Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. C. elegans Maintenance and Bacterial Strains
2.3. Lifespan Assay
2.4. Development Assay
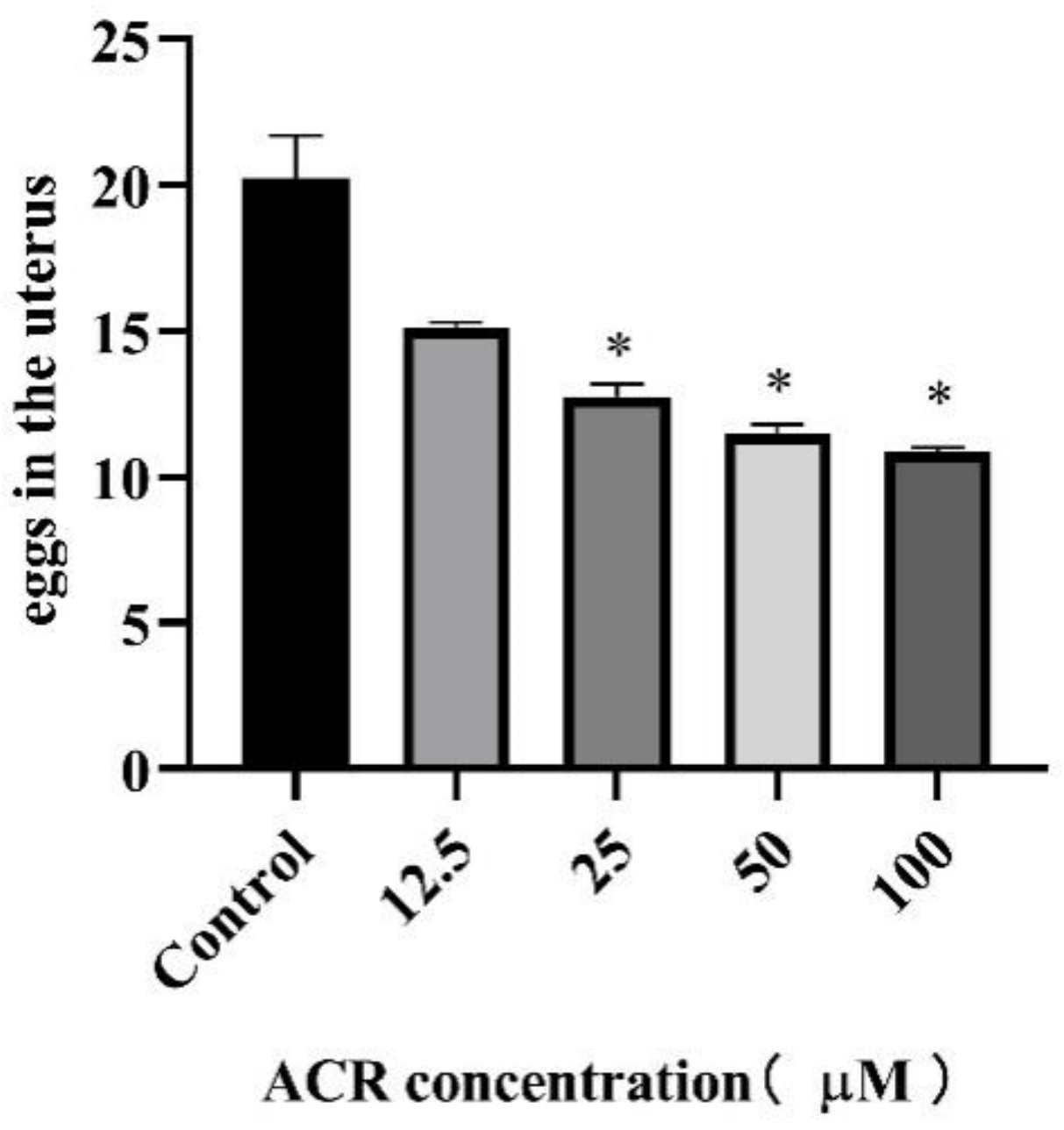
2.5. Reproduction Assay
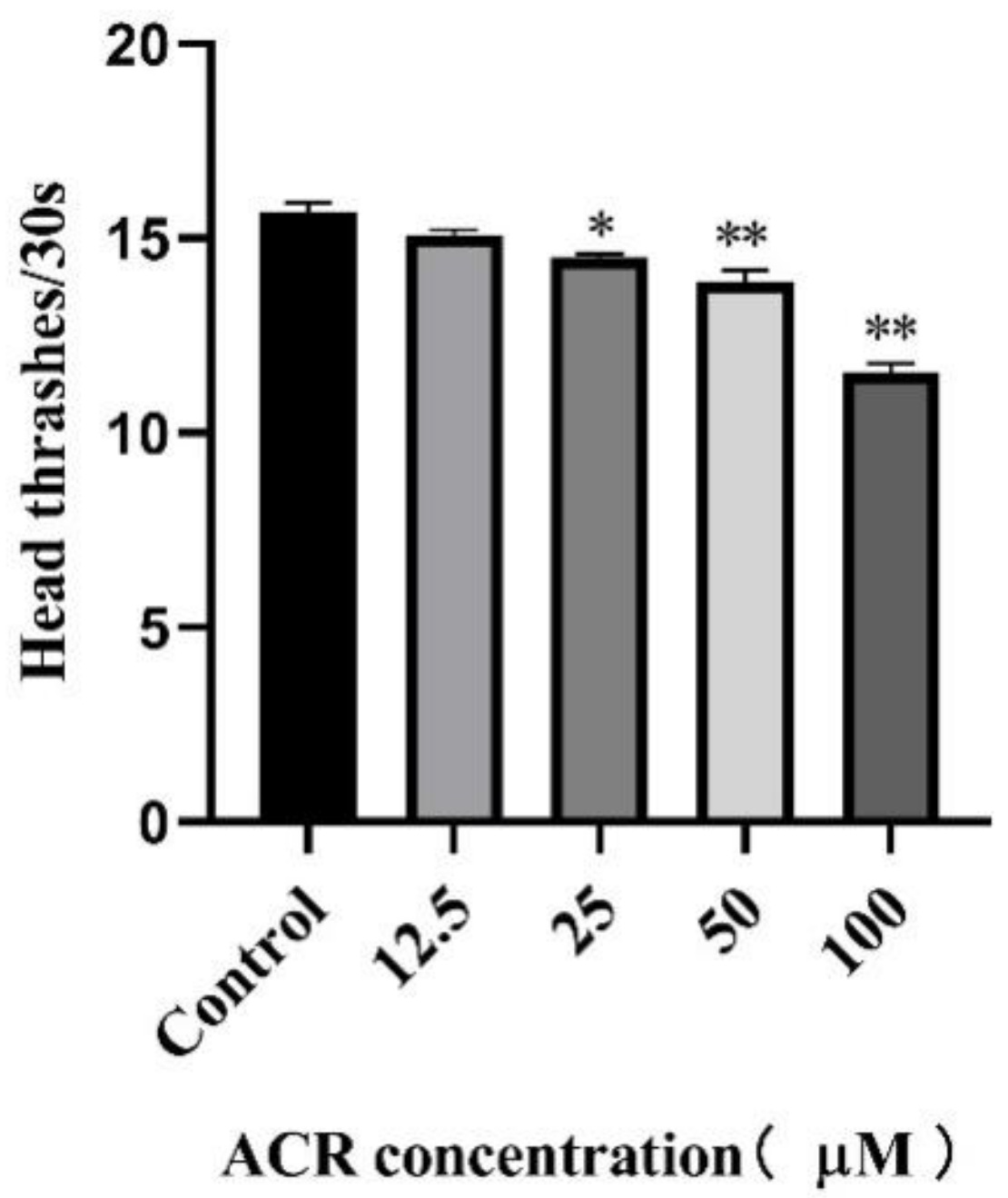
2.6. Locomotion Behavior Assay
2.7. Accumulation of Reactive Oxygen Species (ROS)
2.8. Antioxidant Enzyme Activity
2.9. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.10. Statistical Analysis
3. Results and Discussion
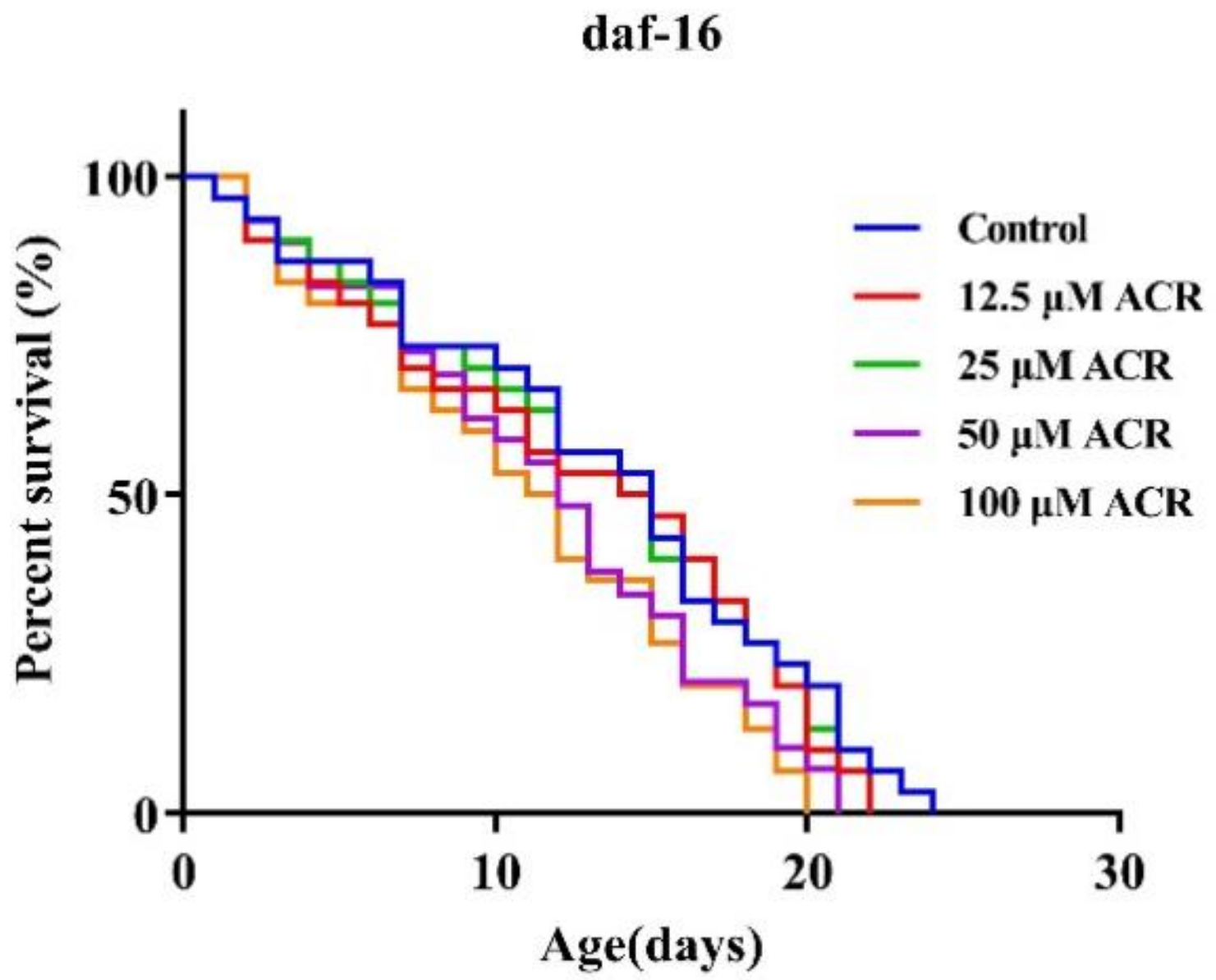
3.1. Effect of ACR Exposure on Lifespan
3.2. Effect of ACR Exposure on Development Indicators and Locomotion Behavior
3.3. Effect of ACR Exposure on Reproduction
3.4. Effect of ACR Exposure on ROS
3.5. Effect of ACR Exposure on Antioxidant Enzymes Activities and Malondialdehyde (MDA) Level
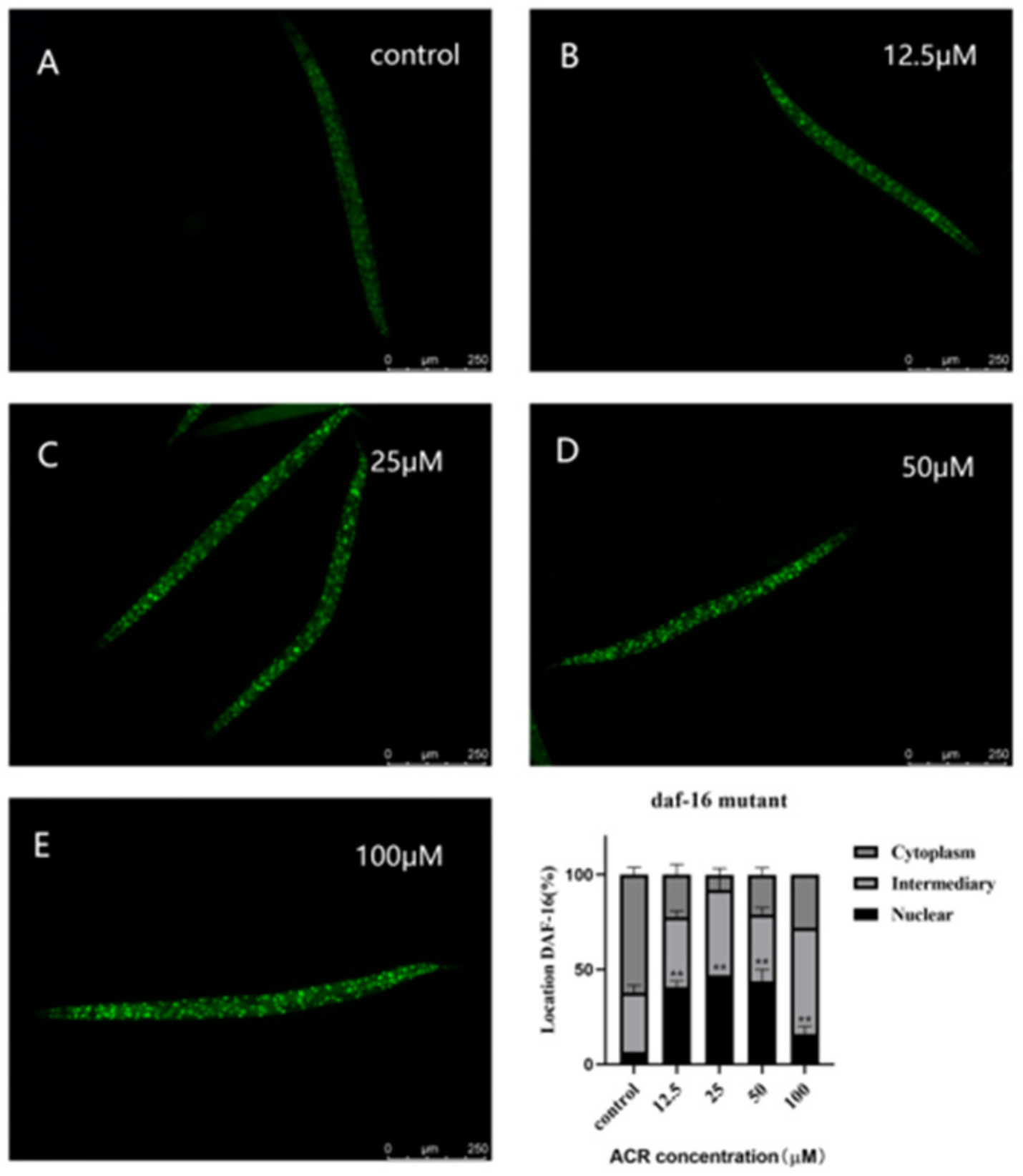
3.6. Effect of ACR Exposure on daf-16 Genetic Strains
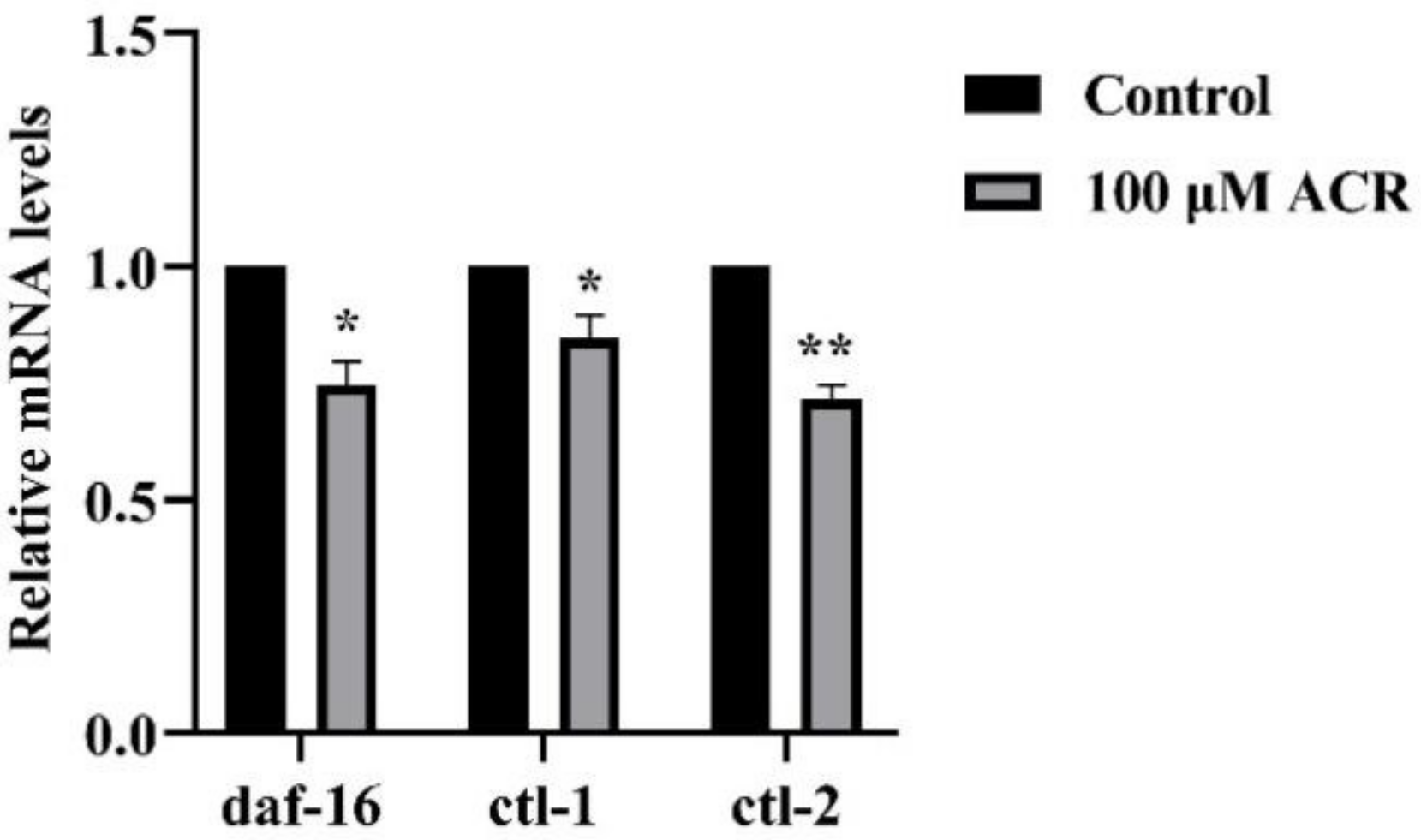
3.7. Effect of ACR Exposure on Expression of daf-16, ctl-1, and ctl-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular Mechanisms of Acrolein Toxicity: Relevance to Human Disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Roney, N.; Taylor, J.; Ashizawa, A.; Lumpkin, M.; Plewak, D. Acrolein health effects. Toxicol. Ind. Health 2008, 24, 447–490. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yin, Z.; Ou, J.; Zheng, J.; Liu, F.; Huang, C.; Ou, S. Identification of adducts formed between acrolein and alanine or serine in fried potato crisps and the cytotoxicity-lowering effect of acrolein in three cell lines. Food Chem. 2021, 361, 130164. [Google Scholar] [CrossRef] [PubMed]
