Microfluidics-Based Time-Resolved Fluorescence Immunoassay for the On-Site Detection of Aflatoxins B1 Zearalenone and Deoxynivalenol in Cereals
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
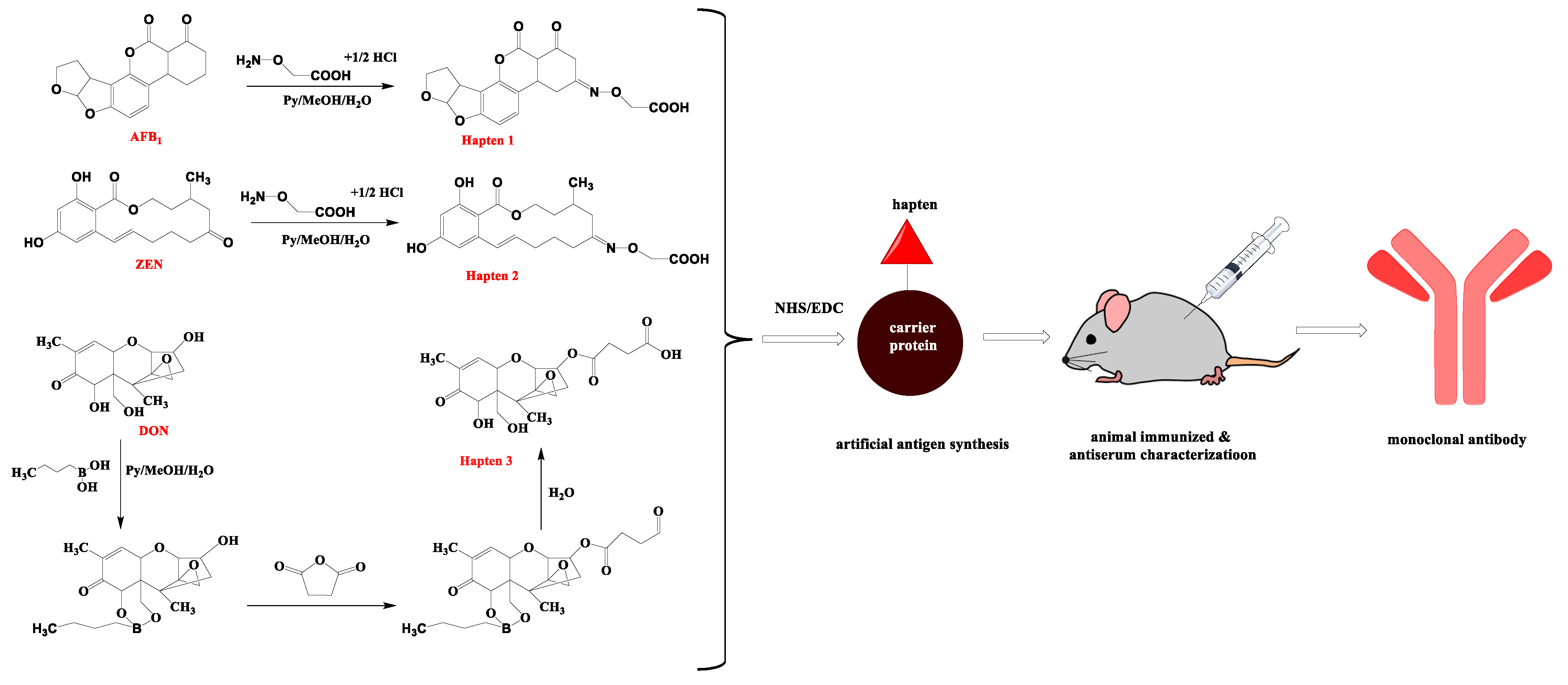
2.2. Preparation of Antigens
2.3. Production of MAb
2.4. Indirect Competitive ELISA
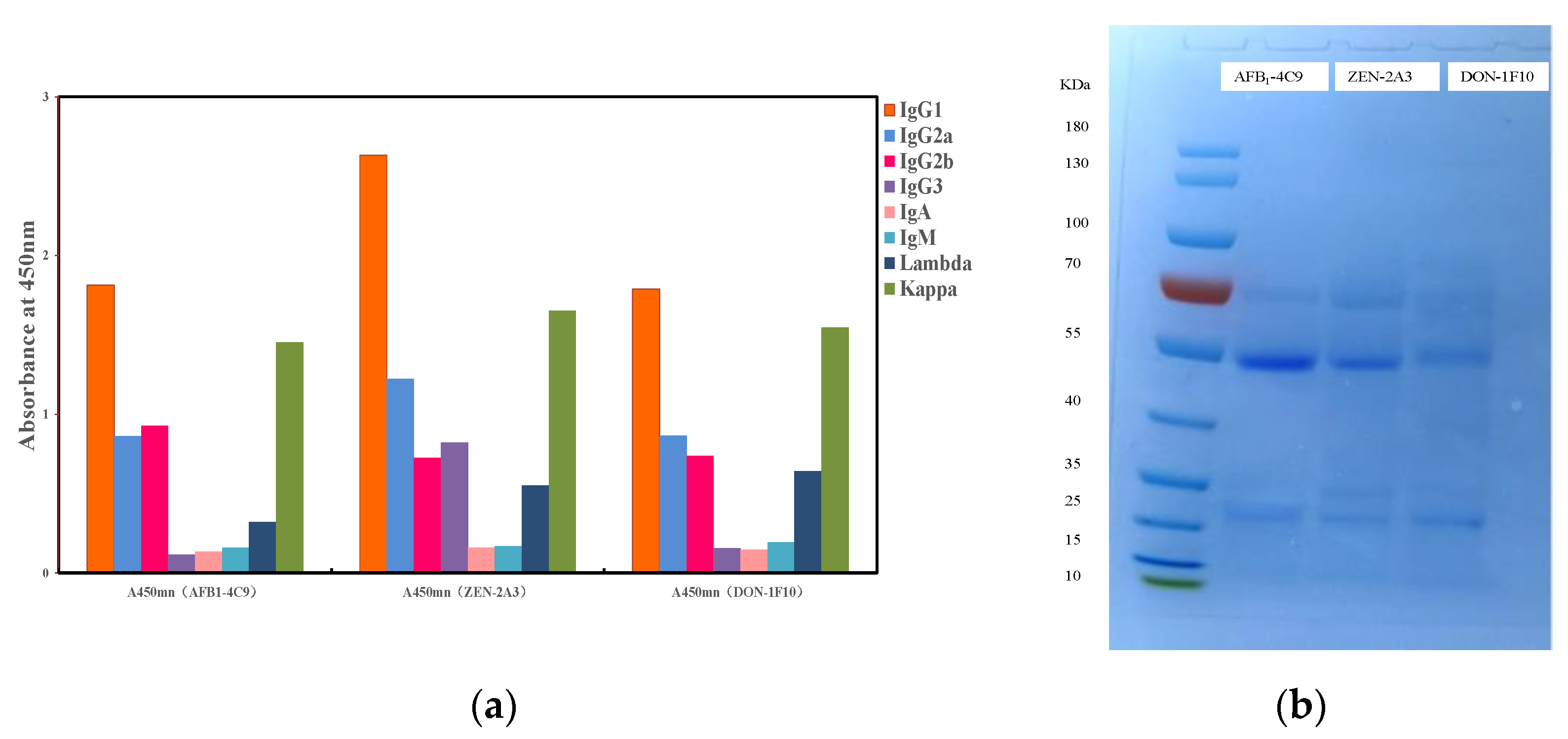
2.5. Characterization of MAb
2.6. Antibody Specificity Determination
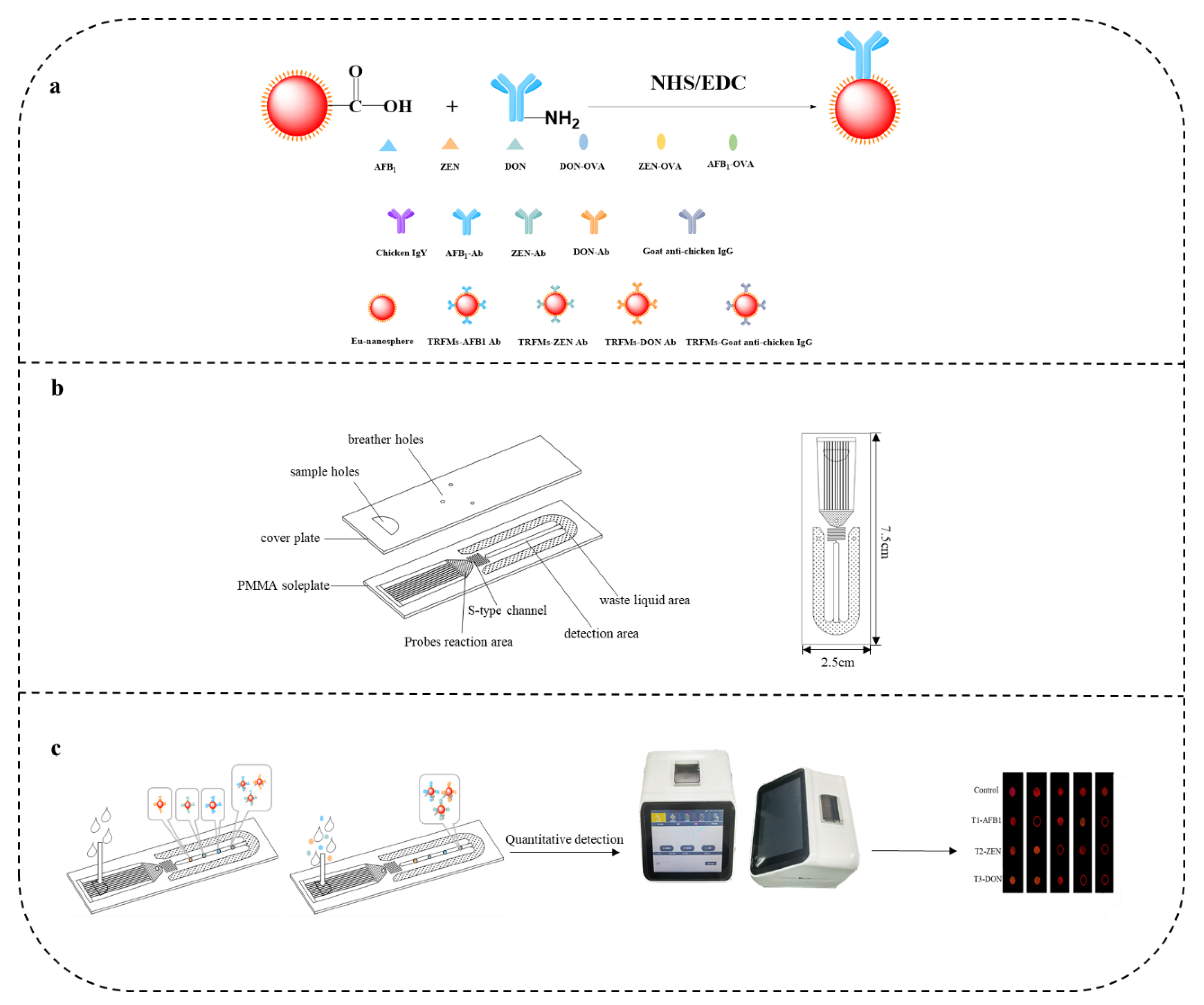
2.7. Prepare TRFMs-Probes
2.8. Assembly of the Microfluidic Chip
2.9. Sample Pretreatment
2.10. Microfluidics Assay Procedure
3. Results and Discussion
3.1. The Mab’s Characterization
3.2. Antibody Specificity Determination
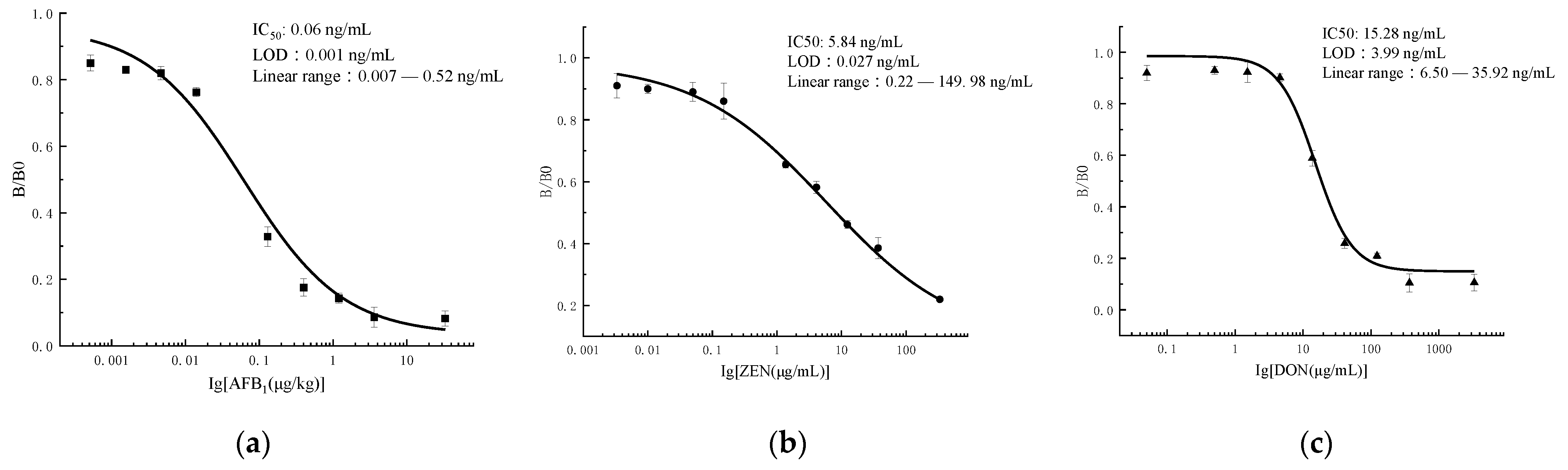
3.3. Indirect Competitive ELISA
3.4. Optimization of the Microfluidics
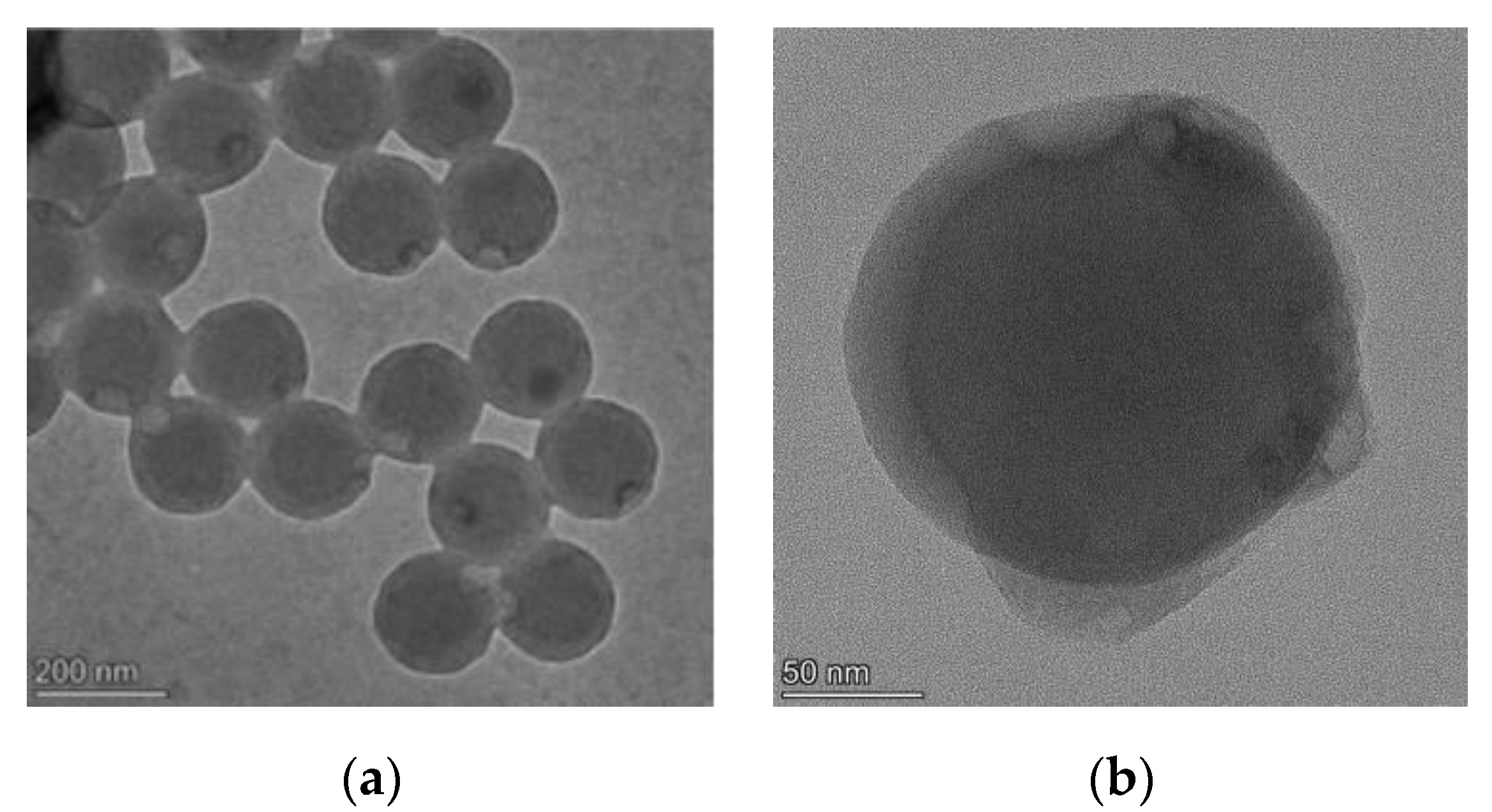
3.4.1. Optimization of the Microspheres
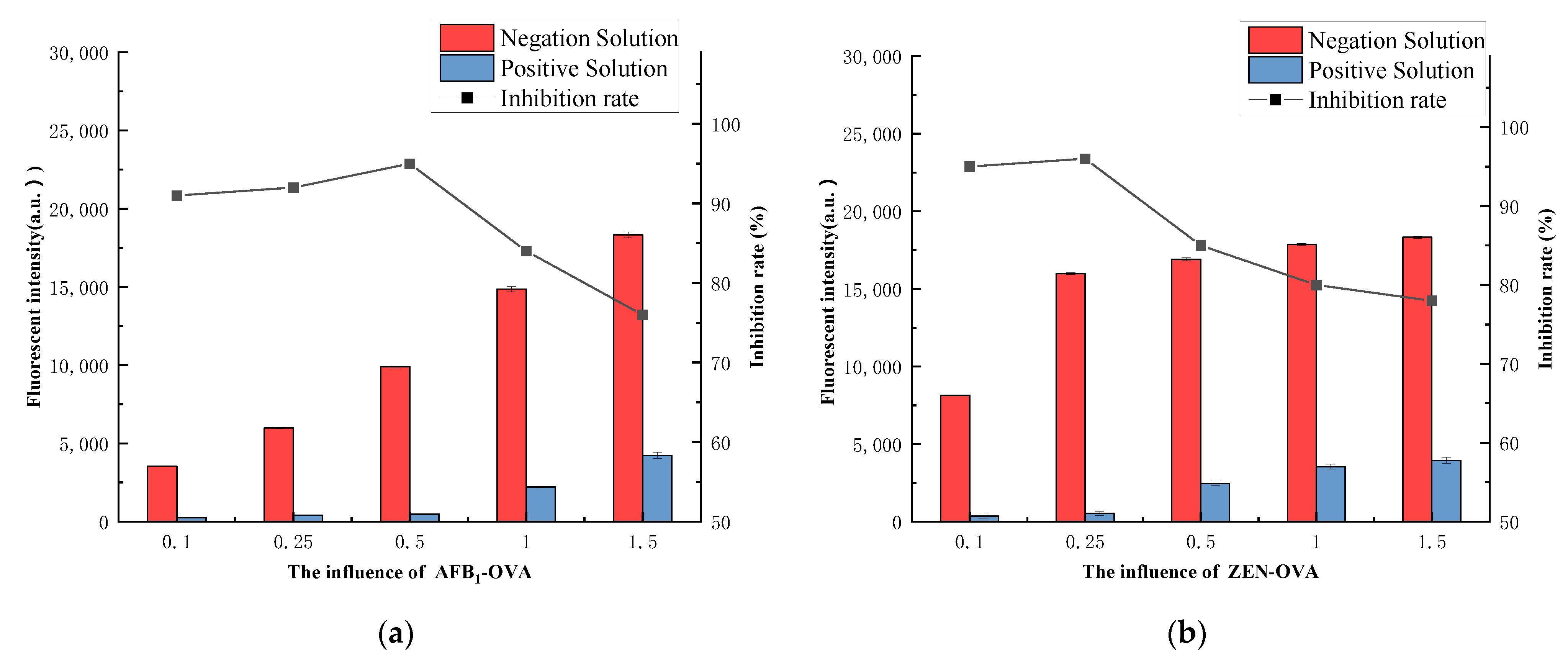
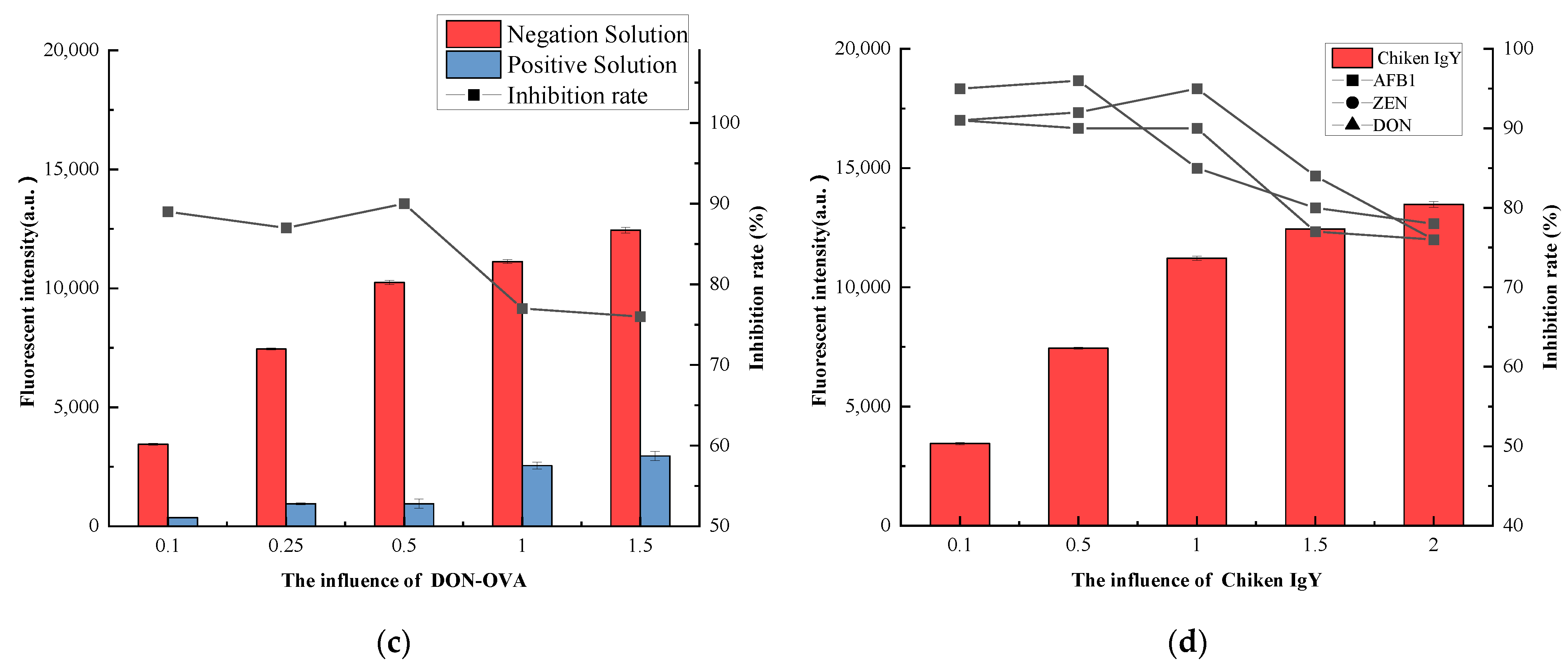
3.4.2. Coating Buffer Selection
3.4.3. Coating Antigen
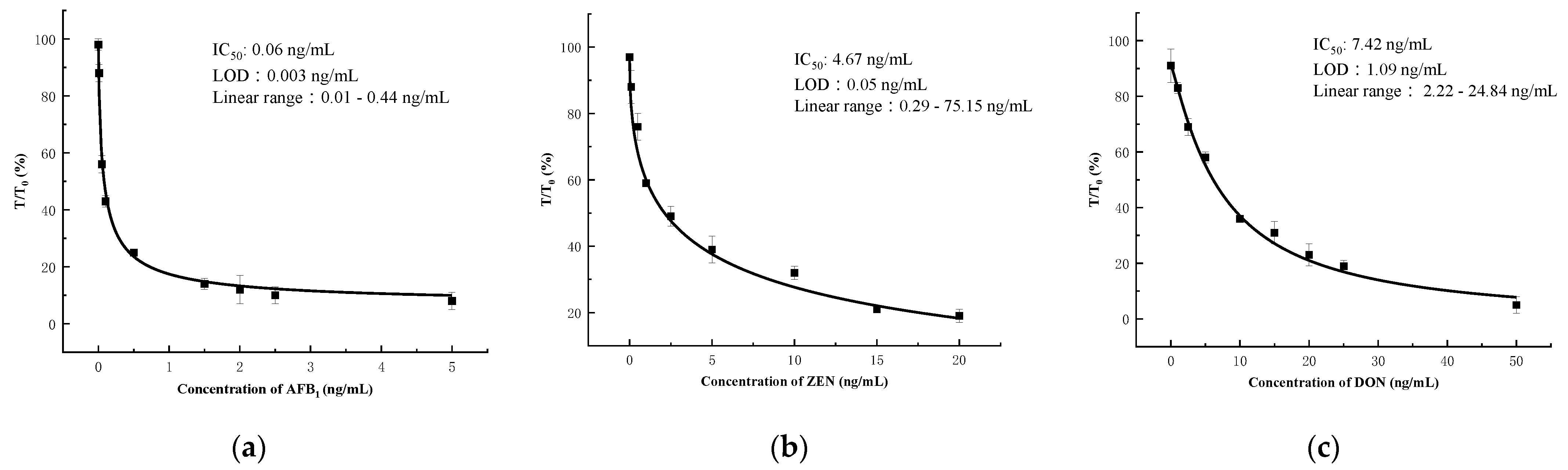
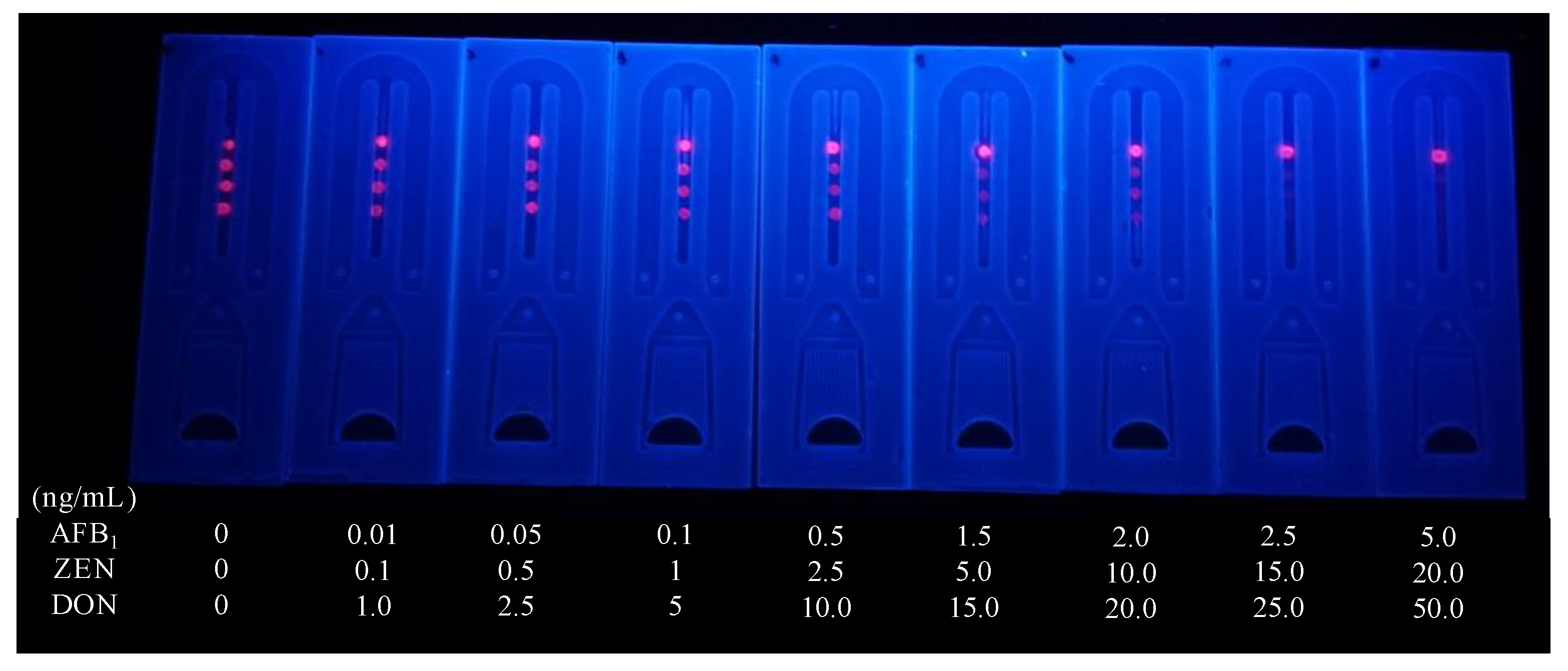
3.5. Sensitivity of the Microfluidics
3.6. Spiked Sample Analysis
3.7. Natural Sample Analysis
3.8. Comparison of the Microfluidics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rice, L.G.; Ross, P.F. Methods for Detection and Quantitation of Fumonisins in Corn, Cereal Products and Animal Excreta. J. Food Potection 1994, 57, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Dobson, A. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 41–158. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, X.; Jian, T.; Zhou, J.; Chu, F. Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int. J. Food Microbiol. 2005, 99, 185–194. [Google Scholar] [CrossRef]
