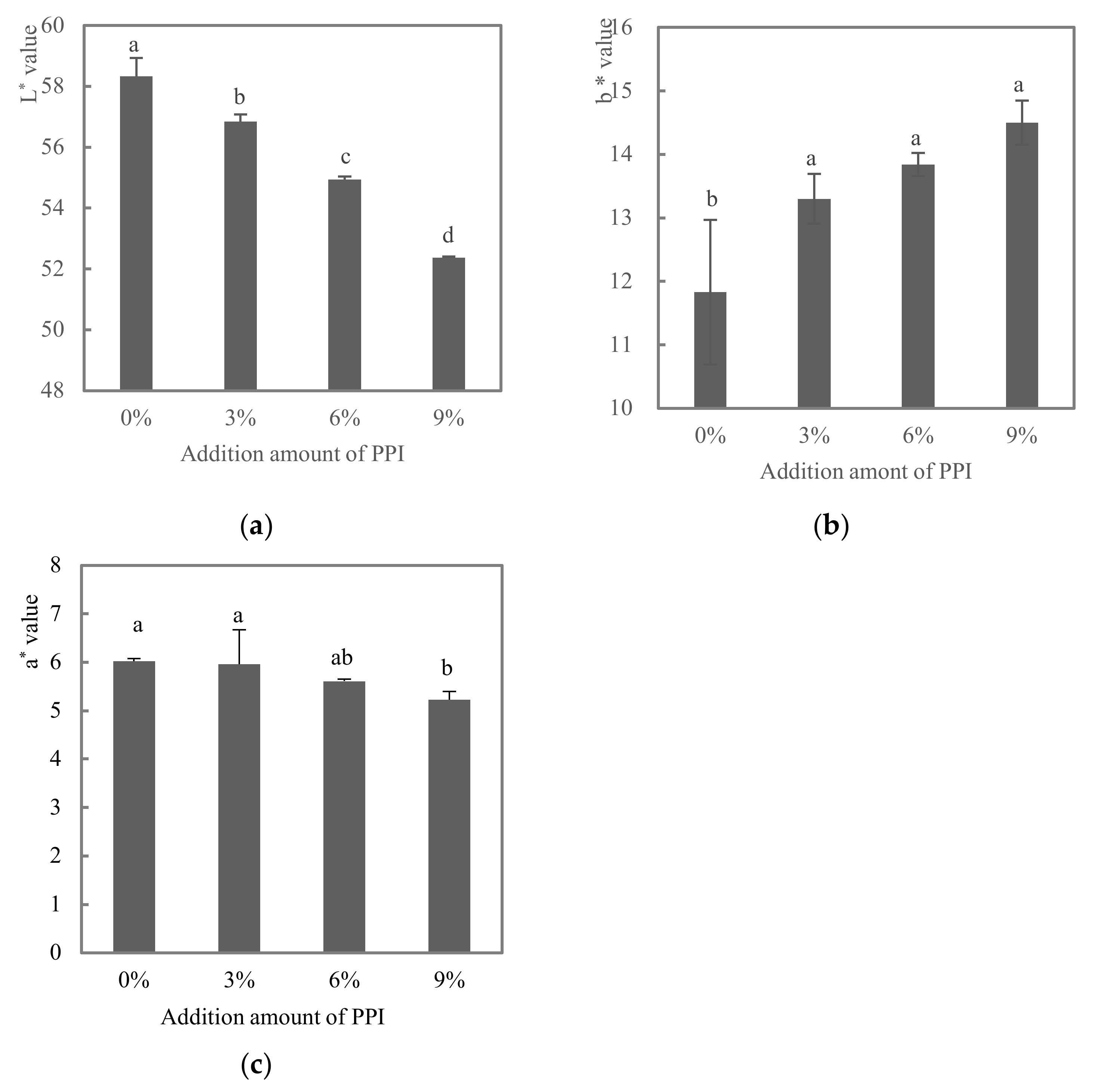
3.1. Color
3.2. Cooking Yield and Water-Holding Capacity (WHC)
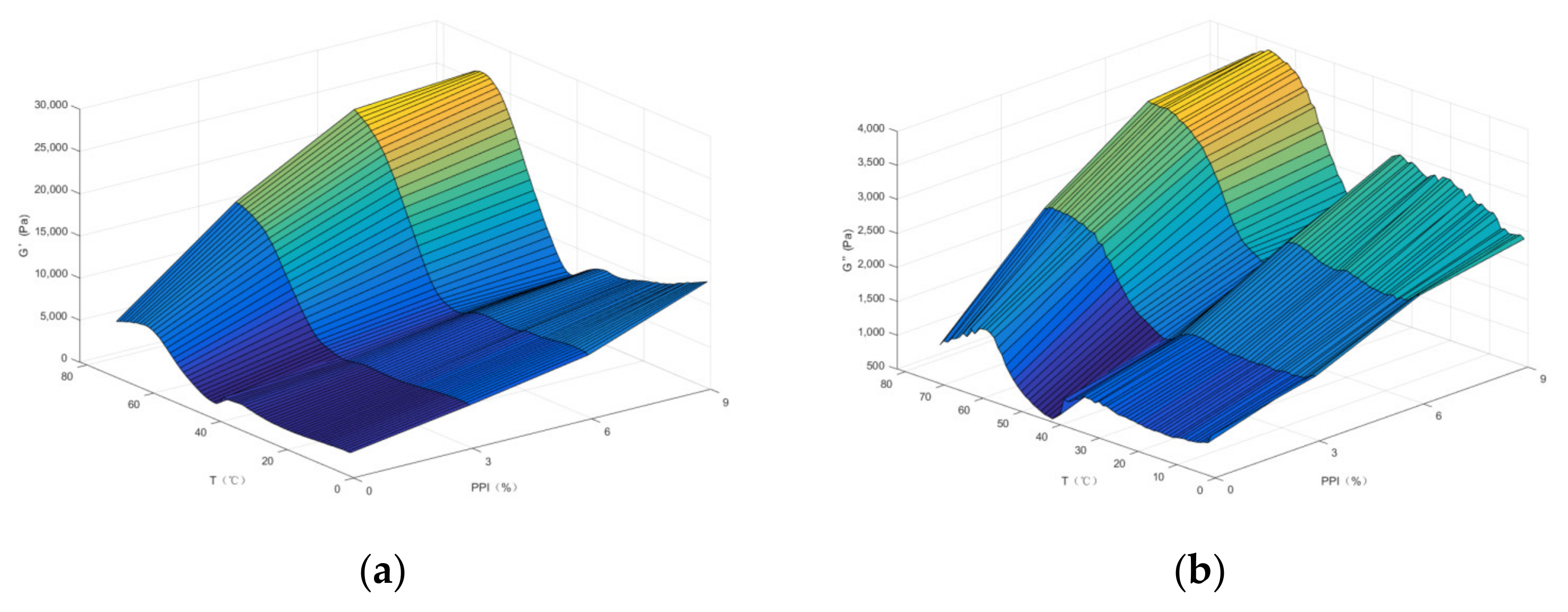
3.3. Dynamic Rheological Properties
3.4. Texture Profile Analysis
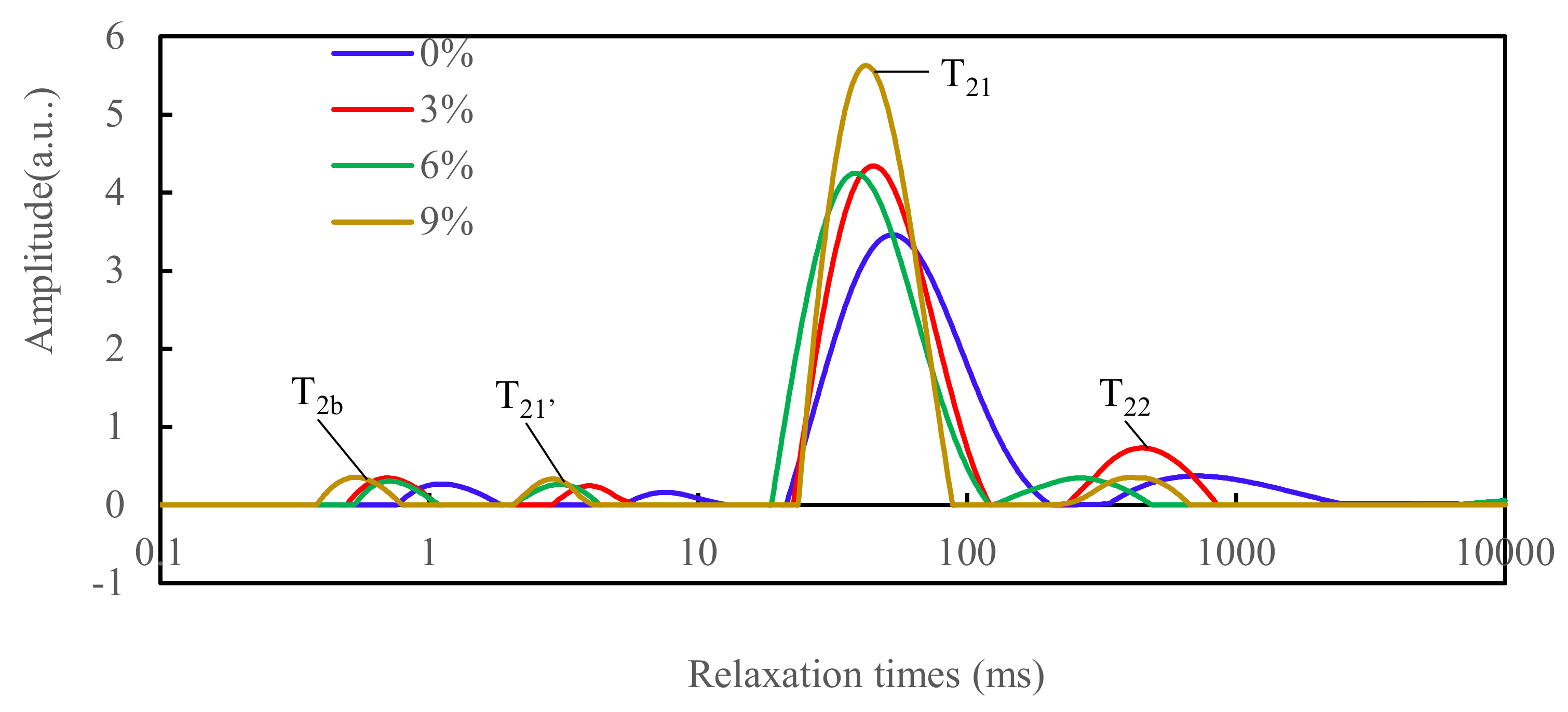
3.5. NMR Proton RELAXATION
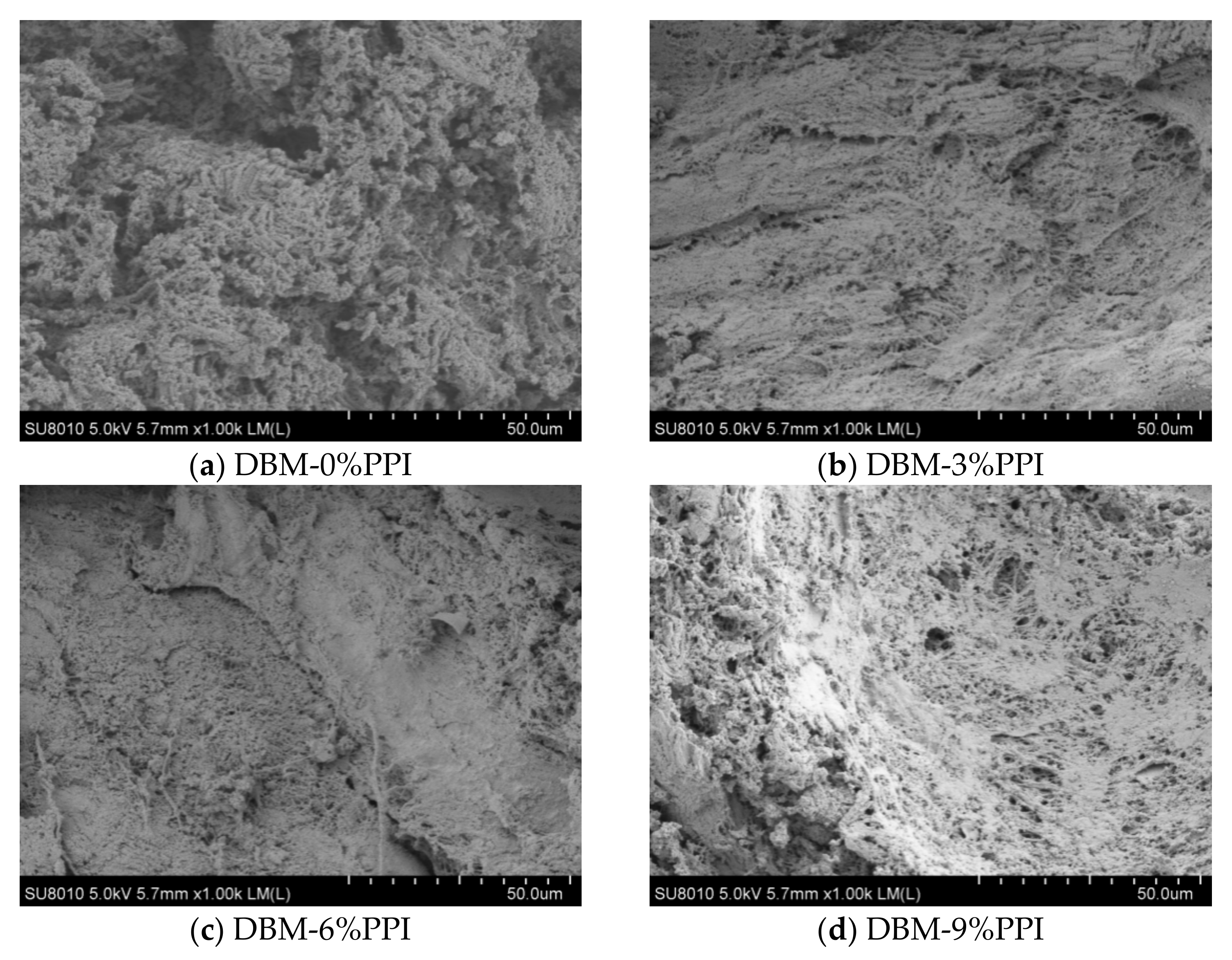
3.6. Microstructure of the Gels
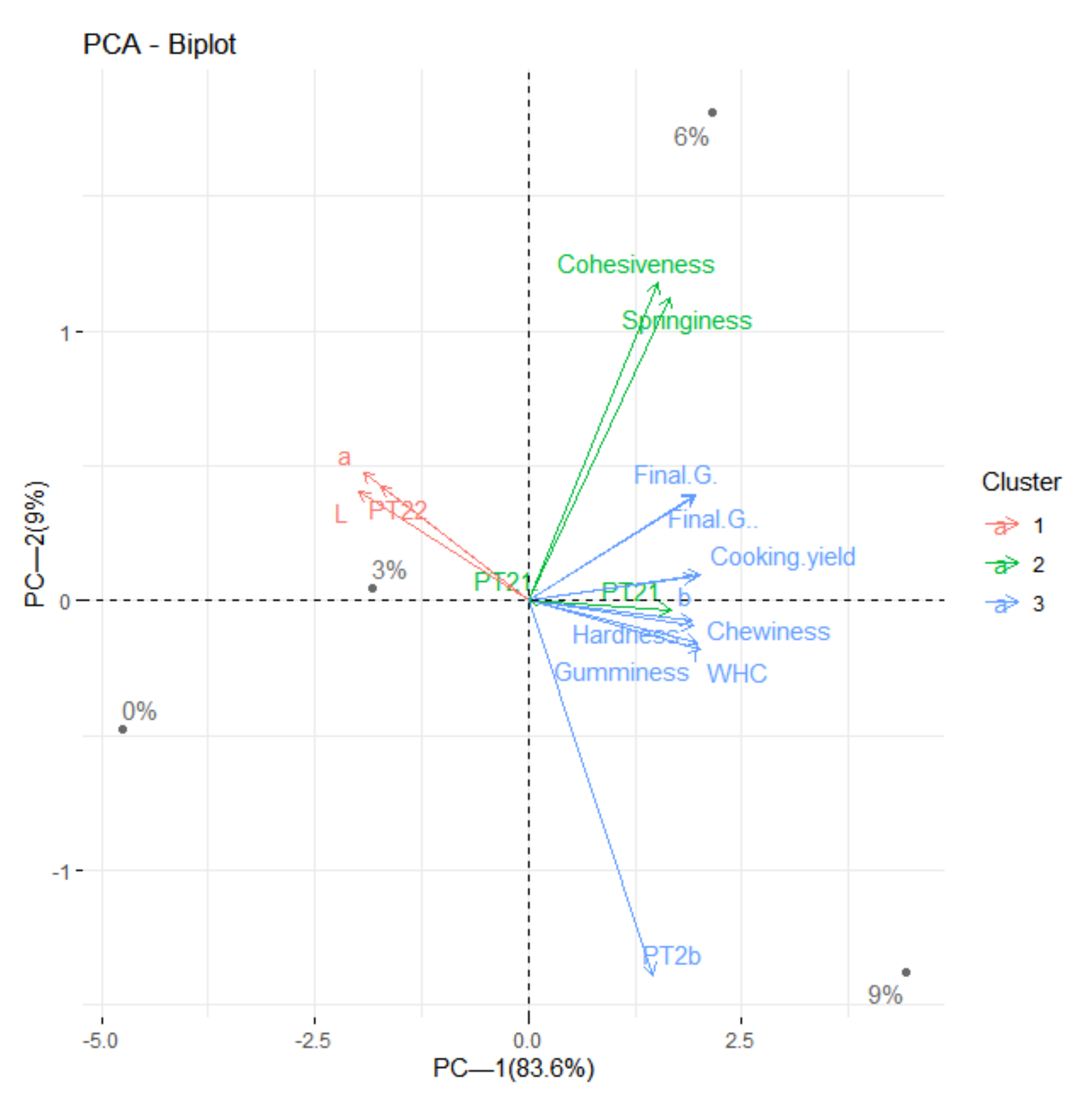
3.7. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Hong, S.; Du, Z.; Chao, M.; O’Quinn, T.; Li, Y. Effect of adding modified pea protein as functional extender on the physical and sensory properties of beef patties. LWT 2022, 154, 112774. [Google Scholar] [CrossRef]
- Ikhlas, B.; Huda, N.; Noryati, I. Chemical Composition and Physicochemical Properties of Meatballs Prepared from Mechanically Deboned Quail Meat Using Various Types of Flour. Int. J. Poult. Sci. 2011, 10, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.C.; Cho, H.; Hong, J.J.; Ryu, R.K.; Hwang, K.T.; Regenstein, J.M. Physicochemical and organoleptic characteristics of seasoned beef patties with added glutinous rice flour. Meat Sci. 2012, 92, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.-S.; Jo, C. Status of meat alternatives and their potential role in the future meat market—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533–1543. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re) formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Baune, M.-C.; Jeske, A.-L.; Profeta, A.; Smetana, S.; Broucke, K.; Van Royen, G.; Gibis, M.; Weiss, J.; Terjung, N. Effect of plant protein extrudates on hybrid meatballs—Changes in nutritional composition and sustainability. Future Foods 2021, 4, 100081. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Janz, J.A.M. Utilization of pea flour, starch-rich and fiber-rich fractions in low fat bologna. Food Res. Int. 2010, 43, 602–608. [Google Scholar] [CrossRef]
- Feiner, G. 27-Burgers, patties and crumbed products. In Meat Products Handbook; Feiner, G., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 481–498. [Google Scholar]
- Baugreet, S.; Kerry, J.P.; Botineştean, C.; Allen, P.; Hamill, R.M. Development of novel fortified beef patties with added functional protein ingredients for the elderly. Meat Sci. 2016, 122, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Liu, S.; Gu, Y.; Yu, X.; Gao, F.; Wang, R. Relationship between Molecular Structure and Heat-Induced Gel Properties of Duck Myofibrillar Proteins Affected by the Addition of Pea Protein Isolate. Foods 2022, 11, 1040. [Google Scholar] [CrossRef]
- Colomer Sellas, M.; de Souza, D.L.; Vila-Marti, A.; Torres-Moreno, M. Effect of pork back-fat reduction and substitution with texturized pea protein on acceptability and sensory characteristics of dry fermented sausages. Cyta-J. Food 2021, 19, 429–439. [Google Scholar] [CrossRef]
- Revilla, I.; Santos, S.; Hernandez-Jimenez, M.; Vivar-Quintana, A.M. The Effects of the Progressive Replacement of Meat with Texturized Pea Protein in Low-Fat Frankfurters Made with Olive Oil. Foods 2022, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Kim, G.D.; Seo, H.W.; Jung, E.Y.; Kim, B.W.; Yang, H.S.; Joo, S.T. Possibility of Making Low-fat Sausages from Duck Meat with Addition of Rice Flour. Asian-Australas. J. Anim. Sci. 2011, 24, 421–428. [Google Scholar] [CrossRef]