- Tsou, H.-H.; Hsu, W.-C.; Fuh, J.-L.; Chen, S.-P.; Liu, T.-Y.; Wang, H.-T. Alterations in Acrolein Metabolism Contribute to Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 61, 571–580. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Hu, Y.; Tang, M.-S. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. USA 2006, 103, 15404–15409. [Google Scholar] [CrossRef] [Green Version]
- Zirak, M.R.; Mehri, S.; Karimani, A.; Zeinali, M.; Hayes, A.W.; Karimi, G. Mechanisms behind the atherothrombotic effects of acrolein, a review. Food Chem. Toxicol. 2019, 129, 38–53. [Google Scholar] [CrossRef]
- Sarkar, A.; Hameed, R.; Mishra, A.; Bhatta, R.S.; Nazir, A. Genetic modulators associated with regulatory surveillance of mitochondrial quality control, play a key role in regulating stress pathways and longevity in C. elegans. Life Sci. 2021, 290, 120226. [Google Scholar] [CrossRef]
- Jaganjac, M.; Poljak-Blazi, M.; Schaur, R.J.; Zarkovic, K.; Borovic, S.; Cipak, A.; Cindric, M.; Uchida, K.; Waeg, G. Elevated neutrophil elastase and acrolein-protein adducts are associated with W256 regression. Clin. Exp. Immunol. 2012, 170, 178–185. [Google Scholar] [CrossRef]