- Balázs, A.; Faisal, Z.; Csepregi, R.; Kőszegi, T.; Kriszt, B.; Szabó, I.; Poór, M. In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein. Int. J. Mol. Sci. 2012, 22, 6281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. 2016, 197, 1271–1274. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Wei, Y.K.; Zhao, X.-M.; Li, M.-M.; Yu, J.-X.; Gurudeeban, S.; Hu, Y.-F.; Ji, G.-F.; Wei, D.-Q. Detoxification of aflatoxins on prospective approach: Effect on structural, mechanical, and optical properties under pressures. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 311–319. [Google Scholar] [CrossRef]
- Gambacorta, S.; Solfrizzo, H.; Visconti, A.; Powers, S.; Cossalter, A.M.; Pinton, P.; Oswald, I.P. Validation study on urinary biomarkers of exposure for aflatoxin B1, ochratoxin A, fumonisin B1, deoxynivalenol and zearalenone in piglet. World Mycotoxin J. 2013, 6, 299. [Google Scholar] [CrossRef]
- Wu, Q.; Kuča, K.; Humpf, H.U.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2016, 33, 79–91. [Google Scholar] [CrossRef]
- EU. Setting Maximum Levels for Certain Contaminants in Food Stuffs; Commission Regulation (EC) No 1881/ 2006; EU: Brussels, Beigium, 2006. [Google Scholar]
