Study of the Efficacy of Probiotic Bacteria to Reduce Acrylamide in Food and In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Strains and Culture Preparation
2.2. Reagents
2.3. Preparation of Acrylamide Working Solutions
2.4. Preparation of Digestive Fluids
2.5. Acrylamide Reduction Ability in Chemical Solutions
2.5.1. Effects of Probiotic Strain, Acrylamide Concentration and Probiotic Concentration
2.5.2. Effects of Incubation Time
2.5.3. Effects of pH
2.6. Acrylamide Reduction Ability in Food Matrices
2.7. Acrylamide Reduction Ability under In-Vitro Digestion Model
2.8. Solid-Phase Extraction
2.9. LC-MS Quantification of Acrylamide
2.10. Statistical Analysis
3. Results and Discussion
3.1. Acrylamide Reduction by Probiotics in Chemical Solution
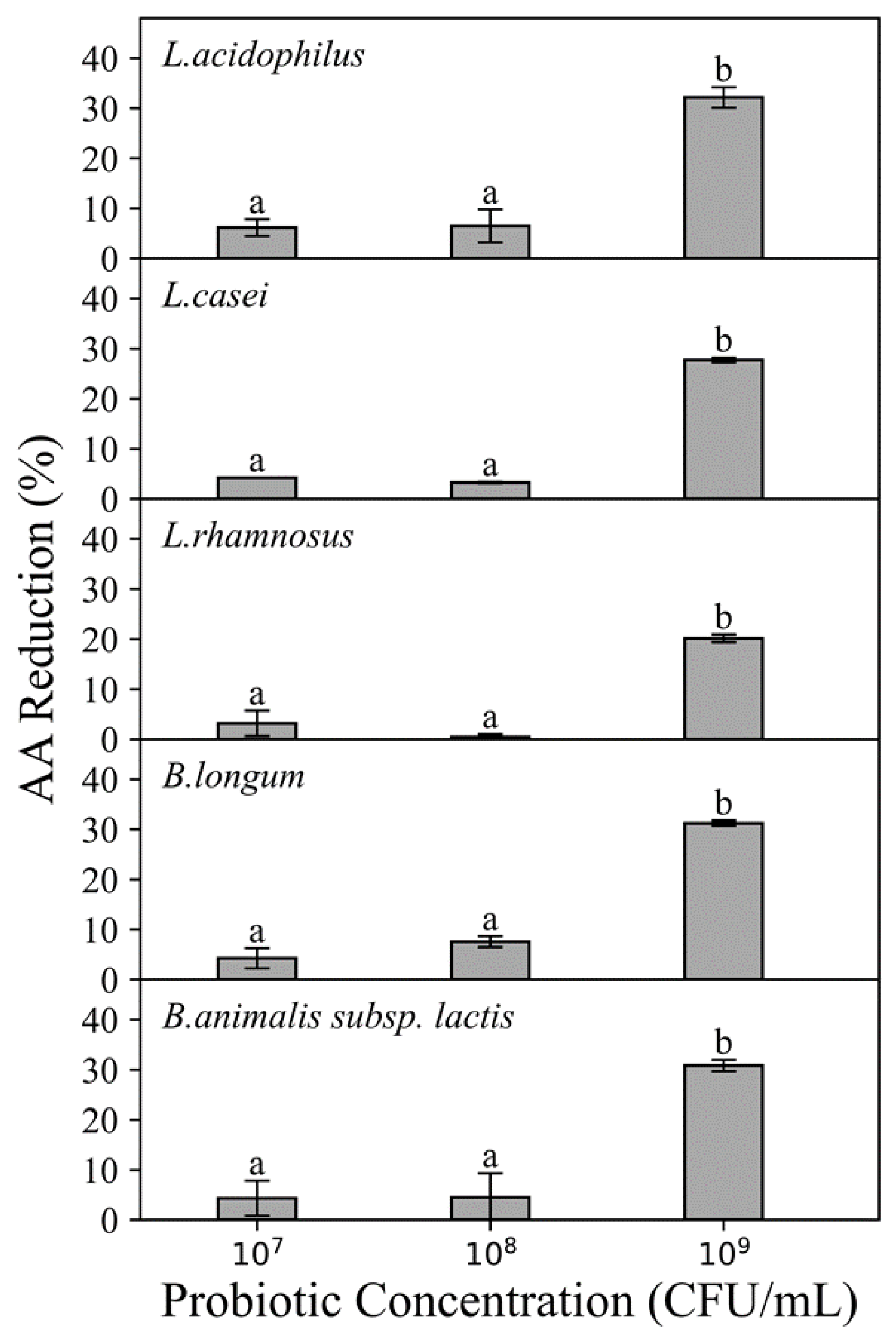
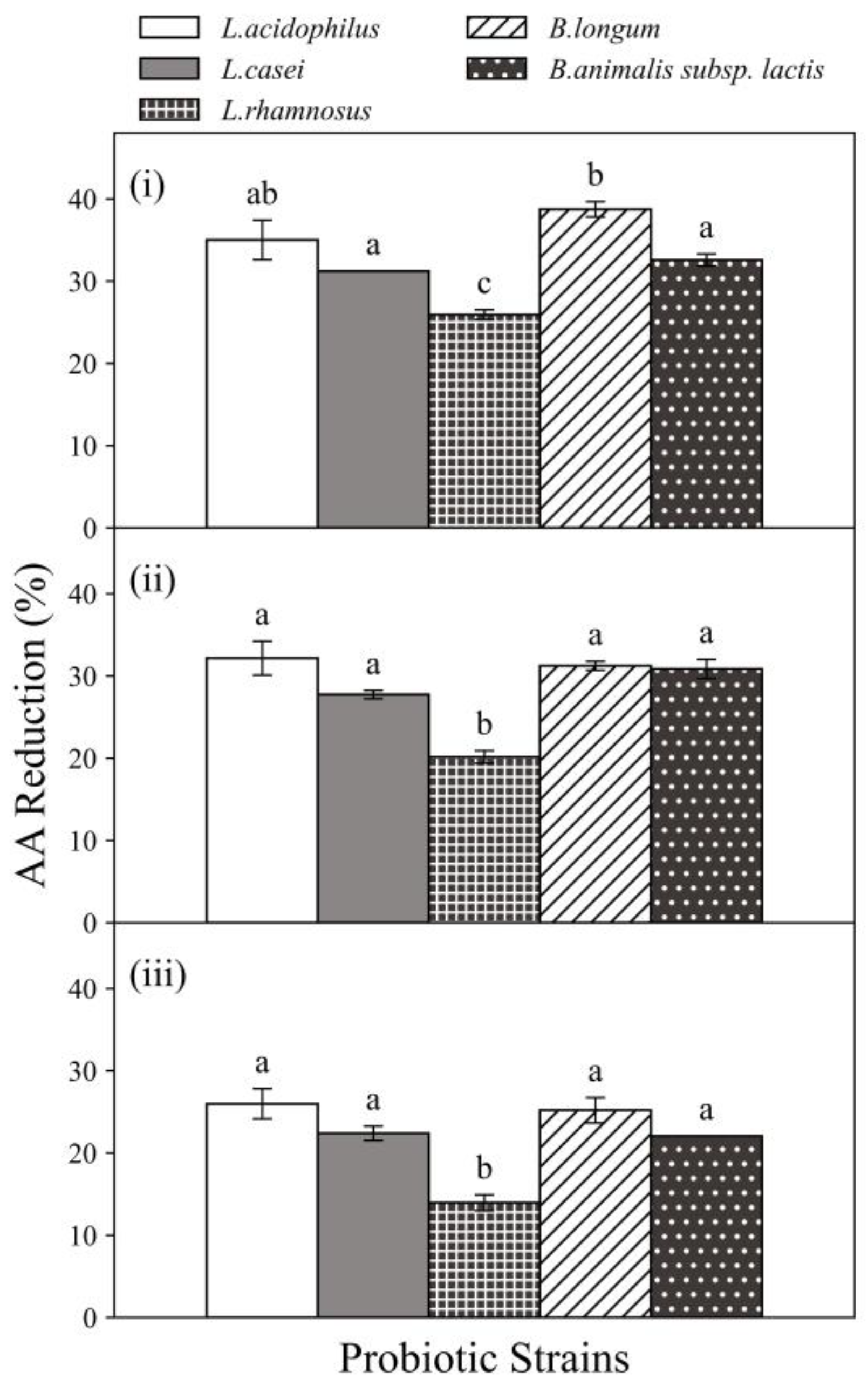
3.1.1. Effects of Probiotic Strains, AA Concentration and Probiotic Concentration
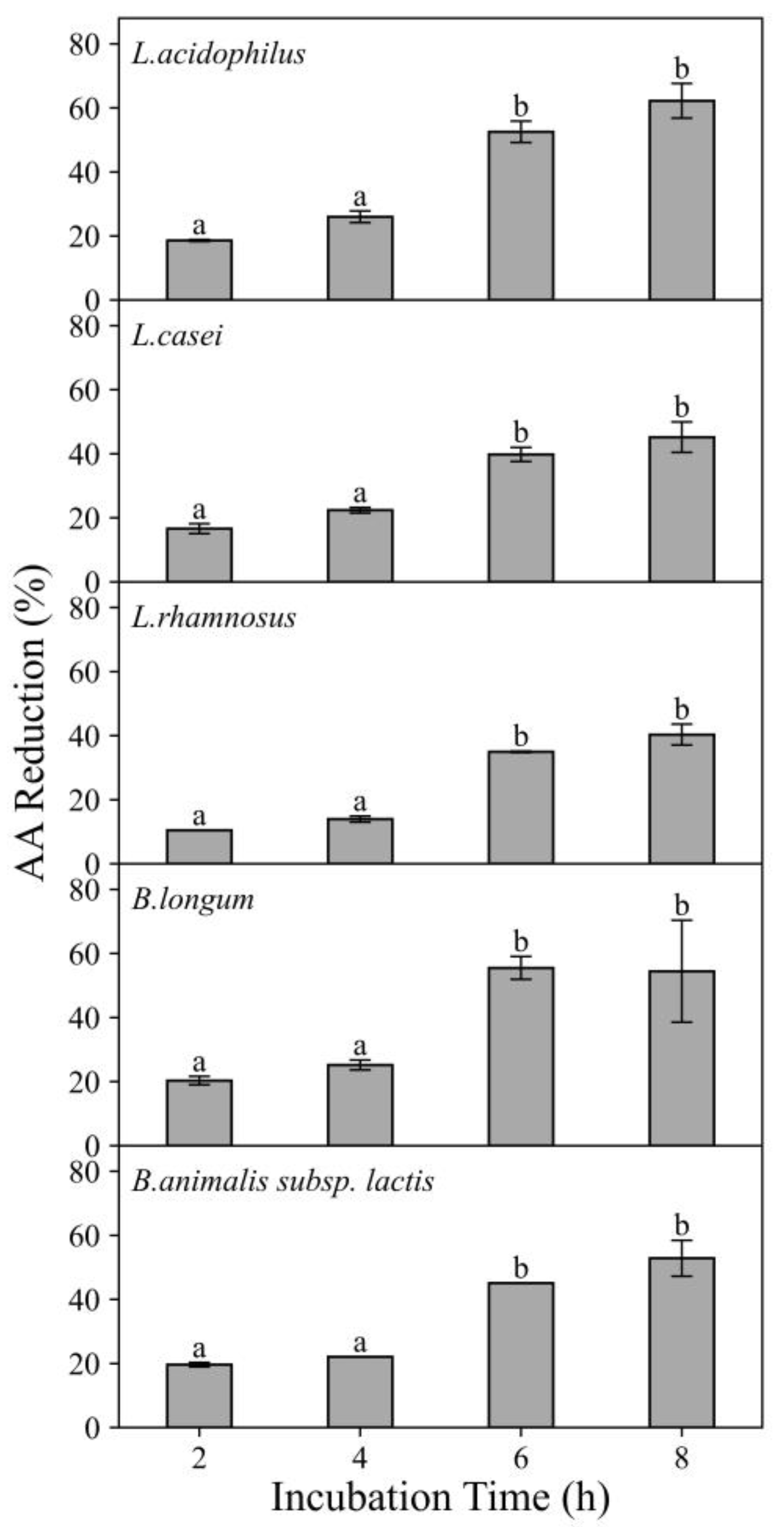
3.1.2. Effects of Incubation Time