- Luo, J.; Robinson, J.P.; Shi, R. Acrolein-induced cell death in PC12 cells: Role of mitochondria-mediated oxidative stress. Neurochem. Int. 2005, 47, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Andres, S.; Palavinskas, R.; Berg, K.; Appel, K.E.; Lampen, A. Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 2011, 55, 1277–1290. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chou, C.-Y.; Ch’Ang, L.-Y.; Liu, C.-S.; Lin, W.-C. Identification of Novel Human Genes Evolutionarily Conserved in Caenorhabditis elegans by Comparative Proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Dai, S.; Lu, Y.; Xiong, L.; Huang, J.; Liu, Z.; Gong, Y. Theanine Improves High-Dose Epigallocate-chin-3-Gallate-Induced Lifespan Reduction in Caenorhabditis elegans. Foods 2021, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y.; Asahi, M.; Otsu, K. Acrolein, an Environmental Toxin, Induces Cardiomyocyte Apoptosis via Elevated Intracellular Calcium and Free Radicals. Cell Biophys. 2011, 61, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, B.; Cawley, T.J. Acute and repeated vapor exposure toxicity of 3-(methylthio)propionaldehyde. Vet. Human Toxicol. 2000, 42, 330–336. [Google Scholar]
- Liu, X.; Zheng, W.; Sivasankar, M.P. Acute Acrolein Exposure Induces Impairment of Vocal Fold Epithelial Barrier Function. PLoS ONE 2016, 11, e0163237. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. DAF-16: FOXO in the Context of C. elegans. In Forkhead Foxo Transcription Factors in Development and Disease; Current Topics in Developmental Biology; Ghaffari, S., Ed.; Academic Press: Cambride, MA, USA; Volume 127, 2018; pp. 1–21. [Google Scholar]