- Kong, W.; Wei, R.; Logrieco, A.F.; Wei, J.; Wen, J.; Xiao, X.; Yang, M. Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and spices in China markets. Food Chem. 2014, 146, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Camenzuli, L.; Van Dam, R.; de Rijk, T.; Andriessen, R.; Van Schelt, J.; Van der Fels-Klerx, H.J. Tolerance and Excretion of the Mycotoxins Aflatoxin B1, Zearalenone, Deoxynivalenol, and Ochratoxin A by Alphitobius diaperinus and Hermetia illucens from Contaminated Substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Development of a GC–MS/MS strategy to determine 15 mycotoxins and metabolites in human urine. Talanta 2014, 128, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhen, Z.; Lili, Q.; Yuanyuan, Z.; Shichun, P. Optimization of ELISA Method Conditions for Detection of AFB1. Acad. Period. Farm Prod. Process. 2009, 30, 205–212. [Google Scholar] [CrossRef]
- Ciccu, R.; Curreli, L.; Giuliani, S.; Manca, P.P.; Massacci, O. Optimization of an Integrated Flowsheet for Barite Processing. Acad. Period. Farm Prod. Process. 1987, 2, 281–293. [Google Scholar]
- Kong, D.; Xie, Z.; Liu, L.; Song, S.; Kuang, H.; Cui, G.; Xu, C. Development of indirect competitive ELISA and lateral-flow immunochromatographic assay strip for the detection of sterigmatocystin in cereal products. Food Agric. Immunol. 2016, 28, 260–273. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.C.; Savard, M.E.; Lau, R. Production of Monoclonal Antibodies for the Specific Detection of Deoxynivalenol and 15-Acetyldeoxynivalenol by ELISA. J. Agric. Food Chem. 1995, 43, 1740–1744. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, X.; Zhu, Y.; Shen, M.; Lu, Y.; Cheng, J.; Xu, Y. Rapid detection of four mycotoxins in corn using a microfluidics and microarray-based immunoassay system. Talanta 2018, 186, 299–305. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Shao, Y.; Huang, X.; Xiong, Y. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sens. Actuators 2019, 291, 411–417. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; De Saeger, S. Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin detection. Anal. Chim. Acta 2016, 955, 48–57. [Google Scholar] [CrossRef]
- Machado, J.M.D.; Soares, R.R.G.; Chu, V.; Conde, J.P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 2017, 99, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.G.; Santos, D.R.; Chu, V.; Azevedo, A.M.; Aires-Barros, M.R.; Conde, J.P. A point-of-use microfluidic device with integrated photodetector array for immunoassay multiplexing: Detection of a panel of mycotoxins in multiple samples. Biosens. Bioelectron. 2017, 87, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.N.; Duan, H.; Zhou, S.; Ma, T.T.; Guo, L.; Huang, X.L.; Xiong, Y.H. Biotin–streptavidin system-mediated ratiometric multiplex immunochromatographic assay for simultaneous and accurate quantification of three mycotoxins. J. Agric. Food Chem. 2019, 67, 9022–9031. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Li, P.W.; Zhang, Q.; Zhang, Z.W.; Zhang, W.; Jiang, J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal. Chem. 2017, 89, 11520–11528. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.L.; Ma, J.J.; Cheng, Y.Q.; Wang, H.G.; Sun, J.H.; Yan, Y.X. One-stop rapid detection of fumonisin B1, dexyonivalenol and zearalenone in grains. Food Control 2020, 117, 107. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.W.; Zhang, Q.; Zhang, W.; Yu, L.; Wang, D.; Li, H.; Li, P.W. An on-site simultaneous semi-Quantification of aflatoxin B1, zearalenone, and T-2 toxin in maize- and cereal-based feed via multicolor immunochromatographic assay. Toxins 2018, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Meneely, J.P.; Quinn, J.G.; Flood, E.M.; Hajslova, J.; Elliott, C.T. Simultaneous screening for T-2/HT-2 and deoxynivalenol in cereals using a surface plasmon resonance immunoassay. World Mycotoxin J. 2012, 5, 117–126. [Google Scholar] [CrossRef]
- Hervas, M.; Angel Lopez, M.; Escarpa, A. Electrochemical microfluidic chips coupled to magnetic bead-based ELISA to control allowable levels of zearalenone in baby foods using simplified calibration. Analyst 2009, 134, 2405–2411. [Google Scholar] [CrossRef]

| Mycotoxin Analyte | Structure | IC50 (ng/mL) | CR (%) |
|---|---|---|---|
| AFB1 | - | 0.08 | 100% |
| AFB2 | 0.67 | 12% | |
| AFM1 | 1.11 | 7% | |
| AFM2 | 10.7 | 1% | |
| AFG1 | 11.56 | 1% | |
| AFG2 | 15.53 | 1% | |
| ZEN, DON, FB1, OTA, T-2 | >10,000 | <0.1 | |
| ZEN | - | 6.41 | 100% |
| ZAN | 28.82 | 22% | |
| α-ZOL | 56.24 | 11% | |
| β-ZOL | 53.31 | 12% | |
| α-ZAL | 44.28 | 14% | |
| β-ZAL | 48.22 | 13% | |
| AFB1, DON, FB1, OTA, T-2 | >10,000 | <0.1 | |
| DON | - | 16.31 | 100% |
| 3-AcDON | 66.28 | 25% | |
| 15-AcDON | >10000 | <0.1 | |
| NIV | >10,000 | <0.1 | |