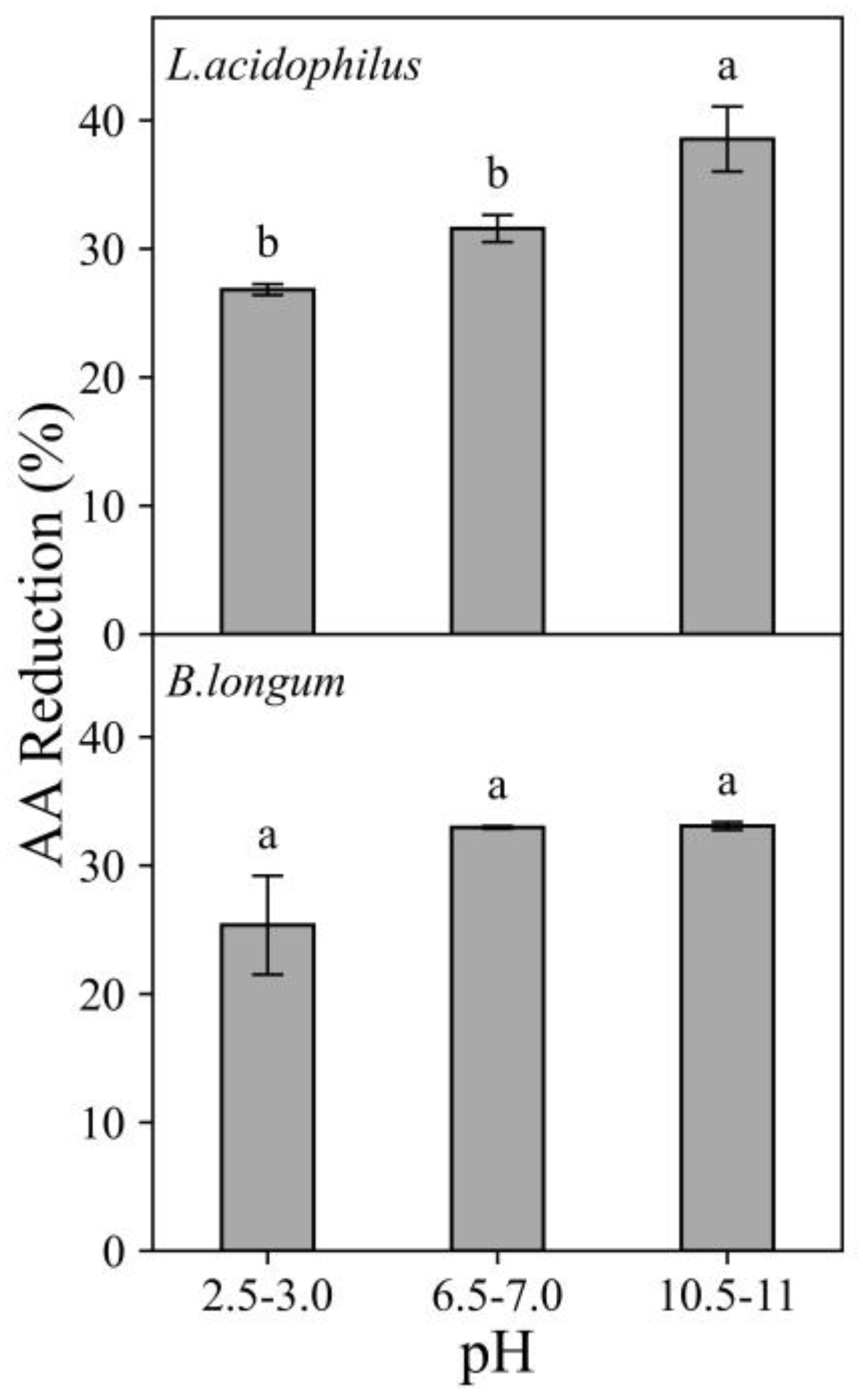
3.1.3. Effects of pH
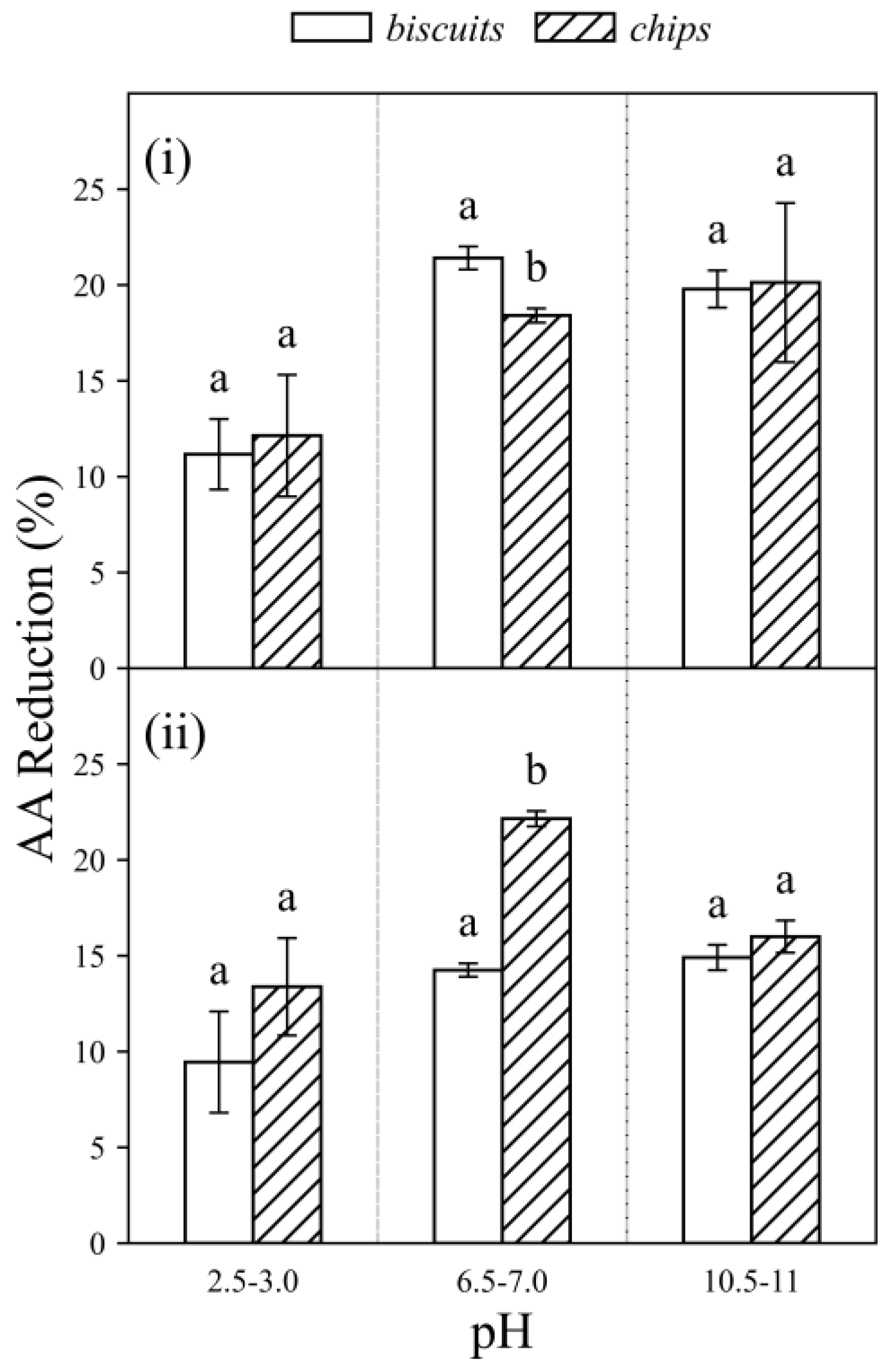
3.2. Acrylamide Reduction by Probiotics in Food Matrices
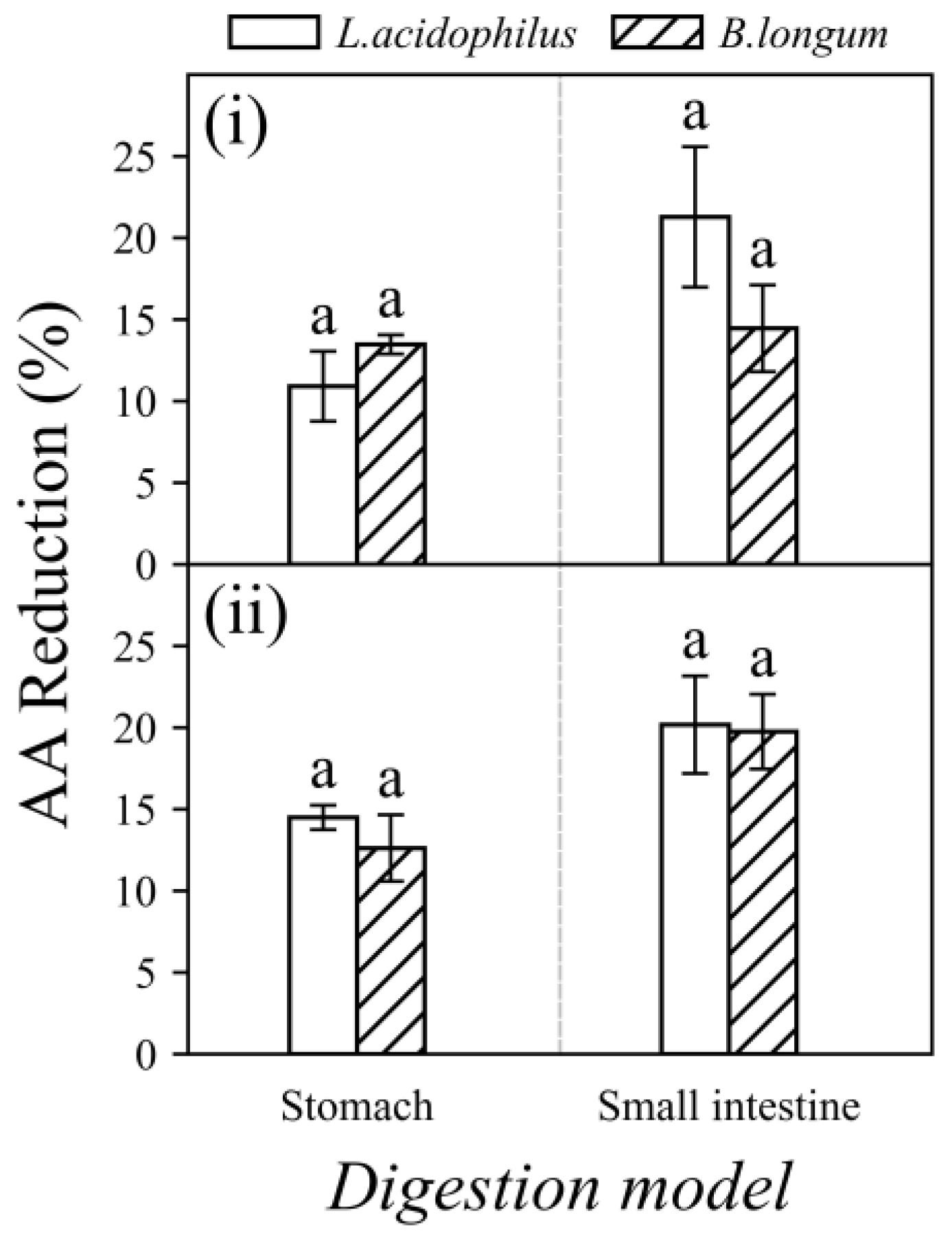
3.3. Acrylamide Reduction by Probiotics in Food Matrices under In Vitro Digestion
3.4. Risk Assessment of Acrylamide after Probiotic Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Duda-Chodak, A.; Wajda Tarko, T.; Sroka, P.; Satora, P. A review of the interactions between acrylamide, microorganisms and food components. Food Funct. 