- Nagashima, T.; Iino, Y.; Tomioka, M. DAF-16/FOXO promotes taste avoidance learning independently of axonal insulin-like signaling. PLoS Genet. 2019, 15, e1008297. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; You, M.; Wang, C.; Zhang, Y.; Fan, C.; Yan, S. Heat shock pretreatment induced cadmium resistance in the nematode Caenorhabditis elegans is depend on transcription factors DAF-16 and HSF-1. Environ. Pollut. 2020, 261, 114081. [Google Scholar] [CrossRef]
- Sga, B.; Ska, B.; Prsa, B.; Jrra, C.; Ssca, B.; Rrmab, C. Glutathione is a potential therapeutic target for acrolein toxicity in the cornea. Toxicol. Lett. 2021, 340, 33–42. [Google Scholar]
- Lithgow, G.J.; Walker, G.A. Stress resistance as a determinate of C. elegans lifespan. Mech. Ageing Dev. 2002, 123, 765–771. [Google Scholar] [CrossRef]
- Kim, S.-J.; Devgan, A.; Miller, B.; Lee, S.M.; Kumagai, H.; Wilson, K.A.; Wassef, G.; Wong, R.; Mehta, H.H.; Cohen, P.; et al. Humanin-induced autophagy plays important roles in skeletal muscle function and lifespan extension. Biochim. Biophys. Acta BBA Gen. Subj. 2022, 1866, 130017. [Google Scholar] [CrossRef]
- Ungurianu, A.; Margină, D.; Grădinaru, D. Assessment of serum oxidative stress biomarkers for smoking patients in different age groups. Toxicol. Lett. 2017, 280, S97. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, J.; Li, H.; Cui, C.; Yu, Y.; Liu, Y.; Lin, K. The chronic toxicity of bisphenol A to Caenorhabditis elegans after long-term exposure at environmentally relevant concentrations. Chemosphere 2016, 154, 546–551. [Google Scholar] [CrossRef]