- Feng, T.; Ye, R.; Zhuang, H.; Rong, Z.; Fang, Z.; Wang, Y.; Gu, Z.; Jin, Z. Physicochemical properties and sensory evaluation of Mesona Blumes gum/rice starch mixed gels as fat-substitutes in Chinese Cantonese-style sausage. Food Res. Int. 2013, 50, 85–93. [Google Scholar] [CrossRef]
- Younis, K.; Yousuf, O.; Qadri, O.S.; Jahan, K.; Osama, K.; Islam, R.U. Incorporation of soluble dietary fiber in comminuted meat products: Special emphasis on changes in textural properties. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100288. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Gibis, M.; Schuh, V.; Weiss, J. Effects of carboxymethyl cellulose (CMC) and microcrystalline cellulose (MCC) as fat replacers on the microstructure and sensory characteristics of fried beef patties. Food Hydrocoll. 2015, 45, 236–246. [Google Scholar] [CrossRef]
- Gujral, H.S.; Kaur, A.; Singh, N.; Sodhi, N.S. Effect of liquid whole egg, fat and textured soy protein on the textural and cooking properties of raw and baked patties from goat meat. J. Food Eng. 2002, 53, 377–385. [Google Scholar] [CrossRef]
- Berry, B.W.; Joseph, R.L.; Stanfield, M.S. Use of electrical stimulation, hot processing and carrageenan for processing low-fat ground beef patties. Meat Sci. 1996, 42, 111–123. [Google Scholar] [CrossRef]
- Kocher, P.N.; Foegeding, E.A. Microcentrifuge-based method for measuring water-holding of protein gels. J. Food Sci. 1993, 58, 1040–1046. [Google Scholar] [CrossRef]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Akesowan, A. Quality characteristics of light pork burgers fortified with soy protein isolate. Food Sci. Biotechnol. 2010, 19, 1143–1149. [Google Scholar] [CrossRef]