2016, 7, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Yaylayan, V.A.; Wnorowski, A.; Perez Locas, C. Why asparagine needs carbohydrates to generate acrylamide. J. Agric. Food Chem. 2003, 51, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Center for Food Safety, Hong Kong. The First Hong Kong Total Diet Study Report No. 6. The First Hong Kong Total Diet Study: Acrylamide; Center for Food Safety, Hong Kong: Hong Kong, China, 2013. Available online: https://www.cfs.gov.hk/english/programme/programme_firm/files/The_first_HKTDS_acrylamide_final_e.pdf (accessed on 1 March 2022).
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; Cantú-Cornelio, F.; González-Córdova, A.F.; Vallejo-Córdoba, B.; Hernández-Mendoza, A.; García, H.S. In vitro reduced availability of aflatoxin B1 and acrylamide by bonding interactions with teichoic acids from Lactobacillus strains. LWT-FOOD Sci. Technol. 2015, 64, 1334–1341. [Google Scholar] [CrossRef]
- Versantvoort, C.H.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.; Sips, A.J. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Center for Food Safety, Hong Kong. Risk Assessment Studies Report No. 43. Dietary Exposure to Acrylamide of Hong Kong Adult Population; Center for Food Safety, Hong Kong: Hong Kong, China, 2010. Available online: https://www.cfs.gov.hk/english/programme/programme_rafs/programme_rafs_fc_01_25.html (accessed on 1 March 2022).
