The Impact of Dry Yeast Rehydrated in Different Plasma Treated Waters (PTWs) on Fermentation Process and Quality of Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plasma Treated Water under Air Atmosphere (PTWAir)
2.2.2. Plasma Treated Water under Nitrogen (PTWN)
2.2.3. Beer production
Milling, Mashing and Lautering
Boiling
Rehydration of Dry Yeast
Fermentation Trials
2.3. Analytical Determinations
2.3.1. The International Bitterness Unit (IBU)
2.3.2. Ethanol, Real and Apparent Extract
2.3.3. Ethanol Yield
2.3.4. The pH of the Obtained Wort and Beer
2.3.5. The Colour of the Obtained Wort and Beer
2.3.6. Free Amino Nitrogen (FAN)
2.3.7. Volatile Compound Analysis SPME-GC/FID
2.3.8. Organic Acid Analysis
2.3.9. Content and Profile of Sugars
2.3.10. Determination of Metal Ions
2.4. Statistical Analyses
3. Results
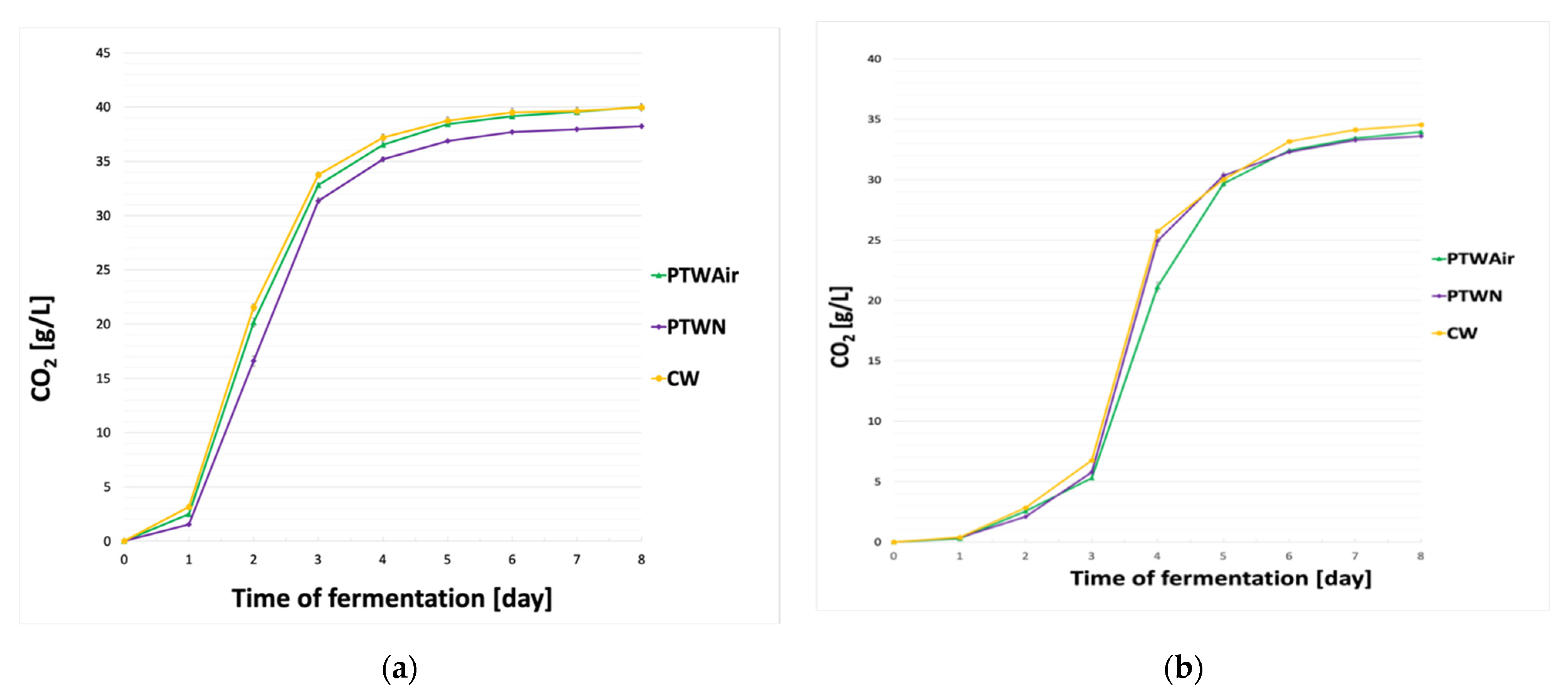
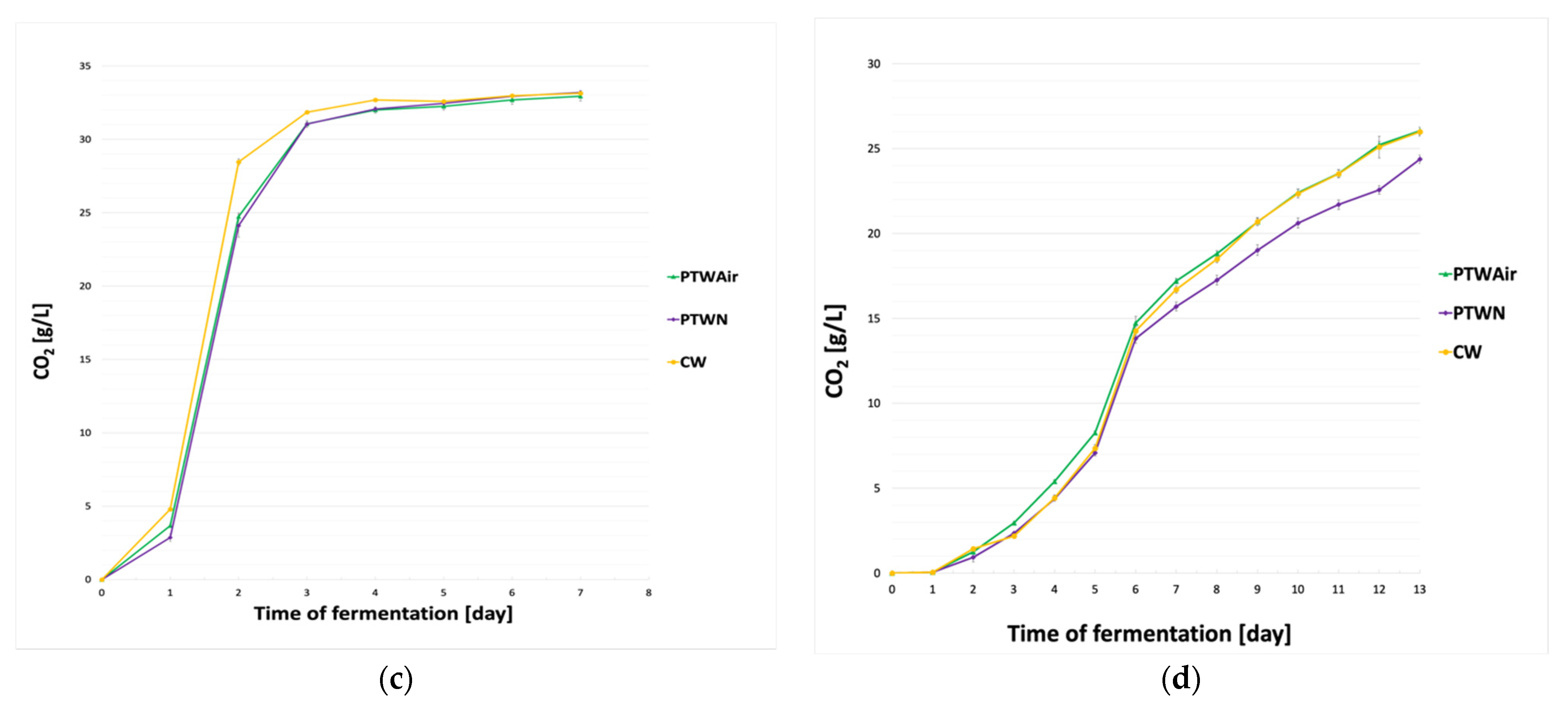
3.1. Fermentation Dynamics
3.2. Ethanol, Real Extract, Apparent Extract and Apparent Degree of Fermentation
3.3. Amount of Biomass
3.4. Colour, pH and FAN
3.5. Ion Content
3.6. Sugars and Organic Acids
3.7. Volatile Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackowski, M.; Trusek, A. Non-alcoholic beer production—An overview. Pol. J. Chem. Technol. 2018, 20, 32–38. [Google Scholar] [CrossRef]
- Bamforth, C.W. Handbook of Alcoholic Beverages Series: Beer a Quality Perspective; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; Volume 347. [Google Scholar]
- Holt, S.; Miks, M.H.; De Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.K. Beer: Production, Sensory Characteristics and Sensory Analysis; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast Responses to Stresses Associated with Industrial Brewery Handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Powell, C.D.; Fischborn, T.; Smart, K.A. Rehydration of active dry brewing yeast and its effect on cell viability. J. Inst. Brew. 2011, 117, 377–382. [Google Scholar] [CrossRef]