- Tabarestani, H.S.; Tehrani, M.M. Optimization of Physicochemical Properties of Low-Fat Hamburger Formulation Using Blend of Soy Flour, Split-Pea Flour and Wheat Starch as Part of Fat Replacer System. J. Food Process. Preserv. 2014, 38, 278–288. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of two types of soy protein isolates, native and preheated whey protein isolates on emulsified meat batters prepared at different protein levels. Meat Sci. 2011, 87, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Dang, Y.; Cao, J.; Pan, D.; Guo, Y.; He, J. Water-insoluble dietary fibers from oats enhance gel properties of duck myofibrillar proteins. Food Chem. 2021, 344, 128690. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, F.E.; Vereijken, J.M.; Gruppen, H.; Van Boekel, M.A.J.S. Gelation Behavior of Protein Isolates Extracted from 5 Cultivars of Pisum sativum L. J. Food Sci. 2005, 70, 132–137. [Google Scholar] [CrossRef]
- Tömösközi, S.; Lásztity, R.; Haraszi, R.; Baticz, O. Isolation and study of the functional properties of pea proteins. Food Nahr. 2001, 45, 399–401. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Jarmoluk, A. Effect of sodium caseinate and κ-carrageenan on binding and textural properties of pork muscle gels enhanced by microbial transglutaminase addition. Food Res. Int. 2003, 36, 285–294. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of myofibrillar/pea protein mixtures induced by transglutaminase crosslinking. Food Hydrocoll. 