Arsenic in Portuguese Rice: Is There Any Risk?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Standards, Chemicals and Materials
2.3. Digestion Procedure and Analysis
2.4. Risk Assessment Calculation
2.5. Statistical Analysis
3. Results
3.1. Validation of the Analytical Methodology
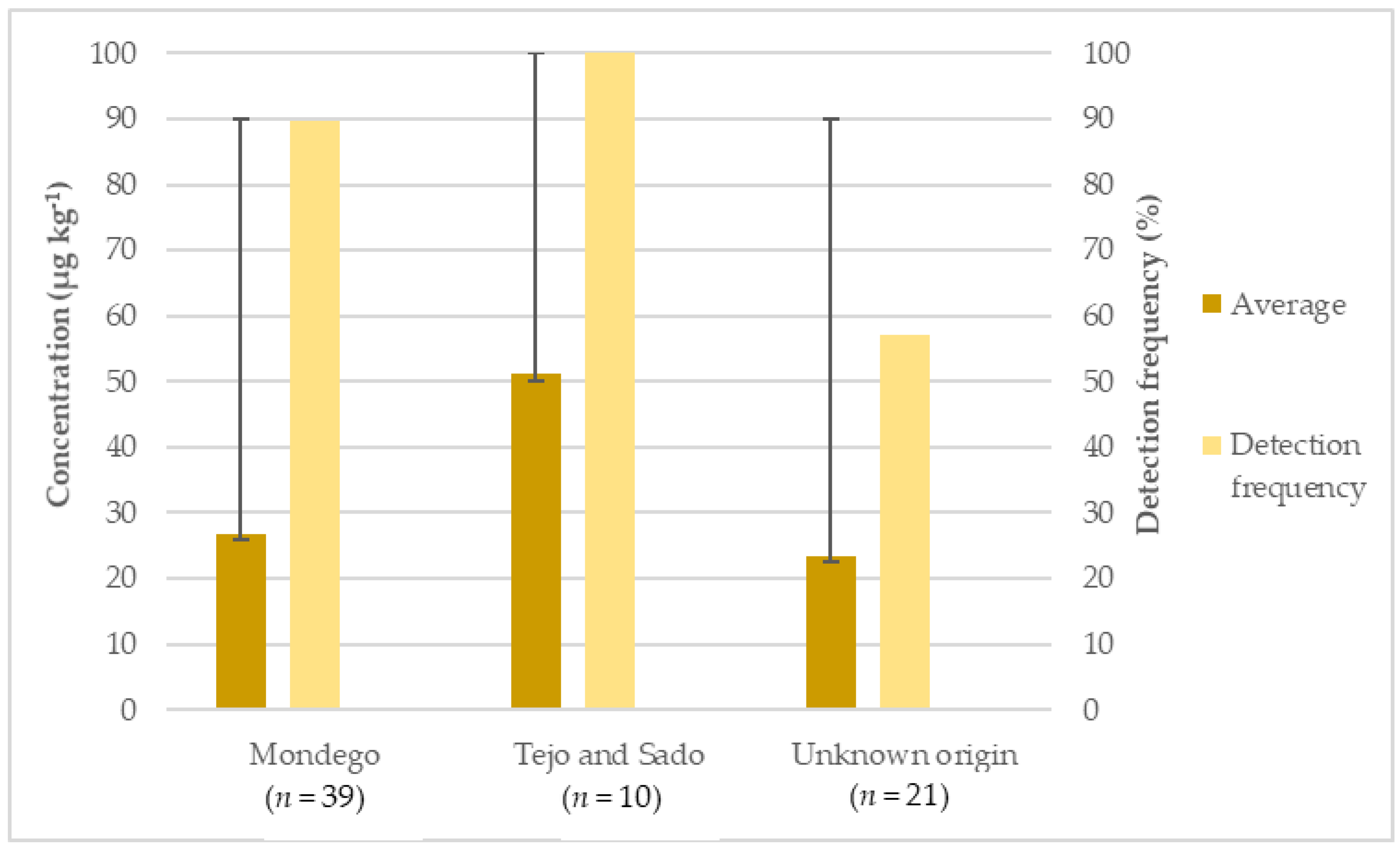
3.2. Occurrence of Inorganic Arsenic in Rice Samples
3.3. Comparison with Other Studies
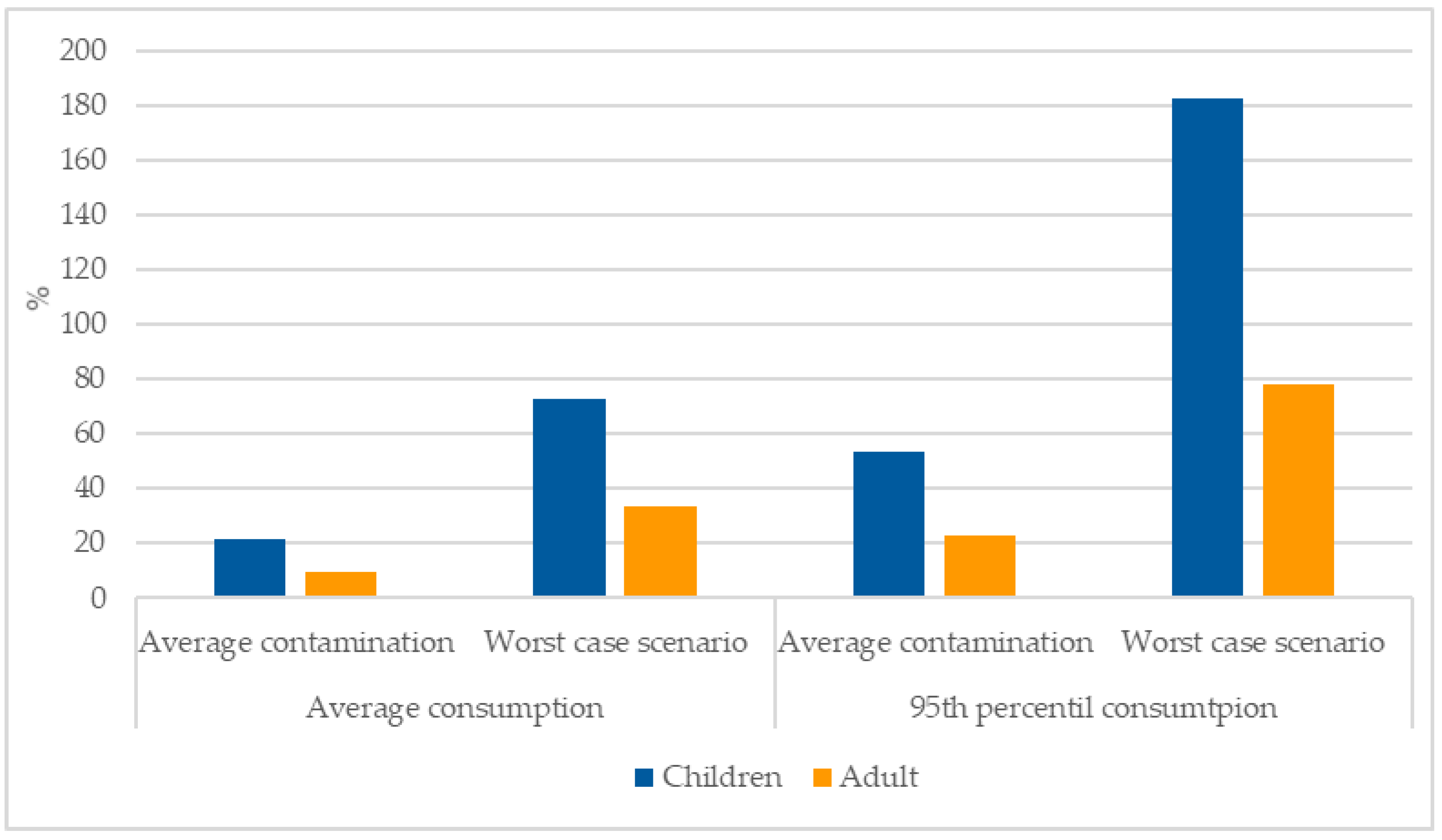
3.4. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreiro, M.; Antunes, H. Segurança alimentar e sustentabilidade: O caso do setor do arroz no Vale do Tejo e Sorraia: Perceção e práticas. Master’s Thesis, University Institue of Lisbon, Lisbon, Portugal, 2017. [Google Scholar] [CrossRef][Green Version]
- Cardoso, M. Segurança alimentar, ajuda pública ao desenvolvimento e pobreza. In Proceedings of the 7th Congress of African Studies, Lisboa, Portugal, 9–11 September 2010. CIEA7 #22. [Google Scholar]
- Souza, J.M.O.; Carneiro, M.F.H.; Carolina, A.; Paulelli, C.; Grotto, D.; Magalhães, A.M. Arsénio e arroz: Toxicidade, metabolismo e segurança alimentar. Quim. Nova 2014, 38, 118–127. [Google Scholar]
- International Agency for Research on Cancer(IARC) Arsenic and Arsenic Compounds Monograph. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 41–93.