- Laroche, C.; Gervais, P. Achievement of rapid osmotic dehydration at specific temperatures could maintain high Saccharomyces cerevisiae viability. Appl. Microbiol. Biotechnol. 2003, 60, 743–747. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef]
- Coutinho, C.; Bernardes, E.; Félix, D.; Panek, A.D. Trehalose as cryoprotectant for preservation of yeast strains. J. Biotechnol. 1988, 7, 23–32. [Google Scholar] [CrossRef]
- Leslie, S.B.; Teter, S.A.; Crowe, L.M.; Crowe, J.H. Trehalose lowers membrane phase transitions in dry yeast cells. Biochim. Biophys. Acta 1994, 1192, 7–13. [Google Scholar] [CrossRef]
- Beker, M.J.; Rapoport, A.I. Conservation of yeasts by dehydration. Adv. Biochem. Eng. Biotechnol. 1987, 35, 128–165. [Google Scholar] [CrossRef]
- Ferrarini, R.; Bocca, E.; Cavazza, A. Mechanical dispersion procedures improve the rehydration of active dry yeast. Enzym. Microb. Technol. 2007, 40, 1251–1255. [Google Scholar] [CrossRef]
- Pater, A.; Zdaniewicz, M.; Satora, P.; Khachatryan, G.; Oszczęda, Z. Application of water treated with low-temperature low-pressure glow plasma for quality improvement of barley and malt. Biomolecules 2020, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Pater, A.; Satora, P.; Zdaniewicz, M.; Khachatryan, K. The Improvement of Reserve Polysaccharide Glycogen Level and Other Quality Parameters of S. cerevisiae Brewing Dry Yeasts by Their Rehydration in Water, Treated with Low-Temperature, Low-Pressure Glow Plasma (LPGP). Appl. Sci. 2022, 12, 2909. [Google Scholar] [CrossRef]
- Elkin, I. Device for plasma treatment of water Pl 216025 B1. 2009, Volume 14. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.389626 (accessed on 10 January 2022).