2012, 27, 394–400. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.-Y.; Bai, Y.-H.; Zhao, Y.-Y.; Zhang, Y.-Y.; Li, J.-G.; Zhang, H.; Zhao, D.-B. Effect of bamboo shoot dietary fiber on gel quality, thermal stability and secondary structure changes of pork salt-soluble proteins. CyTA-J. Food 2019, 17, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Xiong, Y.L.; Chen, J.; Tang, X.; Zhou, G. Rheological and Microstructural Properties of Porcine Myofibrillar Protein–Lipid Emulsion Composite Gels. J. Food Sci. 2009, 74, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Zhang, Q.; Lu, J.; Xu, J.-L.; Zhang, H.; Wang, J.-H. Physicochemical properties of Tremella fuciformis polysaccharide and its interactions with myofibrillar protein. Bioact. Carbohydr. Diet. Fibre 2017, 11, 18–25. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z.; Xu, X.; Li, P. Effect of peanut protein isolate on functional properties of chicken salt-soluble proteins from breast and thigh muscles during heat-induced gelation. Meat Sci. 2012, 91, 88–92. [Google Scholar] [CrossRef]
- Bloukas, J.G.; Paneras, E.D. Substituting Olive Oil for Pork Backfat Affects Quality of Low-Fat Frankfurters. J. Food Sci. 1993, 58, 705–709. [Google Scholar] [CrossRef]
- Ni, N.; Wang, Z.; Wang, L.; He, F.; Liu, J.; Gao, Y.; Zhang, D. Reduction of sodium chloride levels in emulsified lamb sausages: The effect of lamb plasma protein on the gel properties, sensory characteristics, and microstructure. Food Sci. Biotechnol. 2014, 23, 1137–1143. [Google Scholar] [CrossRef]