- Guo, X.; Navetta, A.; Gualberto, D.G.; García, L.R. Behavioral decay in aging male C. elegans correlates with increased cell excitability. Neurobiol. Aging 2012, 33, 1483.e5–1483.e23. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; Waddell, B.M.; Wu, C.-W. Neuron-specific toxicity of chronic acrylamide exposure in C. elegans. Neurotoxicol. Teratol. 2020, 77, 10. [Google Scholar] [CrossRef]
- Ayuda-Durán, B.; González-Manzano, S.; Gil-Sánchez, I.; Moreno-Arribas, M.V.; Bartolomé, B.; Sanz-Buenhombre, M.; Guadarrama, A.; Santos-Buelga, C.; González-Paramás, A.M. Antioxidant Characterization and Biological Effects of Grape Pomace Extracts Supplementation in Caenorhabditis elegans. Foods 2019, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Gubert, P.; Puntel, B.; Lehmen, T.; Bornhorst, J.; Avila, D.; Aschner, M.; Soares, F.A. Reversible reprotoxic effects of manganese through DAF-16 transcription factor activation and vitellogenin downregulation in Caenorhabditis elegans. Life Sci. 2016, 151, 218–223. [Google Scholar] [CrossRef]
- Karacaoglu, E.; Selmanoglu, G.; Ozgun, G. Subchronic reproductive toxicity in prepubertal male rats exposed to food con-taminant acrolein. Toxicol. Lett. 2013, 221, S215. [Google Scholar] [CrossRef]