- Kim, C.T.; Hwang, E.-S.; Lee, H.J. An improved LC-MS/MS method for the quantitation of acrylamide in processed foods. Food Chem. 2007, 101, 401–409. [Google Scholar] [CrossRef]
- Rivas-Jimenez, L.; Ramírez-Ortiz, K.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Garcia, H.S.; Hernandez-Mendoza, A. Evaluation of acrylamide-removing properties of two Lactobacillus strains under simulated gastrointestinal conditions using a dynamic system. Microbiol. Res. 2016, 190, 19–26. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Li, L.; Zhao, H.-Y.; Sun, H.-Y.; Meng, M.-H.; Zhang, S.; Shao, M.-L. Key role of peptidoglycan on acrylamide binding by lactic acid bacteria. Food Sci. Biotechnol. 2017, 26, 271–277. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Zhao, X.; Sun, H.; Shao, M.; Xu, H. In vitro adsorption mechanism of acrylamide by lactic acid bacteria. LWT 2019, 100, 119–125. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014, 13 (Suppl. 1), S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A.; García, H.S. In vitro Study of the Potential Protective Role of Lactobacillus Strains by Acrylamide Binding. J. Food Saf. 2014, 34, 62–68. [Google Scholar] [CrossRef]
- Alrabadi, N.I.; Al-Jubury, E.M.; Thalij, K.M.; Hajeej, J.M. Lactobacillus rhamnosus ability of aflatoxin detoxification. Jordan J. Biol. Sci. 2018, 11, 87–92. [Google Scholar]
- Trinder, M.; McDowell, T.W.; Daisley, B.A.; Ali, S.N.; Leong, H.S.; Sumarah, M.W.; Reid, G. Probiotic Lactobacillus rhamnosus Reduces Organophosphate Pesticide Absorption and Toxicity to Drosophila melanogaster. Appl. Environ. Microbiol. 2016, 82, 6204–6213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Huang, C.P.; Morehart, A.L. Proton competition in Cu(II) adsorption by fungal mycelia. Water Res. 1991, 25, 1365–1375. [Google Scholar] [CrossRef]
- Center for Food Safety, Hong Kong. Acrylamide in Some Popular Foods; Center for Food Safety, Hong Kong: Hong Kong, China, 2010. Available online: https://www.cfs.gov.hk/english/programme/programme_rafs/programme_rafs_fc_01_29_Acrylamide_in_Some_Popular_Foods.html (accessed on 1 March 2022).
- Center for Food Safety, Hong Kong. Trade Guidelines on Reducing Acrylamide in Food; Center for Food Safety, Hong Kong: Hong Kong, China, 2013. Available online: https://www.cfs.gov.hk/english/food_leg/files/Acrylamide_E_New_3.pdf (accessed on 1 March 2022).
- Ibrahim, F.; Halttunen, T.; Tahvonen, R.; Salminen, S. Probiotic bacteria as potential detoxification tools: Assessing their heavy metal binding isotherms. Can. J. Microbiol. 2006, 52, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Hamzalıoğlu, A.; Gökmen, V. Investigation of the reactions of acrylamide during in vitro multistep enzymatic digestion of thermally processed foods. Food Funct. 2015, 6, 108–113. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.M.; Yang, L.; Chang, Y.; Chu, I.K.; Dong, N. Study of the Efficacy of Probiotic Bacteria to Reduce Acrylamide in Food and In Vitro Digestion. Foods 2022, 11, 1263. https://doi.org/10.3390/foods11091263
Choi SM, Yang L, Chang Y, Chu IK, Dong N. Study of the Efficacy of Probiotic Bacteria to Reduce Acrylamide in Food and In Vitro Digestion. Foods. 2022; 11(9):1263. https://doi.org/10.3390/foods11091263
Chicago/Turabian StyleChoi, Siu Mei, Ling Yang, Yuxuan Chang, Ivan K. Chu, and Naiping Dong. 2022. "Study of the Efficacy of Probiotic Bacteria to Reduce Acrylamide in Food and In Vitro Digestion" Foods 11, no. 9: 1263. https://doi.org/10.3390/foods11091263
APA StyleChoi, S. M., Yang, L., Chang, Y., Chu, I. K., & Dong, N. (2022). Study of the Efficacy of Probiotic Bacteria to Reduce Acrylamide in Food and In Vitro Digestion. Foods, 11(9), 1263. https://doi.org/10.3390/foods11091263