- Camou, J.P.; Sebranek, J.G. Gelation characteristics of muscle proteins from pale, soft, exudative (PSE) pork. Meat Sci. 1991, 30, 207–220. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Chung, H.-Y. Effects of κ-carrageenan, salt, phosphates and fat on qualities of low fat emulsified meatballs. J. Food Eng. 2001, 47, 115–121. [Google Scholar] [CrossRef]
- Park, J.W. Temperature-tolerant fish protein gels using konjac flour. J. Muscle Foods 1996, 7, 165–174. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Y.; Fei, Y.; Xu, X.; Zhou, G. Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2009, 228, 665–670. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Dong, X.; Li, K.; Wang, Y.; Wang, Y.; Du, M.; Zhang, J.; Bai, Y. Effect of chickpea (Cicer arietinum L.) protein isolate on the heat-induced gelation properties of pork myofibrillar protein. J. Sci. Food Agric. 2021, 101, 2108–2116. [Google Scholar] [CrossRef]
- Kang, Z.-L.; Zou, X.-L.; Meng, L.; Li, Y.-P. Effects of NaCl and soy protein isolate on the physicochemical, water distribution, and mobility in frankfurters. Int. J. Food Sci. Technol. 2021, 56, 6572–6579. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Moreno, P.; Careche, M. Low field nuclear magnetic resonance (LF-NMR) relaxometry in hake (Merluccius merluccius, L.) muscle after different freezing and storage conditions. Food Chem. 2014, 153, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.; Barbut, S. Effects of protein level and fat/oil on emulsion stability, texture, microstructure and color of meat batters. Meat Sci. 2009, 82, 228–233. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.