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.-R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18. [Google Scholar] [CrossRef]
- Jeelani, R.; Chatzicharalampous, C.; Kohan-Ghadr, H.-R.; Awonuga, A.; Joshi, N.; Morris, R.T.; Abu-Soud, H.M. Acrolein, a commonly found environmental toxin, causes oocyte mitochondrial dysfunction and negatively affects embryo development. Free Radic. Res. 2018, 52, 929–938. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and thera-peutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Hou, K.; Yu, Q.; Hu, X.; Ding, X.; Hong, J.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Protective effect of Ganoderma atrum polysaccharide on acrolein-induced macrophage injury via autophagy-dependent apoptosis pathway. Food Chem. Toxicol. 2019, 133, 8. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Erhan, E.; Salcan, I.; Bayram, R.; Suleyman, B.; Dilber, M.; Yazici, G.N.; Coban, T.A.; Altuner, D.; Suleyman, H. Protective effect of lutein against acrolein-induced ototoxicity in rats. Biomed. Pharmacother. 2021, 137, 7. [Google Scholar] [CrossRef]
- Burgering, B.M.; Kops, G. Cell cycle and death control: Long live Forkheads. Trends Biochem. Sci. 2002, 27, 352–360. [Google Scholar] [CrossRef]
- McElwee, J.; Bubb, K.; Thomas, J.H. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2003, 2, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.T.Y.; Guo, C.F.; Itani, O.A.; Budaitis, B.G.; Williams, T.W.; Hopkins, C.E.; McEachin, R.C.; Pande, M.; Grant, A.R.; Yoshina, S.; et al. Longevity Genes Revealed by Integrative Analysis of Isoform-Specific daf-16/FoxO Mutants of Caenorhabditis elegans. Genetics 2015, 201, 613–629. [Google Scholar] [CrossRef] [Green Version]
- Weinkove, D.; Halstead, J.R.; Gems, D.; Divecha, N. Long-term starvation and ageing induce AGE-1/PI 3-kinase-dependent translocation of DAF-16/FOXO to the cytoplasm. BMC Biology 2006, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Ke, T.; Soares, F.A.A.; Santamaría, A.; Bowman, A.B.; Skalny, A.V.; Aschner, M. N,N′ bis-(2-mercaptoethyl) isophthalamide induces developmental delay in Caenorhabditis elegans by promoting DAF-16 nuclear localization. Toxicol. Rep. 2020, 7, 930–937. [Google Scholar] [CrossRef]
- Heidler, T.; Hartwig, K.; Daniel, H.; Wenzel, U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology 2010, 11, 183–195. [Google Scholar] [CrossRef]
- Huang, C.W.; Liao, W.R.; How, C.M.; Yen, P.L.; Wei, C.C. Chronic exposure of zearalenone inhibits antioxidant defense and results in aging-related defects associated with DAF-16/FOXO in Caenorhabditis elegans. Environ. Pollut. 2021, 285, 117233. [Google Scholar] [CrossRef]
- Lin, X.X.; Sen, I.; Janssens, G.E.; Zhou, X.; Fonslow, B.R.; Edgar, D.; Stroustrup, N.; Swoboda, P.; Yates, J.R., III; Ruvkun, G.; et al. DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and lon-gevity. Nat. Commun. 2018, 9, 4400. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Group | Dose (μM) | Average Lifespan |
