Bioactive Properties, Volatile Compounds, and Sensory Profile of Sauerkraut Are Dependent on Cultivar Choice and Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Sauerkraut Production, and Sampling
2.2. Physicochemical Measurements
2.2.1. pH and EC Measurements
2.2.2. Dry Matter Determination
2.2.3. Color Evaluation
2.3. Phytochemical measurements
2.3.1. Total Antioxidant Capacity
2.3.2. Total Phenolic Content
2.3.3. Volatile Compounds Analysis
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
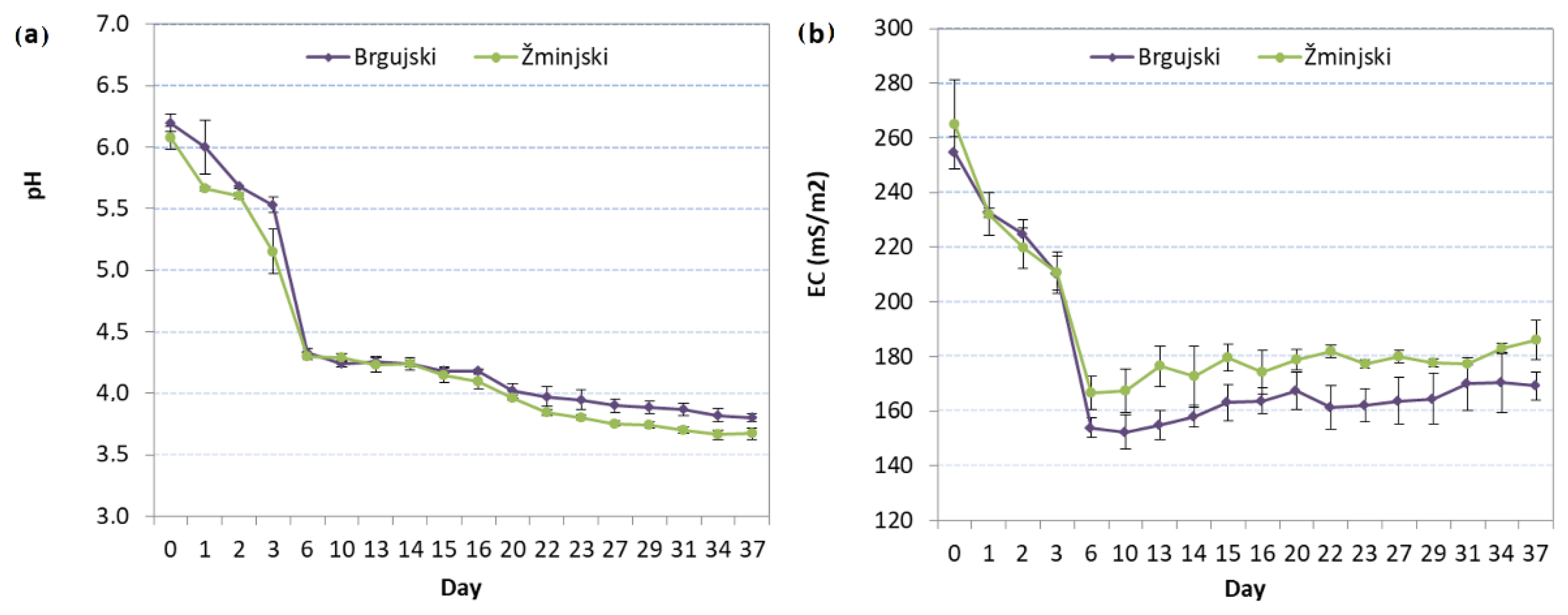
3.1. Changes in Brine pH and EC during Fermentation
3.2. Comparison of Traditional Sauerkraut Cultivars with Commercial Samples
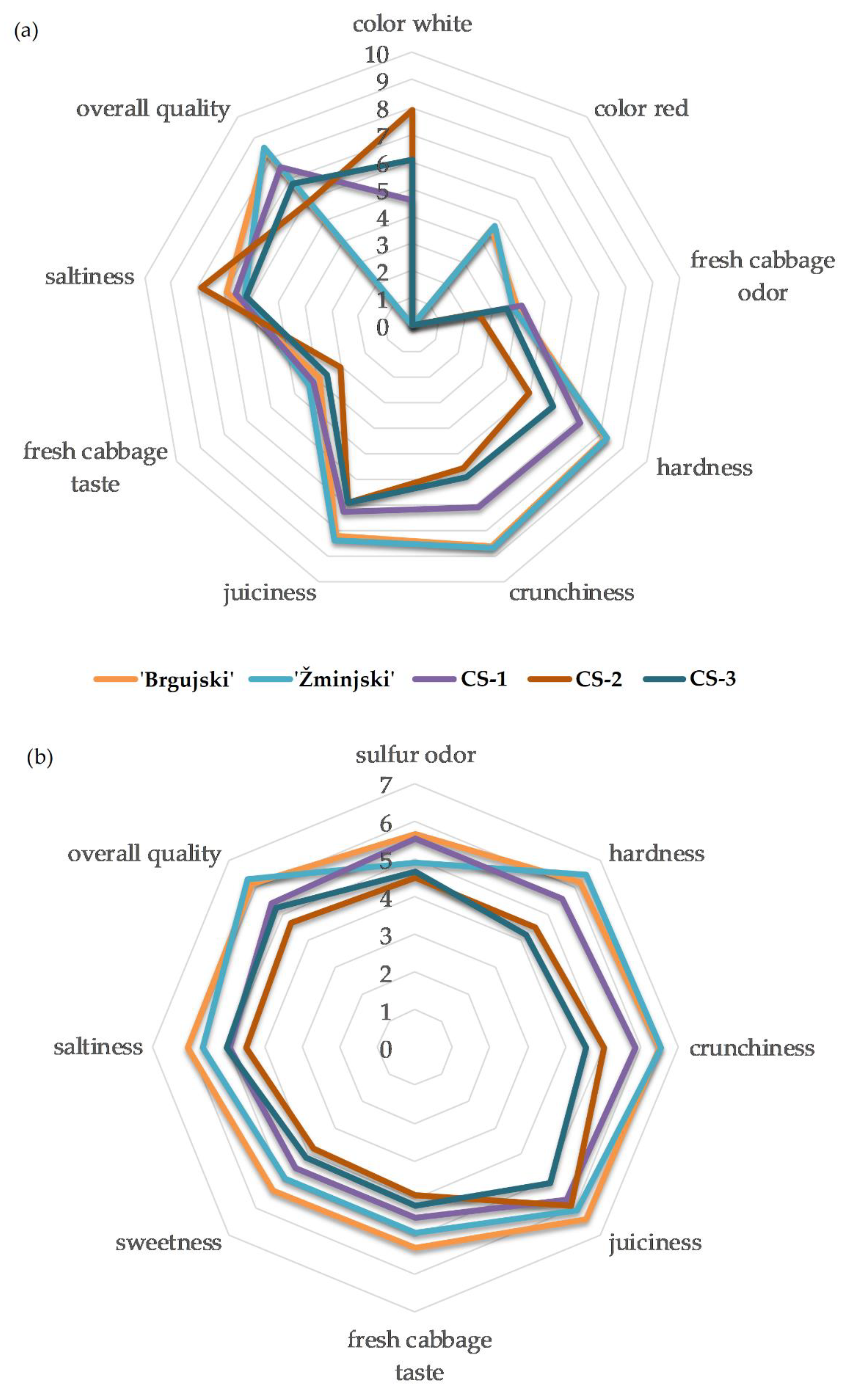
3.3. Sensory Analysis of Traditional and Commercial Sauerkraut Samples
3.4. Influence of Storage Conditions and Cultivar on the Physicochemical and Phytochemical Parameters of Traditional Sauerkraut Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lešić, R.; Borošić, J.; Buturac, I.; Ćustić, M.H.; Poljak, M.; Romić, D. Povrćarstvo (Vegetable Crops), 3rd ed.; Zrnski d.d.: Čakovec, Croatia, 2016. [Google Scholar]
- Lončarić, A.; Marček, T.; Šubarić, D.; Jozinović, A.; Babić, J.; Miličević, B.; Sinković, K.; Šubarić, D.; Ačkar, D. Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages. Molecules 2020, 25, 3696. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data, (accessed on 22 February 2022).