-G.; Zhou, Y.-Z.; Li, P.-J.; Ma, F.; Nishiumi, T.; Suzuki, A. Effects of high pressure processing on the thermal gelling properties of chicken breast myosin containing κ-carrageenan. Food Hydrocoll. 2014, 40, 262–272. [Google Scholar] [CrossRef]
- Ullah, N.; Wang, X.; Chen, L.; Xu, X.; Li, Z.; Feng, X. Influence of biofilm surface layer protein A (BslA) on the gel structure of myofibril protein from chicken breast. J. Sci. Food Agric. 2017, 97, 4712–4720. [Google Scholar] [CrossRef]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.; Xue, C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, W.; Liu, R.; Liu, Y.; Xing, L.; Han, M.; Kang, Z.-L.; Xu, X.-L.; Zhou, G.-H. Improved gel functionality of myofibrillar proteins incorporation with sugarcane dietary fiber. Food Res. Int. 2017, 100, 586–594. [Google Scholar] [CrossRef]


| Addition Level | Hardness (N) | Springiness | Cohesiveness (Ratio) | Gumminess | Chewiness (N) |
|---|---|---|---|---|---|
| 0% | 25.56 c ± 8.07 | 0.907 a ± 0.003 | 0.791 a ± 0.067 | 21.94 c ± 7.22 | 23.20 c ± 6.54 |
| 3% | 49.12 b ± 1.63 | 0.916 a ± 0.064 | 0.792 a ± 0.004 | 35.30 b ± 0.86 | 38.98 b ± 1.05 |
| 6% | 53.29 b ± 2.67 | 0.960 a ± 0.008 | 0.811 a ± 0.004 | 40.90 b ± 2.03 | 43.35 b ± 2.35 |
| 9% | 64.53 a ± 3.32 | 0.938 a ± 0.003 | 0.802 a ± 0.018 | 49.92 a ± 1.56 | 51.80 a ± 1.36 |
| 0% | 3% | 6% | 9% | |
|---|---|---|---|---|
| T2b (ms) | 0.96 a ± 0.26 | 0.91 a ± 0.35 | 0.76 a ± 0.09 | 0.67 a ± 0.29 |
| PT2b (%) | 2.58 a ± 0.82 | 3.03 a ± 0.61 | 2.96 a ± 0.49 | 3.55 a ± 0.60 |
| T21′ (ms) | 4.76 a ± 2.45 | 4.84 a ± 2.26 | 3.33 a ± 0.24 | 2.83 a ± 0.64 |