| ZEN, AFB1, FB1, OTA, T-2 | >10,000 | <0.1 |
| Target Analyte | AFB1 | ZEN | DON |
|---|---|---|---|
| Coating antigen | AFB1-OVA | ZEN-OVA | DON-OVA |
| Coating concentration (μg/mL) | 0.42 | 0.34 | 0.46 |
| Coating buffer | 0.05 M carbonate buffer (pH 9.6) | ||
| Coating condition | 14 h, 4 °C | ||
| MAb | Anti-AFB1 MAb | Anti-ZEN MAb | Anti-DON MAb |
| Standard analyte | AFB1 | ZEN | DON |
| Competition condition | 30 min, 37 °C | ||
| HRP- IgG dilution | 1:5000 | ||
| HRP-IgG incubation condition | 30 min, 37 °C | ||
| Analyte | Spiked Level (µg/kg) | Microfluidics Detection Level (µg/kg) | Visual | Ic-ELISA Detection Level (µg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Recovery Rate (%) | CV (%) | Mean ± SD | Recovery Rate (%) | CV (%) | |||
| AFB1 | 0 | ND | NC | NC | − − − | ND | NC | NC |
| 5 | 4.75 ± 0.01 | 95 | 1 | ± ± ± | 5.33 ± 0.35 | 107 | 7 | |
| 25 | 22.9 ± 1.18 | 92 | 5 | + + + | 25.08 ± 0.21 | 100 | 1 | |
| 50 | 50.65 ± 1.13 | 101 | 2 | + + + | 51.1 ± 0.39 | 102 | 1 | |
| ZEN | 0 | ND | NC | NC | − − − | ND | NC | NC |
| 50 | 52.28 ± 2.92 | 105 | 6 | ± ± ± | 50.66 ± 1.20 | 101 | 2 | |
| 250 | 258.05 ± 8.19 | 103 | 3 | + + + | 257.83 ± 11.11 | 103 | 4 | |
| 500 | 510.00 ± 6.68 | 102 | 1 | + + + | 513.32 ± 8.17 | 103 | 2 | |
| DON | 0 | ND | NC | NC | − − − | ND | NC | NC |
| 50 | 54.20 ± 2.31 | 108 | 4 | ± ± ± | 55.35 ± 2.56 | 111 | 5 | |
| 250 | 258.55 ± 3.45 | 103 | 1 | + + + | 261.65 ± 5.34 | 105 | 2 | |
| 500 | 512.68 ± 4.99 | 103 | 1 | + + + | 501.65 ± 5.13 | 100 | 1 | |