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White Cabbage (Brassica oleracea Var. Capitata f. Alba): Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Faltusová, Z.; Kučera, L.; Ovesná, J. Genetic Diversity of Brassica Oleracea Var. Capitata Gene Bank Accessions Assessed by AFLP. Electron. J. Biotechnol. 2011, 14, 11. [Google Scholar] [CrossRef]
- Jakobek, L.; Tomac, I.; Sabo, M.; Đugum, J.; Šubarić, D. Croatian Journal of Food Science and Technology Bioactive Polyphenolic Compounds from White Cabbage Cultivars. Croat. J. Food Sci. Technol. 2018, 10, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Bažon, I.; Ban, S.G.; Cvitan, D.; Prekalj, B.; Franić, M.; Ban, D. Morphological and Agronomic Traits of Cabbage “Brgujski” Natural Population. In Proceedings of the 54th Croatian and 14th International Symposium on Agriculture, Vodice, Croatia, 17–22 February 2019; Mioč, B., Širić, I., Eds.; pp. 231–235. [Google Scholar]
- Cvetković, B.R.; Pezo, L.L.; Pestorić, M.; Filipčev, B.; Kevrešan, Ž.; Mastilović, J. Comparative Study of White Cabbage, Traditional Variety and Hybrid Intended for Biological Fermentation. J. Appl. Bot. Food Qual. 2014, 87, 286–290. [Google Scholar] [CrossRef]
- Moreb, N.; Murphy, A.; Jaiswal, S.; Jaiswal, A.K. Cabbage. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: London, UK, 2020; pp. 33–54. [Google Scholar] [CrossRef]
- Hunaefi, D.; Akumo, N.; Smetanska, I. Effect of Fermentation on Antioxidant Properties of Red Cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical Composition and Sensory Quality of Sauerkraut Produced from Different Cabbage Varieties. LWT 2021, 136, 110352. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the Content and Composition of Anthocyanins in Red Cabbage and Its Antioxidant Capacity during Fermentation, Storage and Stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. The Effect of Heating and Fermenting on Antioxidant Properties of White Cabbage. Food Chem. 2008, 108, 853–861. [Google Scholar] [CrossRef]
- Ashfaq, F.; Butt, M.S.; Bilal, A.; Tehseen, S.; Suleria, H.A.R. Comparative Assessment of Free Radical Scavenging Ability of Green and Red Cabbage Based on Their Antioxidant Vitamins and Phytochemical Constituents. Curr. Bioact. Compd. 2020, 16, 1231–1241. [Google Scholar] [CrossRef]
- Berger, M.D.; Vakula, A.; Horecki, A.T.; Rakić, D.; Pavlić, B.; Malbaša, R.; Vitas, J.; Jerković, J.; Šumić, Z. Cabbage (Brassica oleracea, L. Var. Capitata) Fermentation: Variation of Bioactive Compounds, Sum of Ranking Differences and Cluster Analysis. LWT 2020, 133, 110038. [Google Scholar] [CrossRef]
- Ghosh, D. Studies on the Changes of Biochemical, Microbiological and Sensory Parameters of Sauerkraut and Fermented Mix Vegetables. J. Homepage 2021, 5, 78–83. [Google Scholar] [CrossRef]
- Dobričević, N.; Voća, S.; Pliestić, S. Kakvoća Kiselog Kupusa “Ribanca” iz Ogulina. Agron. Glas. Glas. Hrvat. Agron. Društva 2006, 68, 459–473. [Google Scholar]