- Andrade, D.; Rocha, M. A toxicidade do arsenio e a sua natureza. Rev. Acadêmica Oswaldo Cruz 2016, 3, 102–111. [Google Scholar]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- WHO. Exposure to Arsenic: A Major Public Health Concern. Agriculture 2010, 79, 90–100. [Google Scholar] [CrossRef]
- de Oliveira, R.M.; Antunes, A.C.N.; Vieira, M.A.; Medina, A.L.; Ribeiro, A.S. Evaluation of sample preparation methods for the determination of As, Cd, Pb, and Se in rice samples by GF AAS. Microchem. J. 2016, 124, 402–409. [Google Scholar] [CrossRef]
- Garcia, I. Influência de Diferentes Variedades de Arroz Carolino no Seu Comportamento em Cozedura. Ph.D. Thesis, Istituto Politécnico de Coimbra, Coimbra, Portugal, 2017. [Google Scholar]
- FAO. Nations–Crops. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 3 November 2021).
- De Jesus, M.G. Caracterização das Diferentes Designações Comerciais de Arroz. Master’s Thesis, University of Oporto, Oporto, Portugal, 2019. [Google Scholar]
- Coelho, I.; Gueifão, S.; Pinto, T.; Castanheira, I. Estudos de especiação de arsénio em arroz. Obs. Epidemiológico 2015, 2, 6–7. [Google Scholar]
- Instituto Nacional de Estatiscica, I. Estatísticas Agrícolas 2018. 2019. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=320461359&PUBLICACOESmodo=2 (accessed on 21 December 2021).
- Hojsak, I.; Braegger, C.; Bronsky, J.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; Mis, N.F.; Mihatsch, W.; et al. Arsenic in rice: A cause for concern. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 142–145. [Google Scholar] [CrossRef]
- Fernandes, R.M. Efeito da temperatura e humidade no rendimento industrial de arroz das variedades Ariete, Euro, Gládio e Sírio. Master’s Thesis, Instituto Superior de Agronomia University of Lisbon, Lisbon, Portugal, 2015. [Google Scholar]
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.S.; Meharg, A.; Bundschuh, J. Arsenic in cooked rice foods: Assessing health risks and mitigation options. Environ. Int. 2019, 127, 584–591. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37. [Google Scholar] [CrossRef]
- Torres-Escribano, S.; Leal, M.; Vélez, D.; Montoro, R. Total and Inorganic Arsenic Concentrations in Rice Sold in Spain, Effect of Cooking, and Risk Assessments. Environ. Sci. Technol. 2008, 42, 3867–3872. [Google Scholar] [CrossRef]
- Simões, A.C.P. Avaliação da Presença de Arsénio em Arroz e Produtos Derivados de Arroz. Master’s Thesis, Instituto Superior de Agronomia University of Lisbon, Lisbon, Portugal, 2014. [Google Scholar]
- Comissão Europeia Regulamento (UE) 2015/1006 da Comissão de 25 de junho de 2015 que altera o Regulamento (CE) no 1881/2006 no que diz respeito aos teores máximos de arsénio na forma inorgânica nos géneros alimentícios. J. Of. da União Eur. 2015, 2015, 1993–1995.