|---|---|---|
| Control | 0 | 16.53 ± 0.94 a |
| ACR | 12.5 | 14.83 ± 0.27 a |
| 25 | 14.32 ± 0.32 b | |
| 50 | 13.32 ± 0.22 b | |
| 100 | 9.97 ± 0.70 c |
| Group | Dose (μM) | SOD | CAT | MDA |
|---|---|---|---|---|
| Control | 0 | 206.20 ± 1.56 a | 16.31 ± 0.08 a | 11.18 ± 0.82 c |
| ACR | 12.5 | 89.46 ± 0.177 b | 14.05 ± 0.08 b | 12.03 ± 0.09 bc |
| 25 | 77.35 ± 1.47 c | 14.87 ± 0.03 c | 12.66 ± 0.46 b | |
| 50 | 79.56 ± 0.35 d | 14.69 ± 0.08 d | 15.34 ± 0.41 a | |
| 100 | 83.17 ± 0.53 e | 9.94 ± 0.09 e | 15.80 ± 0.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Song, Y.; Xie, J.; Xie, J.; Chen, Y.; Li, P.; Liu, D.; Hu, X.; Yu, Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods 2022, 11, 1590. https://doi.org/10.3390/foods11111590
Hong J, Song Y, Xie J, Xie J, Chen Y, Li P, Liu D, Hu X, Yu Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods. 2022; 11(11):1590. https://doi.org/10.3390/foods11111590
Chicago/Turabian StyleHong, Jiaqian, Yiming Song, Jiayan Xie, Jianhua Xie, Yi Chen, Ping Li, Danyang Liu, Xiaobo Hu, and Qiang Yu. 2022. "Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans" Foods 11, no. 11: 1590. https://doi.org/10.3390/foods11111590
APA StyleHong, J., Song, Y., Xie, J., Xie, J., Chen, Y., Li, P., Liu, D., Hu, X., & Yu, Q. (2022). Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods, 11(11), 1590. https://doi.org/10.3390/foods11111590