- Peñas, E.; Pihlava, J.M.; Vidal-Valverde, C.; Frias, J. Influence of Fermentation Conditions of Brassica oleracea L. Var. capitata on the Volatile Glucosinolate Hydrolysis Compounds of Sauerkrauts. LWT-Food Sci. Technol. 2012, 48, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.K.; Gupta, S.; Abu-Ghannam, N. Kinetic Evaluation of Colour, Texture, Polyphenols and Antioxidant Capacity of Irish York Cabbage after Blanching Treatment. Food Chem. 2012, 131, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Sysi-Aho, M.; Katajamaa, M.; Yetukuri, L.; Orešič, M. Normalization Method for Metabolomics Data Using Optimal Selection of Multiple Internal Standards. BMC Bioinform. 2007, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Johanningsmeier, S.; McFeeters, R.F.; Fleming, H.P.; Thompson, R.L. Effects of Leuconostoc Mesenteroides Starter Culture on Fermentation of Cabbage with Reduced Salt Concentrations. J. Food Sci. 2007, 72, M166–M172. [Google Scholar] [CrossRef]
- Ritz, M.; Vojnović, V.; Vahčić, N.; Mahnet, S. Senzorska Procjena Desertnih Mliječnih Proizvoda. Mljekarstvo 1992, 42, 53–60. [Google Scholar]
- Martinez-Villaluenga, C.; Peñas, E.; Sidro, B.; Ullate, M.; Frias, J.; Vidal-Valverde, C. White Cabbage Fermentation Improves Ascorbigen Content, Antioxidant and Nitric Oxide Production Inhibitory Activity in LPS-Induced Macrophages. LWT-Food Sci. Technol. 2012, 46, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Palani, K.; Harbaum-Piayda, B.; Meske, D.; Keppler, J.K.; Bockelmann, W.; Heller, K.J.; Schwarz, K. Influence of Fermentation on Glucosinolates and Glucobrassicin Degradation Products in Sauerkraut. Food Chem. 2016, 190, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L.; Yemoto, K. Salinity: Electrical Conductivity and Total Dissolved Solids. Soil Sci. Soc. Am. J. 2020, 84, 1442–1461. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Luo, S.; Tang, Z.; Liu, Z.; Wei, S.; Liu, F.; Zhao, X.; Yu, J.; Zhong, Y. Comprehensive Evaluation of Amino Acids and Polyphenols in 69 Varieties of Green Cabbage (Brassica oleracea L. Var. capitata L.) Based on Multivariate Statistical Analysis. Molecules 2021, 26, 5355. [Google Scholar] [CrossRef] [PubMed]
- Červenski, J.; Savić, A.; Popović, A.; Petrović, A.; Maksimović, L.; Takac, A.; Popovic, V.; Glogovac, S. Possibility of Exploitation of Serbian Local Cultivars and Landraces of Cabbages (Brassica oleracea Var. Capitata L.): Case of “Futoški Cabbage” from Futog Region. Acta Hortic. 2013, 1005, 127–132. [Google Scholar] [CrossRef]
- Farnworth, E.R. Handbook of Fermented Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429136672. [Google Scholar]
- Lee, M.A.; Choi, Y.J.; Lee, H.; Hwang, S.; Lee, H.J.; Park, S.J.; Chung, Y.B.; Yun, Y.R.; Park, S.H.; Min, S.; et al. Influence of Salinity on the Microbial Community Composition and Metabolite Profile in Kimchi. Ferment 2021, 7, 308. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Drabińska, N. Changes in Glucosinolates and Their Breakdown Products during the Fermentation of Cabbage and Prolonged Storage of Sauerkraut: Focus on Sauerkraut Juice. Food Chem. 2021, 365, 130498. [Google Scholar] [CrossRef]