- Pétursdóttir, Á.H.; Friedrich, N.; Musil, S.; Raab, A.; Gunnlaugsdóttir, H.; Krupp, E.M.; Feldmann, J. Hydride generation ICP-MS as a simple method for determination of inorganic arsenic in rice for routine biomonitoring. Anal. Methods 2014, 6, 5392–5396. [Google Scholar] [CrossRef]
- Ashmore, E.; Molyneux, S.; Watson, S.; Miles, G.; Pearson, A. Inorganic arsenic in rice and rice products in New Zealand and Australia. Food Addit. Contam. Part B Surveill. 2019, 12, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Simões, B.D.F.; Lino, C.; Pena, A. Exposure to nickel through commercial premade baby foods: Is there any risk? J. Food Compos. Anal. 2020, 92, 103541. [Google Scholar] [CrossRef]
- WHO; FAO; UN. Dietary exposure assessment of chemicals in food. Principles and Methods for the Risk Assessment of Chemicals in Food 2009. Available online: http://apps.who.int/iris/bitstream/handle/10665/44027/9789241597470_eng.pdf;jsessionid=5117B891C99D05F7C349286B54C0BC7C?sequence=1 (accessed on 21 December 2021).
- Lopes, C.; Torres, D.; Oliveira, A.; Severo, M.; Alarcão, V.; Guiomar, S.; Mota, J.; Teixeira, P.; Ramos, E.; Rodrigues, S.; et al. Inquérito Alimentar Nacional e de Atividade Física, IAN-AF 2015–2016; University of Oporto: Oporto, Portugal, 2017; ISBN 978-989-746-181-1. [Google Scholar]
- DGS. Circular Normativa N°: 05/DSMIA. Consultas de Vigilância de Saúde Infantil e Juvenil-Actualização das Curvas de Crescimento; Direcção-Geral da Saúde: Lisbon, Portugal, 2006. [Google Scholar]
- Magdeburg, A.; Stalter, D.; Oehlmann, J. Whole effluent toxicity assessment at a wastewater treatment plant upgraded with a full-scale post-ozonation using aquatic key species. Chemosphere 2012, 88, 1008–1014. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Carey, M.; Meharg, A.A. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016, 191, 128–134. [Google Scholar] [CrossRef]
- Zavala, Y.J.; Duxbury, J.M. Arsenic in rice: I. Estimating normal levels of total arsenic in rice grain. Environ. Sci. Technol. 2008, 42, 3856–3860. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Carey, M.; Carbonell-Barrachina, A.A.; Moreno-Jiménez, E.; Green, A.J.; Meharg, A.A. Geographical variation in inorganic arsenic in paddy field samples and commercial rice from the Iberian Peninsula. Food Chem. 2016, 202, 356–363. [Google Scholar] [CrossRef]