- Weltmann, K.D.; Polak, M.; Masur, K.; von Woedtke, T.; Winter, J.; Reuter, S. Plasma Processes and Plasma Sources in Medicine. Contrib. Plasma Phys. 2012, 52, 644–654. [Google Scholar] [CrossRef]
- Murawski, M.; Schwarz, T.; Grygier, J.; Patkowski, K.; Oszczęda, Z.; Jelkin, I.; Kosiek, A.; Gruszecki, T.M.; Szymanowska, A.; Skrzypek, T.; et al. The utility of nanowater for ram semen cryopreservation. Exp. Biol. Med. 2015, 240, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Szymanowicz, J.; Schwarz, T.; Murawski, M.; Małopolska, M.; Oszczeda, Z.; Tuz, R.; Nowicki, J.; Bartlewski, P.M. Storage of boar semen at 16-18 °C in the long-term commercial extender prepared with deionized water or nanowater. Anim. Reprod. 2019, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Pater, A.; Zdaniewicz, M. Application of Water Treated with Low-Temperature Low-Pressure Glow Plasma (LPGP) in Various Industries. Beverages 2022, 8, 8. [Google Scholar] [CrossRef]
- Ciesielska, A.; Ciesielski, W.; Khachatryan, K.; Koloczek, H.; Kulawik, D.; Oszczeda, Z.; Soroka, J.A.; Tomasik, P. Structure and Physicochemical Properties of Water Treated Under Methane with Low-Temperature Glow Plasma of Low Frequency. Water 2020, 12, 1638. [Google Scholar] [CrossRef]
- Białopiotrowciz, T.; Ciesielski, W.; Domanski, J.; Doskocz, M.; Khachatryan, K.; Fiedorowicz, M.; Graz, K.; Kołoczek, H.; Kozak, A.; Oszczeda, Z.T.P. Structure and Physicochemical Properties of Water Treated with Low-Temperature Low-Frequency Glow Plasma. Curr. Phys. Chem. 2016, 6, 312–320. [Google Scholar] [CrossRef]
- Uchida, G.; Takenaka, K.; Takeda, K.; Ishikawa, K.; Hori, M.; Setsuhara, Y. Selective production of reactive oxygen and nitrogen species in the plasma-treated water by using a nonthermal high-frequency plasma jet. Jpn. J. Appl. Phys. 2018, 57, 0102B4. [Google Scholar] [CrossRef]
- Boulton, C. Fermentation Systems; Wiley-Blackwell: UHoboken, NJ, USA, 2007; ISBN 0632054751. [Google Scholar]
- Standard, I.; Methods, T. EBC_4.5.1 Extract of Malt—Congress Mash; BrewUp: Brussels, Belgium, 2004; pp. 1–4. [Google Scholar]
- The, S.; Standard, I.; Method, E.B.C.; The, P.; See, R.; Method, E.B.C.; See, A.; Method, E.B.C.; Clarify, S. Bitterness of Wort 8.8 2004; BrewUp: Brussels, Belgium, 2013; pp. 8–9. [Google Scholar]
- Charles, E.; Wayman, B.H.D. Seventeenth Symposium on Biotechnology for Fuels and Chemicals. Humana Press: Totowa, NJ, USA, 1996; ISBN 9781461266693. [Google Scholar]
- Standard, I.; Methods, T. Colour of Wort: Spectrophotometric Method (IM) 8.5 2000; BrewUp: Brussels, Belgium, 2013; pp. 8–10. [Google Scholar]
- Standard, I.; Methods, T. Free Amino Nitrogen in Beer by Spectrophotometry (IM) 9.10 2000; BrewUp: Brussels, Belgium, 2013; Volume 2013. [Google Scholar]
- Antalick, G.; Perello, M.C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography-mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Pater, A.; Hrabia, O.; Duliński, R.; Cioch-Skoneczny, M. Tritordeum malt: An innovative raw material for beer production. J. Cereal Sci. 2020, 96, 103095. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0849330343. [Google Scholar]
- Finn, D.A.; Stewart, G.G. Fermentation characteristics of dried brewers yeast: Effect of drying on flocculation and fermentation. J. Am. Soc. Brew. Chem. 2002, 60, 135–139. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Pater, A.; Knapik, A.; Duliński, R. The effect of different oat (Avena sativa L) malt contents in a top-fermented beer recipe on the brewing process performance and product quality. J. Cereal Sci. 2021, 101, 103301. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Satora, P.; Pater, A.; Bogacz, S. Low lactic acid-producing strain of lachancea thermotolerans as a new starter for beer production. Biomolecules 2020, 10, 256. [Google Scholar] [CrossRef]