- Peñas, E.; Martínez-Villaluenga, C.; Pihlava, J.-M.; Frias, J. Evaluation of Refrigerated Storage in Nitrogen-Enriched Atmospheres on the Microbial Quality, Content of Bioactive Compounds and Antioxidant Activity of Sauerkrauts. LWT-Food Sci. Technol. 2015, 61, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Piljac-Žegarac, J.; Bogović, M.; Habjanič, K.; Grúz, J. Antioxidant Potency of White (Brassica oleracea L. Var. capitata) and Chinese (Brassica rapa L. Var. pekinensis (Lour.)) Cabbage: The Influence of Development Stage, Cultivar Choice and Seed Selection. Sci. Hortic. 2011, 128, 78–83. [Google Scholar] [CrossRef]
- Šamec, D.; Bogović, M.; Vincek, D.; Martinčić, J.; Salopek-Sondi, B. Assessing the Authenticity of the White Cabbage (Brassica oleracea Var. capitata f. alba) Cv. ‘Varaždinski’ by Molecular and Phytochemical Markers. Food Res. Int. 2014, 60, 266–272. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.R.; Park, S.H.; Lee, M.A. Changes in Volatile and Non-Volatile Compounds of Model Kimchi through Fermentation by Lactic Acid Bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Comparison of Northeast Sauerkraut Fermentation between Single Lactic Acid Bacteria Strains and Traditional Fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef] [PubMed]
- Breidt, F.; McFeeters, R.F.; Perez-Diaz, I.; Lee, C.-H. Fermented Vegetables, 4th ed.; Michael, P.D., Robert, L.B., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2014. [Google Scholar]
- Kang, J.H.; Lee, J.H.; Min, S.; Min, D.B. Changes of Volatile Compounds, Lactic Acid Bacteria, PH, and Headspace Gases in Kimchi, a Traditional Korean Fermented Vegetable Product. J. Food Sci. 2003, 68, 849–854. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregón, S.; Padilla, G.; de Haro, A. Seasonal Variation in Glucosinolate Content in Brassica Oleracea Crops Grown in Northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Gurtner, K.; Carle, R.; Björn Steingass, C. Investigation into the Removal of Glucosinolates and Volatiles from Anthocyanin-Rich Extracts of Red Cabbage. Food Chem. 2019, 278, 406–414. [Google Scholar] [CrossRef]
- Rutnakornpituk, B.; Boonthip, C.; Sutham, S.; Rutnakornpituk, M. Glucosinalate Compound and Antioxidant Activity of Fresh Green Cabbage and Fermented Green Cabbage Products. NU. Int. J. Sci. 2021, 18, 62–75. [Google Scholar]
- Miller-Cebert, R.L.; Sistani, N.A.; Cebert, E. Comparative Mineral Composition among Canola Cultivars and Other Cruciferous Leafy Greens. J. Food Compos. Anal. 2009, 22, 112–116. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, J.; Li, D.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Metabolomics Approaches for the Comprehensive Evaluation of Fermented Foods: A Review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An Overview of Plant-Autochthonous Microorganisms and Fermented Vegetable Foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, l-Ascorbic Acid, Total Phenols, Anthocyanins, Antioxidant Capacities and Colour in Cauliflower (Brassica oleracea L. ssp. botrytis); Effects of Long-Term Freezer Storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of Package Type on Selected Parameters of Nutritional Quality of Chill-Stored White Sauerkraut. Polish J. Food Nutr. Sci. 2017, 67, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.-Y.; Kim, D.-G.; Park, J.-T.; Kandpal, L.M.; Hong, S.; Cho, B.-K.; Lee, W.-H. Physicochemical Quality Changes in Chinese Cabbage with Storage Period and Temperature: A Review. J. Biosyst. Eng. 2016, 41, 373–388. [Google Scholar] [CrossRef] [Green Version]