- Lamont, W.H. Concentration of inorganic arsenic in samples of white rice from the United States. J. Food Compos. Anal. 2003, 16, 687–695. [Google Scholar] [CrossRef]
- Carbonell-barrachina, Á.A.; Wu, X.; Ramírez-gandolfo, A.; Norton, G.J.; Burló, F.; Deacon, C.; Meharg, A.A. Inorganic arsenic contents in rice-based infant foods from Spain. Environ. Pollut. 2012, 163, 77–83. [Google Scholar] [CrossRef]
- Ruttens, A.; Cheyns, K.; Blanpain, A.C.; De Temmerman, L.; Waegeneers, N. Arsenic speciation in food in Belgium. Part 2: Cereals and cereal products. Food Chem. Toxicol. 2018, 118, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Guillod-Magnin, R.; Brüschweiler, B.J.; Aubert, R.; Haldimann, M. Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1164–1178. [Google Scholar] [CrossRef]
- Panebianco, F.; Nava, V.; Giarratana, F.; Gervasi, T.; Cicero, N. Assessment of heavy- and semi-metals contamination in edible seaweed and dried fish sold in ethnic food stores on the Italian market. J. Food Compos. Anal. 2021, 104, 104150. [Google Scholar] [CrossRef]
- Mac Monagail, M.; Morrison, L. Arsenic Speciation in a Variety of Seaweeds and Associated Food Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–310. [Google Scholar]
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 2021, 263, 127983. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3597. [Google Scholar] [CrossRef]
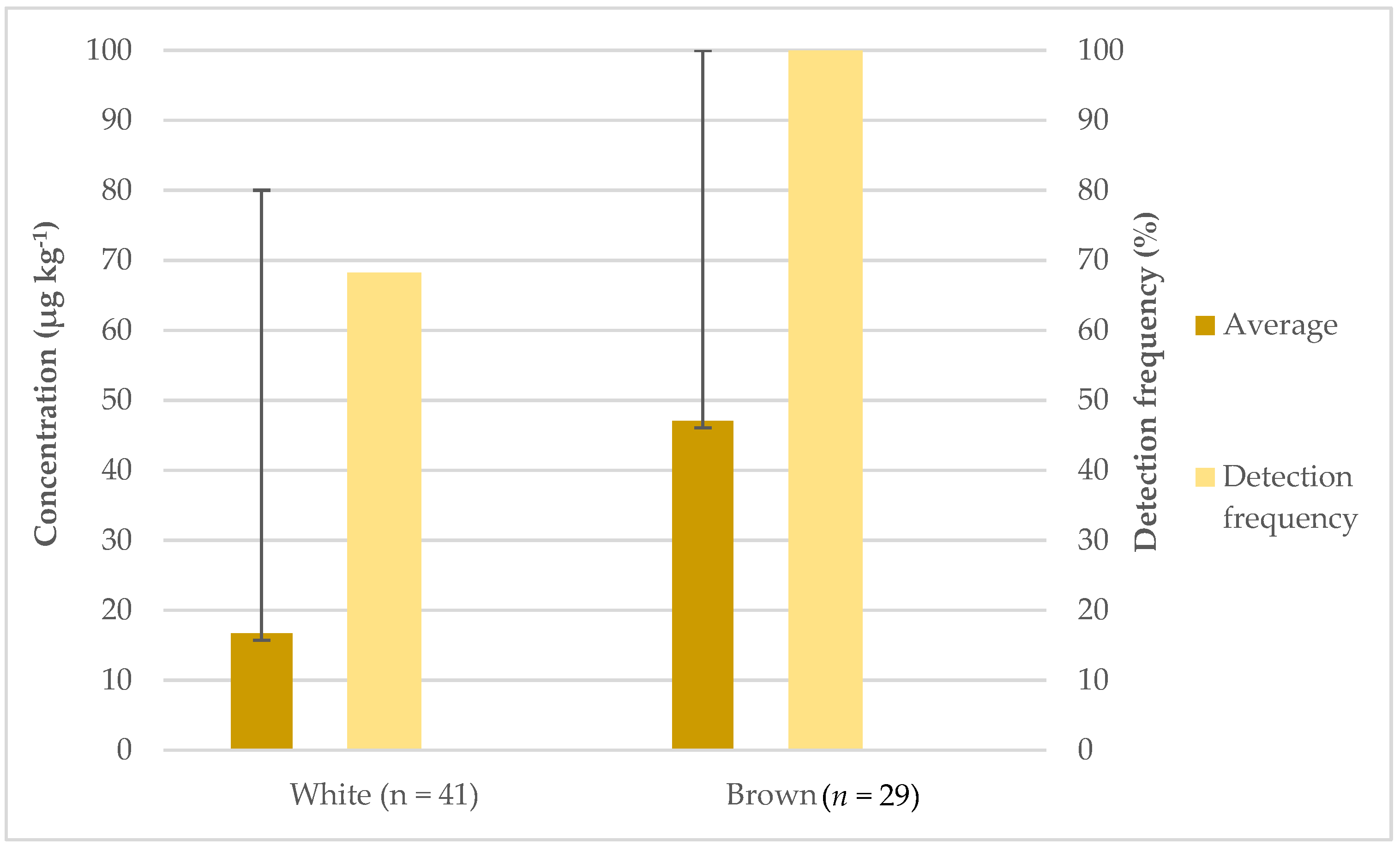

| Foodstuff | Maximum Residue Limit (mg kg−1) |
|---|---|
| Non-parboiled milled rice (polished or white rice) | 0.20 |
| Parboiled rice and husked rice | 0.25 |
| Rice waffles, rice wafers, rice crackers and rice cakes | 0.30 |
| Rice destined for the production of food for infants and young children | 0.10 |
| Type of Rice | Country | Year | Number of Samples | Detection Frequency (%) | Range and Standard Deviation (µg kg−1) | Mean Concentration and Standard Deviation (µg kg−1) | References |
|---|---|---|---|---|---|---|---|
| Long | Scotland a | 2014 | 44 b | 100 | NA | 94 ± 1 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 52 ± 10 | [22] | |
| Scotland a | 2014 | 44 b | 100 | NA | 61 ± 4 | [22] | |