- Lei, H.; Xu, H.; Feng, L.; Yu, Z.; Zhao, H.; Zhao, M. Fermentation performance of lager yeast in high gravity beer fermentations with different sugar supplementations. J. Biosci. Bioeng. 2016, 122, 583–588. [Google Scholar] [CrossRef]
- Walkey, C.J.; Luo, Z.; Borchers, C.H.; Measday, V.; Van Vuuren, H.J.J. The Saccharomyces cerevisiae fermentation stress response protein Igd1p/Yfr017p regulates glycogen levels by inhibiting the glycogen debranching enzyme. FEMS Yeast Res. 2011, 11, 499–508. [Google Scholar] [CrossRef][Green Version]
- Bolat, I. The importance of trehalose in brewing yeast survival. Innov. Rom. Food Biotechnol. 2008, 2, 1–10. [Google Scholar]
- Meier-Dörnberg, T.; Hutzler, M.; Michel, M.; Methner, F.J.; Jacob, F. The importance of a comparative characterization of Saccharomyces cerevisiae and Saccharomyces pastorianus strains for brewing. Fermentation 2017, 3, 41. [Google Scholar] [CrossRef]
- Portno, B.A.D. Continuous fermentation of brewer’s wort. J. Inst. Brew. 1968, 74, 55–63. [Google Scholar] [CrossRef]
- Verbelen, P.J.; De Schutter, D.P.; Delvaux, F.; Verstrepen, K.J.; Delvaux, F.R. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol. Lett. 2006, 28, 1515–1525. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Bamforth, C.W. Assessing color quality of beer. ACS Symp. Ser. 2008, 983, 192–202. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Rautio, J.; Londesborough, J. Maltose Transport by Brewer’s Yeasts in Brewer’s Wort. J. Inst. Brew. 2003, 109, 251–261. [Google Scholar] [CrossRef]
- Alves, S.L.; Herberts, R.A.; Hollatz, C.; Miletti, L.C.; Stambuk, B.U. Maltose and maltotriose active transport and fermentation by Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 2007, 65, 99–104. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Magalhães, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and maltotriose utilisation by group I strains of the hybrid lager yeast Saccharomyces pastorianus. FEMS Yeast Res. 2016, 16, fow053. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing oxygen pulses to modulate Lachancea thermotolerans–Saccharomyces cerevisiae Chardonnay fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor impacts of glycerol in the processing of yeast fermented beverages: A review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Nevoigt, E.; Stahl, U. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1997, 21, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Van Laere, S.D.M.; Vercammen, J.; Derdelinkx, G.; Dufour, J.P.; Pretorius, I.S.; Winderickx, J.; Thevelein, J.M.; Delvaux, F.R. The Saccharomyces cerevisiae alcohol acetyl transferase Atflp is localized in lipid particles. Yeast 2004, 21, 367–377. [Google Scholar] [CrossRef]
- Langstaff, S.A.; Guinard, J.; Lewis, M.J. Sensory Evaluation of the Mouthfeel of Beer. J. Am. Soc. Brew. Chem. 1991, 49, 54–59. [Google Scholar] [CrossRef]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding flavor to beverages with non-conventional yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- Renger, B.R.S.; Hateren, S.H.V.a.n.; Luyben, K.C.A.M. The Formation of Esters and Higher Alcohols during Brewery Fermentation; The Effect of Carbon Dioxide Pressure. J. Inst. Brew. 1992, 98, 509–513. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Keukeleire, D.D. Fundamentals of beer and hop chemistry. Divulgacao 2000, 23, 108–112. [Google Scholar] [CrossRef]
- Wang, G.; Tian, L.; Aziz, N.; Broun, P.; Dai, X.; He, J.; King, A.; Zhao, P.X.; Dixon, R.A. Terpene biosynthesis in glandular trichomes of hop. Plant Physiol. 2008, 148, 1254–1266. [Google Scholar] [CrossRef]
- Kishimoto, T.; Wanikawa, A.; Kono, K.; Shibata, K. Comparison of the odor-active compounds in unhopped beer and beers hopped with different hop varieties. J. Agric. Food Chem. 2006, 54, 8855–8861. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; Ebeler, S.E. The occurrence of glycosylated aroma precursors in vitis vinifera fruit and humulus lupulus hop cones and their roles in wine and beer volatile aroma production. Foods 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