- Martínez, S.; Armesto, J.; Gómez-Limia, L.; Carballo, J. Impact of Processing and Storage on the Nutritional and Sensory Properties and Bioactive Components of Brassica spp. A Review. Food Chem. 2020, 313, 126065. [Google Scholar] [CrossRef]
- Galani, J.H.Y.; Patel, J.S.; Patel, N.J.; Talati, J.G. Storage of Fruits and Vegetables in Refrigerator Increases Their Phenolic Acids but Decreases the Total Phenolics, Anthocyanins and Vitamin C with Subsequent Loss of Their Antioxidant Capacity. Antioxidants 2017, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Gao, Y.Y.; Tao, F.; Liu, H.Y.; Xu, P. Temperature-Directed Biocatalysis for the Sustainable Production of Aromatic Aldehydes or Alcohols. Angew. Chem. Int. Ed. 2018, 57, 1214–1217. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Czapski, J. Colour Changes of a Preparation from Red Cabbage during Storage in a Model System. Food Chem. 2007, 104, 709–714. [Google Scholar] [CrossRef]
- Suriyaphan, O.; Cadwallader, K.R.; Drake, M.A. Lecithin Associated Off-Aromas in Fermented Milk. J. Food Sci. 2001, 66, 517–523. [Google Scholar] [CrossRef]
- Kamp, F.; Hamilton, J.A. How Fatty Acids of Different Chain Length Enter and Leave Cells by Free Diffusion. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 149–159. [Google Scholar] [CrossRef]
- Morales-López, J.; Centeno-Álvarez, M.; Nieto-Camacho, A.; López, M.G.; Pérez-Hernández, E.; Pérez-Hernández, N.; Fernández-Martínez, E. Evaluation of Antioxidant and Hepatoprotective Effects of White Cabbage Essential Oil. Pharm. Biol. 2016, 55, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kihara, H.; Tanaka, M.; Yamato, K.T.; Horibata, A.; Yamada, A.; Kita, S.; Ishizaki, K.; Kajikawa, M.; Fukuzawa, H.; Kohchi, T.; et al. Arachidonic Acid-Dependent Carbon-Eight Volatile Synthesis from Wounded Liverwort (Marchantia polymorpha). Phytochemistry 2014, 107, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, R.M.; Peterson, M.E.; Danson, M.J.; Price, N.C.; Kelly, S.M.; Monk, C.R.; Weinberg, C.S.; Oudshoorn, M.L.; Lee, C.K. The Molecular Basis of the Effect of Temperature on Enzyme Activity. Biochem. J. 2010, 425, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Żywica, R.; Modzelewska-Kapituła, M.; Zadroga, I.; Tkacz, K. Influence of Selected Mineral Components and Dry Matter Contents on the Electrical Conductivity of Apple Juice. Towarozn. Probl. Jakości 2017, 3, 86–94. [Google Scholar] [CrossRef]


| Sauerkraut Samples | ||||||
|---|---|---|---|---|---|---|
| ‘Brgujski’ | ‘Žminjski’ | CS-1 | CS-2 | CS-3 | p-Value | |
| Physicochemical parameters | ||||||
| DM (%) | 9.93 ± 0.02 a | 9.05 ± 0.02 b | 8.17 ± 0.17 c | 9.7 ± 0.21 ab | 7.44 ± 0.64 c | *** |