| Long-Agulha | Spain | 2007 | 39 b | 97 | NA | 350 ± 16 | [19] |
| United States of America | 2003 | 40 b | NA | 33–271 | NA | [33] | |
| Long-Agulha brown | Spain | 2007 | 39 b | 97 | NA | 230 ± 20 | [19] |
| Long-Basmati | Scotland a | 2014 | 44 b | 100 | NA | 53 ± 7 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 69 ± 9 | [22] | |
| Punjab, India | 2014 | 10 b | 100 | NA | 12.0 ± 5.48 | [20] | |
| India | 2007 | 39 b | 97 | NA | 67 ± 1 | [19] | |
| Belgium a | 2018 | 5 | 100 | 19 ± 8–48 ± 21 | 30 ± 10 | [35] | |
| Long-Basmati Brown | Spain | 2007 | 39 b | 97 | NA | 148 ± 4 | [19] |
| Long-Steamed | Iberian Peninsula | 2016 | 11 | 100 | 22–70 | 83 (median) | [30] |
| Long-Thai | Thailand | 2007 | 39 b | 97 | NA | 175 ± 8 | [19] |
| Belgium a | 2018 | 7 | 100 | 63 ± 16–147 ± 37 | 77 ± 32 | [35] | |
| Long-Thai jasmine | Scotland a | 2014 | 44 b | 100 | NA | 64 ± 3 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 62 ± 3 | [22] | |
| NA | 2014 | 10 b | 100 | NA | 85.5 ± 10.39 | [20] | |
| Thailand | 2014 | 10 b | 100 | NA | 62.6 ± 6.21 | [20] | |
| Medium grain | Spain | 2007 | 39 b | 97 | NA | 367 ± 4 | [19] |
| United States of America | 2003 | 40 b | NA | 46–114 | NA | [33] | |
| Medium grain-Brown | Spain | 2007 | 39 b | 97 | NA | 145 ± 5 | [19] |
| NA-Baby rice | United Kingdom | 2014 | 29 | 100 | 63–268 | NA | [30] |
| NA-White | Switzerland a | 2018 | 27 | NA | 5.6–188 | 94 | [36] |
| New Zealand and Australia a | 2019 | 36 | 100 | 40–100 | 70 | [23] | |
| Belgium a | 2018 | 7 | 100 | 67 ± 18–245 ± 64 | 172 ± 81 | [35] | |
| NA-Brown | Scotland a | 2014 | 44 b | 100 | NA | 137 ± 5 | [22] |
| Iberian Peninsula | 2016 | 20 | 100 | 53–47 | 157 (median) | [30] | |
| Switzerland a | 2018 | 4 | NA | 117–172 | 152 | [36] | |
| New Zealand and Australia a | 2019 | 21 | 85 | <20–120 | 90 | [23] | |
| Belgium a | 2018 | 5 | 100 | 119 ± 32–243 ± 67 | 167 ± 47 | [35] | |
| NA-Infant Rice | China | 2011 | 14 | 100 | 52–247 | 114 ± 15 | [34] |
| USA | 2011 | 5 | 100 | 93–159 | 125 ± 14 | [34] | |
| United Kingdom | 2011 | 5 | 100 | 107–267 | 162 ± 29 | [34] | |
| Spain | 2011 | 7 | 100 | 10–111 | 85 ± 10 | [34] | |
| New Zealand and Australia a | 2019 | 15 | 100 | 40–140 | 70 | [23] | |
| NA-Polished | Iberian Peninsula | 2016 | 113 | 100 | 27–75 | 71 (median) | [30] |