| Original Gravity [°P] | IBU | pH | Colour [EBC] | FAN [mg/L] | Ca2+ [mg/L] | Zn2+ [mg/L] | Mg2+ [mg/L] | Fructose [g/L] | Glucose [g/L] | Saccharose [g/L] | Maltose [g/L] | Citric Acid [g/L] | Malic Acid [g/L] | Succinic Acid [g/L] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11.9 ± 0.09 | 26 ± 0.04 | 5.75 ± 0.02 | 14.3 ± 1.09 | 191 ± 31.36 | 21.6 ± 2.42 | 0.91 ± 0.17 | 93.7 ± 15.9 | 8.92 ± 1.17 | 7.74 ± 0.74 | 5.12 ± 0.46 | 37.1 ± 3.51 | 3.5 ± 2.48 | 2.7 ± 1.03 | 1.3 ± 0.21 | |
| Analytical Determinations | 2.3.1. | 2.3.4. | 2.3.5. | 2.3.6. | 2.3.10. | 2.3.9. | 2.3.8. | ||||||||
| Parameters | Ethanol [% v/v] | Real Extract [% w/w] | Apparent Extract [% w/w] | Ethanol Yield [g/g] | Amount of Biomass [g of Dry Mass/dm] | Colour [EBC Units] | pH | FAN [mg/L] | Mg [mg/L] | Ca [mg/L] | Zn [mg/L] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae Safbrew US-05 | PTWAir | 5.33 a ± 0.02 | 4.12 ba ± 0.01 | 2.20 a ± 0.01 | 53.6 a ± 0.29 | 2.77 a ± 0.48 | 5.62 a ± 0.71 | 4.24 b ± 0.04 | 44.8 a ± 7.39 | 65.1 a ± 2.52 | 17.4 a ± 2.49 | 0.00 c ± 0.00 |
| PTWN | 5.26 a ± 0.03 | 4.10 a ± 0.02 | 2.19 a ± 0.02 | 52.9 a ± 0.59 | 1.69 b ± 0.1 | 6.67 b ± 0.35 | 4.29 b ± 0.01 | 52.6 a ± 5.21 | 62.8 a ± 4.00 | 16.8 ab ± 1.72 | 0.14 a ± 0.19 | |
| CW | 5.35 a ± 0.05 | 4.17 b ± 0.03 | 2.24 a ± 0.01 | 54.2 a ± 0.81 | 2.75 a ± 0.68 | 6.81 b ± 0.08 | 4.26 b ± 0.02 | 51.9 a ± 6.64 | 71.8 b ± 2.39 | 22.4 a ± 2.03 | 0.27 a ± 0.08 | |
| Saccharomyces cerevisiae Safbrew T58 | PTWAir | 4.69 b ± 0.04 | 4.55 c ± 0.04 | 2.86 b ± 0.03 | 49.5 c ± 0.67 | 2.46 c ± 0.53 | 7.87 b ± 0.38 | 4.65 a ± 0.03 | 73.1 b ± 13.05 | 85.8 b ± 2.87 | 17.7 a ± 3.23 | 0.09 a ± 0.05 |
| PTWN | 4.66 b ± 0.03 | 4.61 d ± 0.09 | 2.92 c ± 0.09 | 49.3 c ± 0.68 | 1.54 d ± 0.49 | 7.22 b ± 0.55 | 4.56 a ± 0.04 | 77.2 b ± 8.18 | 75.7 b ± 4.54 | 20.8 a ± 1.78 | 0.29 a ± 0.17 | |
| CW | 4.78 c ± 0.04 | 4.50 c ± 0.05 | 2.76 b ± 0.06 | 50.5 d ± 0.38 | 2.16 c ± 0.28 | 8.12 c ± 1.34 | 4.64 a ± 0.02 | 72.7 b ± 8.54 | 78.8 b ± 4.54 | 21.9 a ± 3.92 | 0.04 c ± 0.03 | |
| Saccharomyces cerevisiae Safbrew S33 | PTWAir | 4.38 f ± 0.01 | 5.32 e ± 0.02 | 3.75 d ± 0.02 | 51.8 f ± 0.62 | 2.38 a ± 0.27 | 8.57 c ± 0.22 | 4.59 a ± 0.06 | 61.4 a ± 6.96 | 75.0 b ± 1.85 | 16.9 ab ± 0.38 | 0.43 ab ± 0.02 |
| PTWN | 4.30 f ± 0.01 | 5.31 e ± 0.02 | 3.73 d ± 0.04 | 51.8 f ± 0.14 | 1.15 b ± 0.30 | 8.95 c ± 0.06 | 4.59 a ± 0.01 | 66.2 a ± 4.83 | 80.4 b ± 4.87 | 20.6 a ± 1.1 | 0.18 ab ± 0.11 | |
| CW | 4.38 f ± 0.04 | 5.30 f ± 0.02 | 3.71 d ± 0.03 | 51.9 f ± 0.41 | 2.76 a ± 0.21 | 9.20 c ± 1.3 | 4.58 a ± 0.01 | 59.2 a ± 8.13 | 74.8 b ± 14.2 | 16.9 ab ± 1.59 | 0.36 ab ± 0.04 | |
| Saccharomyces pastorianus Saflager W34/70 | PTWAir | 4.79 c ± 0.06 | 4.38 c ± 0.11 | 2.67 b ± 0.15 | 49.6 c ± 1.67 | 1.95 e ± 0.19 | 7.95 b ± 1.24 | 4.63 a ± 0.08 | 136 c ± 7.99 | 80.5 c ± 9.33 | 21.7 a ± 2.24 | 0.64 b ± 0.05 |
| PTWN | 4.51 b ± 0.08 | 4.66 d ± 0.13 | 3.00 c ± 0.16 | 48.8 e ± 0.15 | 0.76 f ± 0.06 | 6.32 a ± 0.2 | 4.56 a ± 0.01 | 155 c ± 12.9 | 72.9 bc ± 1.98 | 18.1 a ± 1.29 | 0.59 b ± 0.07 | |
| CW | 4.77 c ± 0.03 | 4.39 c ± 0.13 | 2.66 b ± 0.13 | 49.6 c ± 1.07 | 2.21 e ± 0.08 | 6.22 a ± 0.31 | 4.59 a ± 0.03 | 150 c ± 3.34 | 70.5 bc ± 3.32 | 17.6 a ± 0.67 | 0.47 b ± 0.02 | |
| Significance | Strain | *** | *** | *** | *** | *** | *** | *** | * | *** | ns | ** |
| Water | *** | *** | *** | *** | ** | *** | ns | ns | ns | ns | ns | |
| Strain × Water | *** | *** | *** | *** | *** | *** | * | ** | *** | ** | ** | |
 Values with different superscript roman letters (a–i) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 *. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.