| EC (mS/m) | 110 ± 3 c | 143 ± 4 b | 83 ± 1 d | 208 ± 15 a | 96 ± 1 cd | *** |
| pH | 3.61 ± 0.01 c | 3.79 ± 0.01 b | 3.84 ± 0.01 a | 3.58 ± 0.01 e | 3.67 ± 0.01 cd | *** |
| (L) | 50.6 ± 1.7 d | 57.0 ± 3.2 c | 61.8 ± 1.9 ab | 59.7 ± 1.4 bc | 64.7 ± 2.8 a | *** |
| (a) | 23.7 ± 4.9 a | 9.7 ± 0.4 b | 1.7 ± 0.3 d | 4.0 ± 0.2 c | 1.4 ± 0.3 d | *** |
| (b) | 14.3 ± 1.9 c | 26.6 ± 1.8 a | 28.4 ± 6.6 a | 19.3 ± 3.5 b | 25.3 ± 1.8 a | *** |
| Phytochemical parameters | ||||||
| TPC (mg GAE/100 g FW) | 34.9 ± 2.7 b | 61.4 ± 4.6 a | 12.7 ± 0.8 cd | 14.8 ± 1.4 c | 6.1 ± 0.6 d | *** |
| FRAP (nmol TE/100 g FW) | 152 ± 13 a | 143 ± 5 a | 52 ± 2 c | 62 ± 1 c | 100 ± 8 b | *** |
| DPPH (nmol TE/100 g FW) | 118 ± 6 a | 122 ± 3 a | 59 ± 6 d | 78 ± 4 c | 102 ± 1 b | *** |
| Volatile Compounds (Peak Area) | Sauerkraut Samples | |||||
|---|---|---|---|---|---|---|
| ‘Brgujski’ | ‘Žminjski’ | CS-1 | CS-2 | CS-3 | p-Value | |
| Esters | ||||||
| ethyl acetate | 1,395,228 ± 295,733 a | 1,078,893 ± 113,810 ab | 596,995 ± 256,186 bc | 45,486 ± 10,233 c | 1,479,426 ± 235,640 a | *** |
| n-propyl acetate | 203,543 ± 31,519 a | 111,616 ± 6090 b | 54,663 ± 3490 c | 16,184 ± 6650 c | 25,751 ± 2671 c | *** |
| butyl acetate | 36,442 ± 36,690 c | 54,892 ± 17,787 c | 286,608 ± 7828 a | 182,097 ± 1565 b | 203571 ± 24,430 b | *** |
| pentyl acetate | 27,786,255 ± 9,993,811 a | 37,351,541 ± 584,954 a | 419,590 ± 24,673 b | 468,125 ± 24,911 b | 11,292,836 ± 2,047,968 b | *** |
| (E)-3-hexen-1-yl acetate | 1,248,049 ± 228,779 b | 3,615,000 ± 539,369 a | 19,495 ± 15,791 c | 28,931 ± 982 c | 87,931 ± 12,928 c | *** |
| hexyl acetate | 424,619 ± 64,423 b | 2,256,204 ± 218,496 a | 37,024 ± 6511 c | 62,431 ± 21,654 c | 391,433 ± 114,293 b | *** |
| (Z)-3-octen-1-yl acetate | 172,678 ± 36,116 bc | 470,875 ± 73,075 a | 113,805 ± 9591 c | 220,182 ± 8788 bc | 273,790 ± 98,313 b | *** |
| octyl acetate | 200,231 ± 31,786 b | 545,995 ± 54,052 b | 477,884 ± 32,921 b | 208,571 ± 36,727 b | 1,231,124 ± 436,888 a | *** |
| 2-phenylethyl acetate | 7,123,750 ± 563,048 a | 4,615,988 ± 592,564 b | 171,843 ± 23,010 c | 22,569 ± 331 c | 290,101 ± 44,164 c | *** |
| nonyl acetate | 872,016 ± 158,370 bc | 1,550,844 ± 165,832 ab | 34,304 ± 12,129 c | 42,746 ± 23,539 c | 1,875,895 ± 703,985 a | *** |
| Neryl acetate | 37,341 ± 11,302 b | 143,376 ± 31,860 a | 25,183 ± 2742 b | 24,130 ± 4007 b | 52,416 ± 17,119 b | *** |
| decyl acetate | 38,558 ± 10,982 b | 38,029 ± 5532 b | 26,003 ± 1784 b | 23,013 ± 4517 b | 126,374 ± 41,098 a | *** |
| ethyl octanoate | 24,789 ± 6243 c | 35,458 ± 395 c | 117,050 ± 5893 b | 223,767 ± 14,962 a | 38,834 ± 5295 c | *** |
| ethyl hexadecanoate | 2,149,623 ± 96,135 a | 384,260 ± 14,824 c | 1,031,981 ± 65,346 b | 297,926 ± 12,398 c | 418,161 ± 23,793 c | *** |
| Alcohols | ||||||
| 1-pentanol | 2152,915 ± 264,400 a | 321,111 ± 8094 bc | 605,969 ± 18,756 b | 115,464 ± 1806 c | 40,570 ± 15,435 c | *** |
| 1-hexanol | 122,337 ± 17,402 cd | 162,648 ± 42,423 bc | 228,462 ± 10,241 b | 345,102 ± 25,981 a | 85,997 ± 16,499 d | *** |
| 1-heptanol | 288,268 ± 30,967 ab | 215,781 ± 20,872 bc | 94,463 ± 7183 d | 111,842 ± 4062 cd | 404,260 ± 92,168 a | *** |