| Sample | LC-MS (μg/kg) | Microfluidics (μg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AFB1 | ZEN | DON | AFB1 | AFB1-Visual | ZEN | ZEN-Visual | DON | DON-Visual | |
| 1 * corn | 1.31 | 388.33 | 715.54 | 1.48 | ± a | - c | + | - | + |
| 2 * corn | 2.44 | 133.11 | 189.15 | 256 | ± | - | + | - | + |
| 3 * corn | 1.53 | 298.54 | 85.07 | 1.66 | ± | - | + | - | + |
| 4 * wheat | 52.81 | 634.36 | 2258.37 | - | + b | - | + | - | + |
| 5 * wheat | 3.62 | 1105.28 | 3125.8 | 4.02 | ± | - | + | - | + |
| 6 * wheat | 85.31 | 18.36 | 276.81 | - | + | 18.66 | ± | - | + |
| 7 * brown rice | 2.11 | 33.84 | 71.26 | 2.46 | ± | - | + | 70.55 | ± |
| 8 * brown rice | 2.32 | 18.42 | 65.54 | 2.64 | ± | 19.16 | + | 66.39 | ± |
| Method | Target Analytes | Test Sample | Mechanical System | vLOD | cLOD | Quantitative Device | Reference |
|---|---|---|---|---|---|---|---|
| Microfluidics | T2/OTA/AFB1/ ZEN | Corn, wheat | Syringe pump | - | 0.12/0.03/1.24/0.58 μg/kg (sample) | Fluorescent strip reader | [21] |
| Microfluidics | AFB1/OTA/ DON | Maize, wheat | Magnetic valves | - | 3/100/100 μg/kg (sample) | [22] | |
| Microfluidics | AFB1/OTA/ DON | feed | Syringe valves | - | <40/0.1~0.2/<10 ng/mL (buffer) | Smart phone | [23] |
| QDs-LFIA | FB1/AFB1/ OTA | Maize, rice, wheat | capillarity | - | 1.58/0.002/0.059 ng/mL (buffer) | ESE-Quant LFR Fluorescence reader | [24] |
| TRFMs-LFIA | ZEN/AFB1 | Maize | capillarity | - | 0.07/0.05 ng/mL (buffer) | Homemade portable Fluorescence | [25] |
| GNPs-LFIA | FB1/ZEN/ DON | Maize, wheat | capillarity | 60/6/12.5 ng/mL (buffer) | - | - | [26] |