Values with different superscript roman letters (a–i) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 *. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.| Parameters | Maltose [g/L] | Fructose [g/L] | Glucose [g/L] | Saccharose [g/L] | Glycerol [g/L] | Acetic Acid [g/L] | Lacid Acid [g/L] | Malic Acid [g/L] | Citric Acid [g/L] | Succinic Acid [g/L] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae Safbrew US-05 | PTWAir | 0.51 a ± 0.36 | 6.54 c ± 0.15 | 1.94 acd ± 0.07 | 1.36 abe ± 0.03 | 5.49 a ± 1.01 | 0.08 a ± 0.01 | 0.03 ± 0.00 | 0.12 c ± 0.01 | 0.13 ± 0.01 | 0.03 a ± 0.00 |
| PTWN | 1.78 c ± 0.21 | 4.81 b ± 0.07 | 2.73 be ± 0.21 | 1.31 abe ± 0.87 | 4.42 b ± 0.11 | 0.09 ab ± 0.003 | 0.02 ± 0.00 | 0.09 bc ± 0.00 | 0.02 ± 0.00 | 0.03 a ± 0.01 | |
| CW | 0.06 a ± 0.01 | 2.43 a ± 0.04 | 2.37 ab ± 0.44 | 0.15 c ± 0.13 | 5.55 a ± 0.43 | 0.07 ab ± 0.01 | 0.03 ± 0.00 | 0.09 b ± 0.00 | 0.02 ± 0.00 | 0.02 a ± 0.00 | |
| Saccharomyces cerevisiae Safbrew T58 | PTWAir | 0.07 a ± 0.02 | 6.91 c ± 0.85 | 3.69 f ± 0.05 | 1.07 ade ± 1.3 | ND ± 0.00 | 0.11 ab ± 0.01 | 0.02 ± 0.00 | 0.01 a ± 0.00 | 0.05 ± 0.00 | 0.01 bc ± 0.01 |
| PTWN | 0.06 a ± 0.01 | 8.74 d ± 0.45 | 2.59 abe ± 0.06 | 0.38 cd ± 0.23 | ND ± 0.00 | 0.08 ab ± 0.01 | 0.07 ± 0.01 | 0.02 a ± 0.01 | 0.04 ± 0.00 | 0.03 a ± 0.01 | |
| CW | 1.62 c ± 0.39 | 6.81 c ± 0.34 | 3.10 ef ± 0.67 | 3.09 f ± 0.02 | ND ± 0.00 | 0.11 ab ± 0.01 | 0.03 ± 0.00 | 0.02 a ± 0.00 | 0.31 ± 0.01 | 0.04 c ± 0.01 | |
| Saccharomyces cerevisiae Safbrew S33 | PTWAir | 0.26 ab ± 0.20 | 3.28 b ± 0.91 | 0.06 g ± 0.00 | 1.68 ab ± 0.01 | 6.68 c ± 1.06 | 0.08 ab ± 0.01 | 0.03 ± 0.00 | 0.03 a ± 0.01 | 0.04 ± 0.01 | 0.03 a ± 0.01 |
| PTWN | 4.16 d ± 0.59 | 2.27 ab ± 1.4 | 1.29 c ± 0.08 | 0.49 cde ± 0.08 | 5.94 a ± 0.85 | 0.05 ab ± 0.01 | 0.03 ± 0.00 | 0.03 a ± 0.00 | 0.05 ± 0.00 | 0.04 c ± 0.00 | |
| CW | 6.81 e ± 0.58 | 0.33 a ± 0.01 | 1.95 acd ± 0.01 | 1.52 ab ± 0.08 | 6.11 a ± 0.52 | 0.09 ab ± 0.004 | 0.03 ± 0.00 | 0.03 a ± 0.01 | 0.05 ± 0.00 | 0.04 c ± 0.00 | |
| Saccharomyces pastorianus Saflager W34/70 | PTWAir | 4.42 d ± 0.44 | 0.39 a ± 0.02 | 2.21 abd ± 0.79 | 1.82 ab ± 0.61 | 8.93 d ± 0.64 | 0.12 b ± 0.005 | 0.02 ± 0.01 | ND ± 0.00 | 0.02 ± 0.00 | ND ± 0.00 |
| PTWN | 8.55 f ± 1.31 | ND ± 0.00 | 2.52 abe ± 0.19 | 2.07 b ± 0.14 | 8.46 e ± 0.74 | 0.11 b ± 0.01 | 0.02 ± 0.01 | ND ± 0.00 | 0.03 ± 0.00 | 0.01 b ± 0.00 | |
| CW | 5.41 e ± 0.93 | ND ± 0.00 | 1.49 cd ± 0.71 | 0.24 cd ± 0.02 | 8.03 e ± 0.55 | 0.11 b ± 0.01 | 0.19 ± 0.11 | ND ± 0.00 | 0.03 ± 0.00 | ND ± 0.00 | |
| Significance | Strain | *** | *** | ** | ** | *** | * | ns | *** | ns | *** |
| Water | *** | *** | ns | * | * | ns | ns | ns | ns | ns | |
| Strain × Water | **** | *** | * | *** | *** | * | ns | *** | ns | *** | |
 Values with different superscript roman letters (a–f) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 *. ND—Not detected. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.