| 2-heptenol | 61,442 ± 26,990 b | 53,295 ± 9081 b | 92,208 ± 3460 b | 254,405 ± 1543 a | 71,346 ± 17,979 b | *** |
| 1-octen-3-ol | 137,631 ± 18,890 c | 180,286 ± 21,721 bc | 664,844 ± 48,478 a | 136,092 ± 12,520 c | 303,109 ± 108,969 b | *** |
| 2-octen-1-ol | 50,729 ± 3057 b | 55,909 ± 11,587 b | 369,649 ± 22,875 a | 67,852 ± 7470 b | 108,273 ± 61,388 b | *** |
| 1-decanol | 19,322 ± 3443 c | 15,656 ± 2510 c | 41,728 ± 11,006 c | 304,241 ± 5693 a | 78,027 ± 20,450 b | *** |
| 2-undecanol | 608,142 ± 12,494 c | 490,000 ± 32,633 c | 633,783 ± 79,916 a | 44,555 ± 14,489 b | 215,613 ± 13,970 b | *** |
| Organosulfur compounds | ||||||
| dimethyl disulfide | 2,152,915 ± 264,400 a | 913,856 ± 37,479 bc | 638,656 ± 52,597 bc | 151,616 ± 19,805 d | 477,490 ± 24,467 cd | *** |
| allyl isothiocyanate | 100,499 ± 580 a | 87,984 ± 45,420 a | 27,750 ± 2897 b | 46,326 ± 4637 ab | 68,401 ± 4752 ab | ** |
| 2-isothiocyanatobutane | 13,840 ± 3336 c | 11,175 ± 527 c | 23,557 ± 2257 b | 7701 ± 2213 c | 47,456 ± 4184 a | *** |
| dimethyl trisulfide | 72,585 ± 11,457 c | 70,762 ± 35,987 c | 92,208 ± 3460 c | 254,405 ± 1543 a | 176,472 ± 21,729 b | *** |
| 4-isothiocyanatobut-1-ene | 117,438 ± 14,676 c | 180,286 ± 21,721 c | 747,624 ± 102,540 a | 467,811 ± 12,336 b | 294,755 ± 122,098 bc | *** |
| Aldehydes and ketones | ||||||
| 3-octanone | 179,080 ± 36,785 c | 259,322 ± 41,905 c | 903,077 ± 117,834 b | 886,598 ± 88,863 b | 1,805,712 ± 341,480 a | *** |
| 2-heptenal, (Z)- | 85,250 ± 61,199 c | 85,685 ± 44,289 c | 1,576,219 ± 127,593 a | 276,669 ± 40,495 b | 73,943 ± 22,875 c | *** |
| nonanal | 56,889 ± 6595 b | 79,719 ± 4696 b | 1,906,507 ± 1,010,828 a | 1,116,308 ± 970,319 ab | 339,365 ± 16,105 ab | *** |
| 2-undecenal | 32,541 ± 11,638 c | 37,433 ± 11,719 c | 2,320,481 ± 141,942 a | 509,651 ± 63,846 b | 319,381 ± 161,272 b | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, N.; Bažon, I.; Išić, N.; Kovačević, T.K.; Ban, D.; Radeka, S.; Goreta Ban, S. Bioactive Properties, Volatile Compounds, and Sensory Profile of Sauerkraut Are Dependent on Cultivar Choice and Storage Conditions. Foods 2022, 11, 1218. https://doi.org/10.3390/foods11091218
Major N, Bažon I, Išić N, Kovačević TK, Ban D, Radeka S, Goreta Ban S. Bioactive Properties, Volatile Compounds, and Sensory Profile of Sauerkraut Are Dependent on Cultivar Choice and Storage Conditions. Foods. 2022; 11(9):1218. https://doi.org/10.3390/foods11091218
Chicago/Turabian StyleMajor, Nikola, Iva Bažon, Nina Išić, Tvrtko Karlo Kovačević, Dean Ban, Sanja Radeka, and Smiljana Goreta Ban. 2022. "Bioactive Properties, Volatile Compounds, and Sensory Profile of Sauerkraut Are Dependent on Cultivar Choice and Storage Conditions" Foods 11, no. 9: 1218. https://doi.org/10.3390/foods11091218
APA StyleMajor, N., Bažon, I., Išić, N., Kovačević, T. K., Ban, D., Radeka, S., & Goreta Ban, S. (2022). Bioactive Properties, Volatile Compounds, and Sensory Profile of Sauerkraut Are Dependent on Cultivar Choice and Storage Conditions. Foods, 11(9), 1218. https://doi.org/10.3390/foods11091218