| PT21′ (%) | 2.03 a ± 0.71 | 1.96 a ± 0.68 | 2.31 a ± 0.51 | 2.11 a ± 0.11 |
| T21 (ms) | 50.98 a ± 1.50 | 45.13 b ± 0.87 | 38.69 d ± 0.01 | 41.79 c ± 0.00 |
| PT21 (%) | 85.64 c ± 0.52 | 83.57 d ± 0.38 | 88.62 b ± 0.12 | 89.68 a ± 0.11 |
| T22 (ms) | 687.52 a ± 33.09 | 447.25 b ± 4.98 | 264.47 d ± 0.00 | 393.51 c ± 8.79 |
| PT22 (%) | 8.86 b ± 0.94 | 11.43 a ± 1.29 | 5.87 c ± 0.40 | 3.32 d ± 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Tan, B.; Li, K.; Liu, S.; Gu, Y.; Xia, T.; Bai, Y.; Wang, P.; Wang, R. The Impacts of Different Pea Protein Isolate Levels on Functional, Instrumental and Textural Quality Parameters of Duck Meat Batters. Foods 2022, 11, 1620. https://doi.org/10.3390/foods11111620
Zhu X, Tan B, Li K, Liu S, Gu Y, Xia T, Bai Y, Wang P, Wang R. The Impacts of Different Pea Protein Isolate Levels on Functional, Instrumental and Textural Quality Parameters of Duck Meat Batters. Foods. 2022; 11(11):1620. https://doi.org/10.3390/foods11111620
Chicago/Turabian StyleZhu, Xueshen, Beibei Tan, Ke Li, Shaohua Liu, Ying Gu, Tianlan Xia, Yun Bai, Peng Wang, and Renlei Wang. 2022. "The Impacts of Different Pea Protein Isolate Levels on Functional, Instrumental and Textural Quality Parameters of Duck Meat Batters" Foods 11, no. 11: 1620. https://doi.org/10.3390/foods11111620
APA StyleZhu, X., Tan, B., Li, K., Liu, S., Gu, Y., Xia, T., Bai, Y., Wang, P., & Wang, R. (2022). The Impacts of Different Pea Protein Isolate Levels on Functional, Instrumental and Textural Quality Parameters of Duck Meat Batters. Foods, 11(11), 1620. https://doi.org/10.3390/foods11111620