Values with different superscript roman letters (a–f) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 *. ND—Not detected. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.| Compound [ug/L] | Ethyl Acetate | Ethyl Hexanoate | Ethyl Octanoate | 2-phenylethyl Acetate | Ethyl Decanoate | Ethyl Dodecanoate | |
|---|---|---|---|---|---|---|---|
| LRI | 614 | 986 | 1180 | 1228 | 1397 | 1581 | |
| Saccharomyces cerevisiae Safbrew US-05 | PTWAir | 1102 c | 3.37 a | 5.81 a | 378.2 c | 35.1 ab | 0.42 b |
| PTWN | 775.7 b | 2.51 a | 5.89 a | 680.8 c | 35.1 ac | 1.15 a | |
| CW | 1140 c | 3.37 a | 5.89 a | 983.6 b | 41.7 c | 1.22 a | |
| Saccharomyces cerevisiae Safbrew T58 | PTWAir | 447.8 ab | ND | 5.74 a | 213.2 a | 10.5 c | 0.56 b |
| PTWN | 372.7 ab | ND | 4.41 a | 257.4 a | 29.6 d | 1.29 ab | |
| CW | 427.7 ab | ND | ND | 169.5 d | 25.7 c | 1.17 ab | |
| Saccharomyces cerevisiae Safbrew S33 | PTWAir | 249.5 a | ND | ND | 685.5 c | 20.3 ab | 1.00 b |
| PTWN | 720 abc | ND | ND | 641.8 c | 24.2 b | 1.29 ab | |
| CW | 685.2 abc | ND | ND | 912.2 b | 31.8 ab | 1.32 ab | |
| Saccharomyces pastorianus Saflager W34/70 | PTWAir | 407.7 ab | ND | ND | 125.6 a | 18.6 a | 1.33 a |
| PTWN | 362 ab | ND | ND | 106.3 a | 10.1 ab | 1.31 a | |
| CW | 248.7 a | ND | ND | 164.3 d | 17.2 ab | 1.31 a | |
| OT | 5000 | 6.4 | 15 | 3940 | 510 | 1000 | |
| Significance | Strain | ** | ** | *** | ** | * | * |
| Water | ns | ns | ** | ** | ns | ** | |
| Strain × Water | * | * | * | * | ns | * | |
 Values with different superscript roman letters (a–d) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 * ND—Not detected. LRI—linear retention index; the amount of components was determined. OT—Odour thresholds in beer [32]. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.
Values with different superscript roman letters (a–d) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 * ND—Not detected. LRI—linear retention index; the amount of components was determined. OT—Odour thresholds in beer [32]. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.| Compound [ug/L] | 2-Methyl-1-propanol | 2-Methyl-1-butanol | 2,3-Butanediol | 1-Hexanol | 2-Phenylethanol | 1-Nonanol | |
|---|---|---|---|---|---|---|---|
| LRI | 617 | 740 | 768 | 865 | 1084 | 1156 | |
| Saccharomyces cerevisiae Safbrew US-05 | PTWAir | 6.05 a | 2933 ac | 0.66 b | 17.4 a | 115 cd | 3.59 a |
| PTWN | 12.4 bc | 2917 ac | 0.67 b | 22.4 ab | 153 d | 25.7 b | |
| CW | 14.8 bc | 3100 a | 0.67 b | 21.2 ab | 194 e | 25.6 b | |
| Saccharomyces cerevisiae Safbrew T58 | PTWAir | ND | 3049 ac | 0.51 bcd | 16.4 a | 57.5 a | 28.8 b |
| PTWN | ND | 2917 ac | 0.17 acd | 15.7 a | 77.5 ab | 27.5 b | |
| CW | ND | 3481 a | 0.67 b | 14.9 a | 75.9 a | 32.9 b | |
| Saccharomyces cerevisiae Safbrew S33 | PTWAir | 6.49 a | 1765 cd | 0.33 abcd | 20.5 ab | 73.5 a | 8.41 e |
| PTWN | 12.2 bc | 1047 d | 0.16 ac | 21.9 ab | ND | 9.91 d | |
| CW | 19.1 c | 1226 d | ND | 26.7 b | ND | 11.6 d | |
| Saccharomyces pastorianus Saflager W34/70 | PTWAir | ND | 400.2 b | ND | 21.8 ab | 83.1 ab | 3.82 f |
| PTWN | ND | 346.8 b | ND | 22.3 ab | 82.4 ab | 3.30 a | |
| CW | ND | 930.9 b | ND | 17.9 a | 86.8 ab | 3.10 a | |
| OT | 4000 | 400 | 31 | 100 | - | 50 | |
| Significance | Strain | ** | *** | ** | ** | *** | *** |
| Water | * | ns | ns | ns | ns | ** | |
| Strain × Water | ** | ** | ** | * | *** | *** | |
 Values with different superscript roman letters (a–d) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 * ND- Not detected. LRI—linear retention index; the amount of components was determined. OT—Odour thresholds in beer [32]. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.