| Multicolor-LFIA | ZEN/T-2/AFB1 | Maize, feed | capillarity | 2/30/0.5 ng/mL (buffer) | - | - | [27] |
| SPR chip | T-2/HT-2, DON | Wheat, maize | - | - | 12.1/29/31 ng/mL (sample) | SPR reader- | [28] |
| Microchip coupled with ELISA and electrochemical | ZEA | Corn | - | - | <1 μg/L | MK3 microplate reader- | [29] |
| Microfluidics | AFB1/ZEN/ DON | Corn, wheat, rice | capillarity | 1.5/5.0/10 ng/mL (buffer) | 0.003/0.05/1.09 ng/mL (buffer) | Fluorescent strip reader | present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lu, D.; Huang, Q.; Yang, J. Microfluidics-Based Time-Resolved Fluorescence Immunoassay for the On-Site Detection of Aflatoxins B1 Zearalenone and Deoxynivalenol in Cereals. Foods 2022, 11, 1319. https://doi.org/10.3390/foods11091319
Wang X, Lu D, Huang Q, Yang J. Microfluidics-Based Time-Resolved Fluorescence Immunoassay for the On-Site Detection of Aflatoxins B1 Zearalenone and Deoxynivalenol in Cereals. Foods. 2022; 11(9):1319. https://doi.org/10.3390/foods11091319
Chicago/Turabian StyleWang, Xu, Disha Lu, Qingfeng Huang, and Jinyi Yang. 2022. "Microfluidics-Based Time-Resolved Fluorescence Immunoassay for the On-Site Detection of Aflatoxins B1 Zearalenone and Deoxynivalenol in Cereals" Foods 11, no. 9: 1319. https://doi.org/10.3390/foods11091319
APA StyleWang, X., Lu, D., Huang, Q., & Yang, J. (2022). Microfluidics-Based Time-Resolved Fluorescence Immunoassay for the On-Site Detection of Aflatoxins B1 Zearalenone and Deoxynivalenol in Cereals. Foods, 11(9), 1319. https://doi.org/10.3390/foods11091319