| Short-Carolino | Tejo, Portugal | 2014 | 10 b | 100 | NA | 300.8 ± 31.79 | [20] |
| Mondego, Portugal | 2014 | 10 b | 100 | NA | 242.6 ± 32.97 | [20] | |
| Portugal | 2014 | 10 b | 100 | NA | 217.5 ± 15.94 | [20] | |
| Tejo, Portugal | 2014 | 10 b | 100 | NA | 224.3 ± 32.97 | [20] | |
| Short-Japanese | Scotland a | 2014 | 44 b | 100 | NA | 99 ± 5 | [22] |
| Short-Paella | Scotland a | 2014 | 44 b | 100 | NA | 70 ± 3 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 67 ± 3 | [22] | |
| Short-Risotto | Scotland a | 2014 | 44 b | 100 | NA | 120 ± 18 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 114 ± 10 | [22] | |
| Italy | 2014 | 10 b | 100 | NA | 53.2 ± 8.21 | [20] | |
| Long | Scotland a | 2014 | 44 b | 100 | NA | 94 ± 1 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 52 ± 10 | [22] | |
| Scotland a | 2014 | 44 b | 100 | NA | 61 ± 4 | [22] | |
| Long-Agulha | Spain | 2007 | 39 b | 97 | NA | 350 ± 16 | [19] |
| United States of America | 2003 | 40 b | NA | 33–271 | NA | [33] | |
| Long-Agulha brown | Spain | 2007 | 39 b | 97 | NA | 230 ± 20 | [19] |
| Long-Basmati | Scotland a | 2014 | 44 b | 100 | NA | 53 ± 7 | [22] |
| Scotland a | 2014 | 44 b | 100 | NA | 69 ± 9 | [22] | |
| Punjab, India | 2014 | 10 b | 100 | NA | 12.0 ± 5.48 | [20] | |
| India | 2007 | 39 b | 97 | NA | 67 ± 1 | [19] | |
| Belgium a | 2018 | 5 | 100 | 19 ± 8–48 ± 21 | 30 ± 10 | [35] | |
| Long-Basmati Brown | Spain | 2007 | 39 b | 97 | NA | 148 ± 4 | [19] |
| Long-Steamed | Iberian Peninsula | 2016 | 11 | 100 | 22–70 | 83 (median) | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Pereira, A.; Silva, L.; Pena, A. Arsenic in Portuguese Rice: Is There Any Risk? Foods 2022, 11, 277. https://doi.org/10.3390/foods11030277
Silva A, Pereira A, Silva L, Pena A. Arsenic in Portuguese Rice: Is There Any Risk? Foods. 2022; 11(3):277. https://doi.org/10.3390/foods11030277
Chicago/Turabian StyleSilva, Alexandra, André Pereira, Liliana Silva, and Angelina Pena. 2022. "Arsenic in Portuguese Rice: Is There Any Risk?" Foods 11, no. 3: 277. https://doi.org/10.3390/foods11030277
APA StyleSilva, A., Pereira, A., Silva, L., & Pena, A. (2022). Arsenic in Portuguese Rice: Is There Any Risk? Foods, 11(3), 277. https://doi.org/10.3390/foods11030277