Values with different superscript roman letters (a–d) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 * ND- Not detected. LRI—linear retention index; the amount of components was determined. OT—Odour thresholds in beer [32]. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each column is in the darkest red and the highest content is in the darkest green.| Compound [ug/L] | β-Myrcene | Linalool | Geraniol | β-Damascenone | Humulene | β-Farnesene | Nerolidol | |
|---|---|---|---|---|---|---|---|---|
| LRI | 991 | 1092 | 1257 | 1384 | 1455 | 1462 | 1562 | |
| Saccharomyces cerevisiae Safbrew US-05 | PTWAir | 0.09 a | 0.15 b | 2.35 a | 0.31 b | 2.50 | 1.79 a | 0.68 |
| PTWN | 0.09 a | 0.08 a | 0.32 b | 0.59 b | 2.91 | 0.89 b | 0.71 | |
| CW | 0.19 c | 0.07 a | 0.46 b | 0.14 b | 2.17 | 0.44 b | 0.73 | |
| Saccharomyces cerevisiae Safbrew T58 | PTWAir | 0.08 a | 0.1 a | 1.55 c | ND | 2.72 | 1.34 ab | 0.51 |
| PTWN | 0.09 a | 0.18 b | 6.43 a | ND | 2.41 | 1.78 a | 0.69 | |
| CW | 0.08 a | 0.38 b | 0.94 b | ND | 2.41 | 1.8 a | 0.52 | |
| Saccharomyces cerevisiae Safbrew S33 | PTWAir | 0.11 b | 0.15 a | 5.72 a | 0.24 ab | 2.64 | 1.34 ab | 0.68 |
| PTWN | 0.11 b | 0.03 a | 0.56 b | 0.08 a | 2.43 | 0.44 ab | 0.77 | |
| CW | 0.09 a | 0.03 a | 0.23 b | 0.09 a | 2.41 | 0.00 ab | 0.69 | |
| Saccharomyces pastorianus Saflager W34/70 | PTWAir | 0.08 a | 0.14 a | 1.27 a | ND | 2.25 | 1.79 a | 0.66 |
| PTWN | 0.07 d | 0.12 a | 1.91 a | ND | 2.23 | 1.34 ab | 0.69 | |
| CW | 0.08 a | 0.08 a | 3.12 d | ND | 2.24 | 0.44 b | 0.69 | |
| OT | 30 | 2.2 | 36 | 25 | 10 | - | - | |
| Significance | Strain | * | ** | ** | ** | ns | * | ns |
| Water | ** | * | ** | ns | ns | * | ns | |
| Strain × Water | * | * | ** | * | ns | * | ns | |
 Values with different superscript roman letters (a–d) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 * ND—Not detected LRI—linear retention index; the amount of components was determined. OT—Odour thresholds in beer [2]. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each row is in the darkest red and the highest content is in the darkest green.
Values with different superscript roman letters (a–d) in the same column indicate statistically significant differences at p < 0.05; n = 4; ns—not significant; 0.001 ***; 0.01 **; 0.05 * ND—Not detected LRI—linear retention index; the amount of components was determined. OT—Odour thresholds in beer [2]. Color determination from lowest (0%) to highest (100%) concentration of parameters. The lowest concentration of a specific parameter in each row is in the darkest red and the highest content is in the darkest green.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pater, A.; Satora, P.; Zdaniewicz, M.; Sroka, P. The Impact of Dry Yeast Rehydrated in Different Plasma Treated Waters (PTWs) on Fermentation Process and Quality of Beer. Foods 2022, 11, 1316. https://doi.org/10.3390/foods11091316
Pater A, Satora P, Zdaniewicz M, Sroka P. The Impact of Dry Yeast Rehydrated in Different Plasma Treated Waters (PTWs) on Fermentation Process and Quality of Beer. Foods. 2022; 11(9):1316. https://doi.org/10.3390/foods11091316
Chicago/Turabian StylePater, Aneta, Paweł Satora, Marek Zdaniewicz, and Paweł Sroka. 2022. "The Impact of Dry Yeast Rehydrated in Different Plasma Treated Waters (PTWs) on Fermentation Process and Quality of Beer" Foods 11, no. 9: 1316. https://doi.org/10.3390/foods11091316
APA StylePater, A., Satora, P., Zdaniewicz, M., & Sroka, P. (2022). The Impact of Dry Yeast Rehydrated in Different Plasma Treated Waters (PTWs) on Fermentation Process and Quality of Beer. Foods, 11(9), 1316. https://doi.org/10.3390/foods11091316







