Influence of Varying Fermentation Parameters of the Yeast Strain Cyberlindnera saturnus on the Concentrations of Selected Flavor Components in Non-Alcoholic Beer Focusing on (E)-β-Damascenone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Wort
2.2. Yeast Propagation and Fermentation
2.3. Response Surface Methodology (RSM)
2.4. Analytical Methods
2.4.1. Analytical Standard Methods
2.4.2. Headspace-Gas Chromatography (HS-GC) with Olfactometry (-O), Flame Ionization (-FID) und Mass Spectrometry (-MS)
2.4.3. GC × GC-MS System
2.5. Sensory Evaluation
3. Results
3.1. Analytical Results
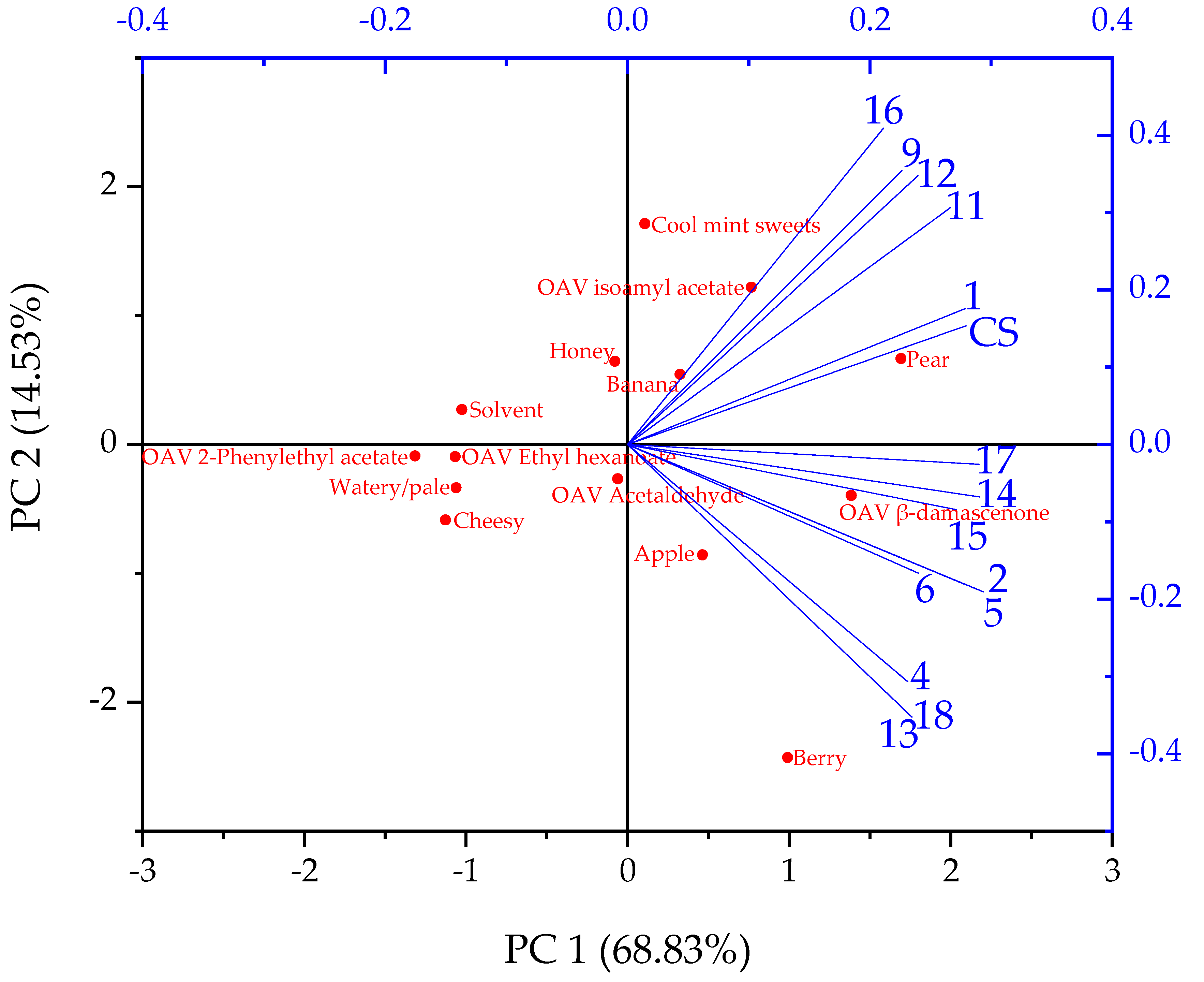
3.2. Sensory Evaluation Results
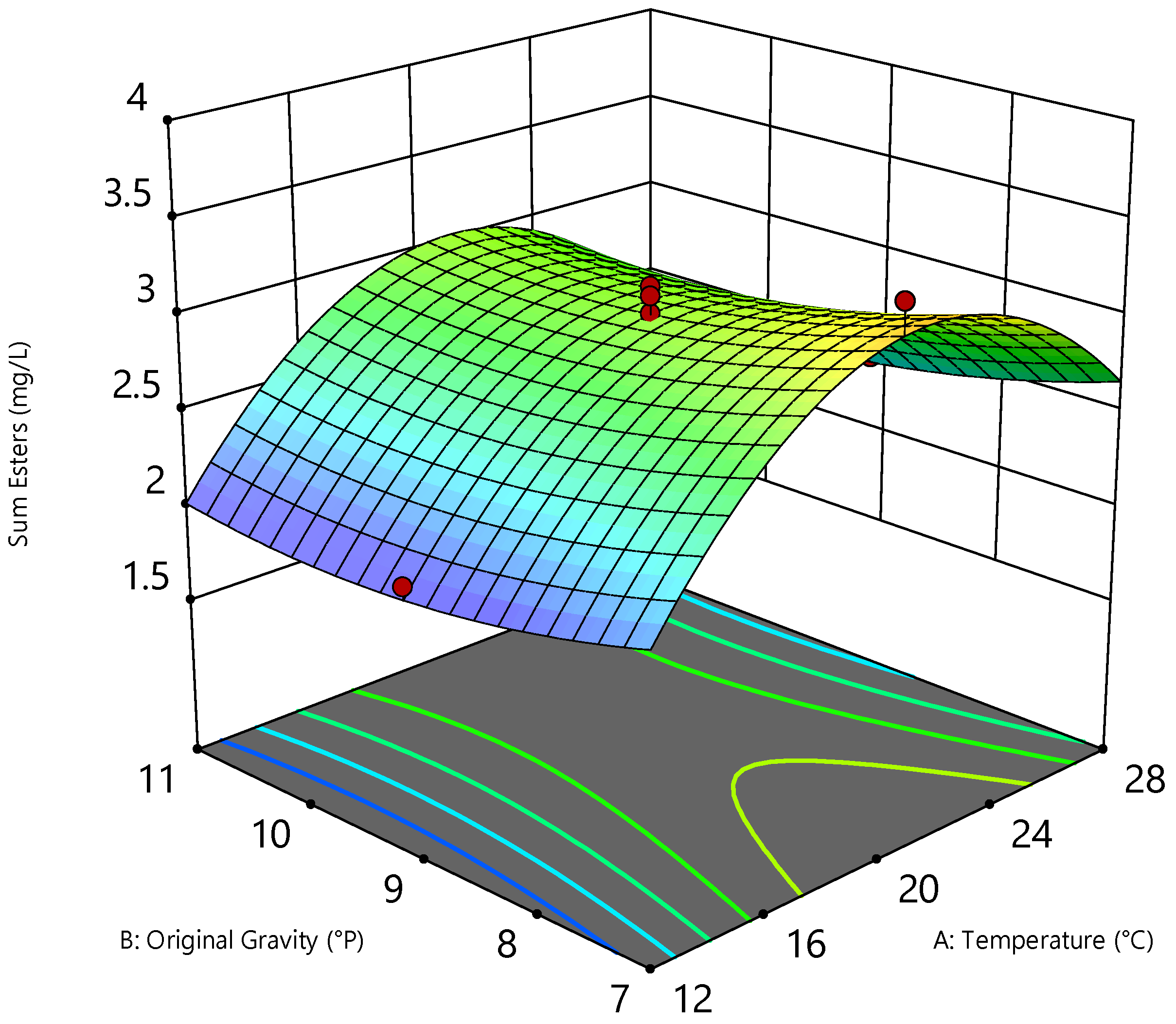
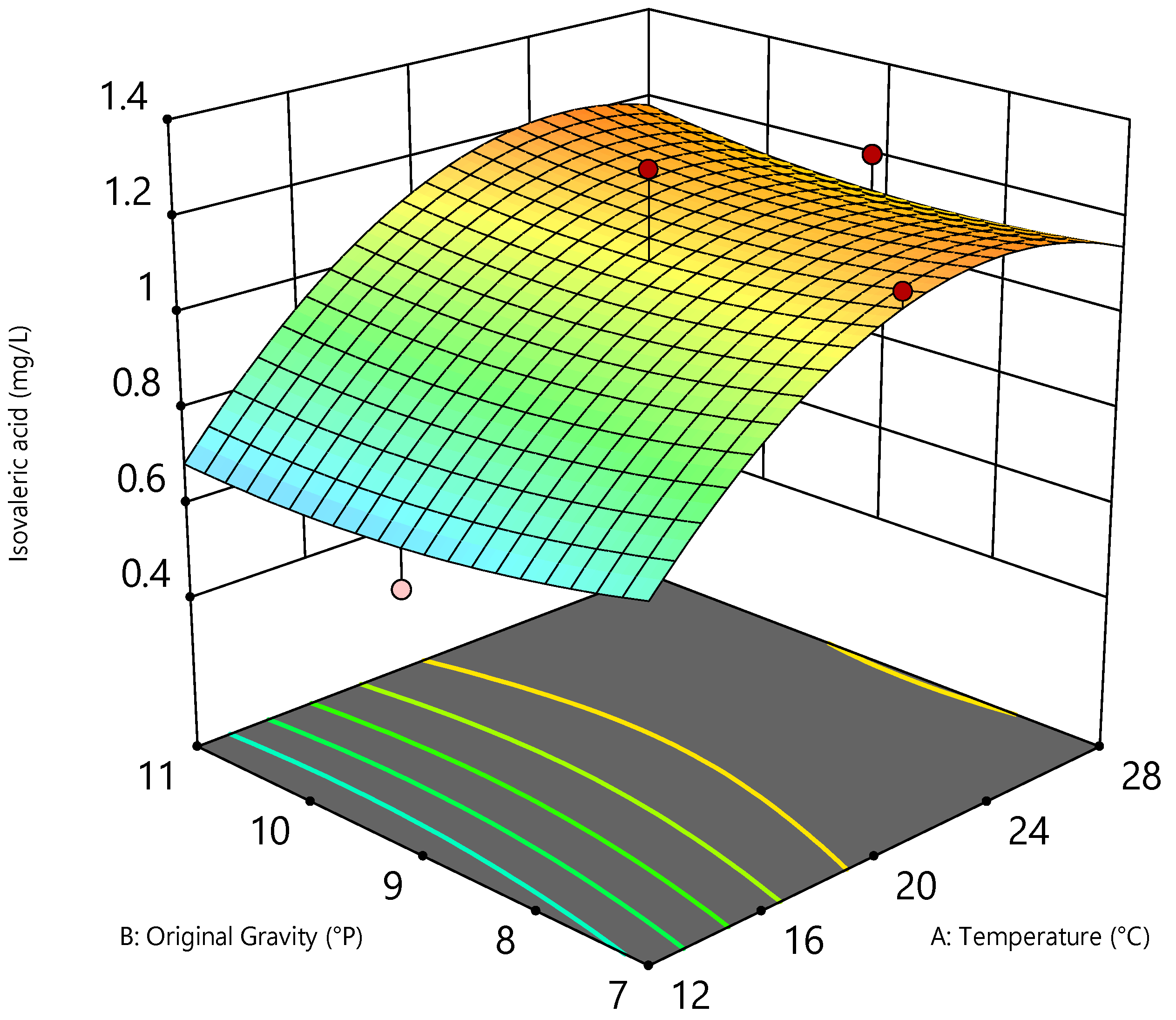
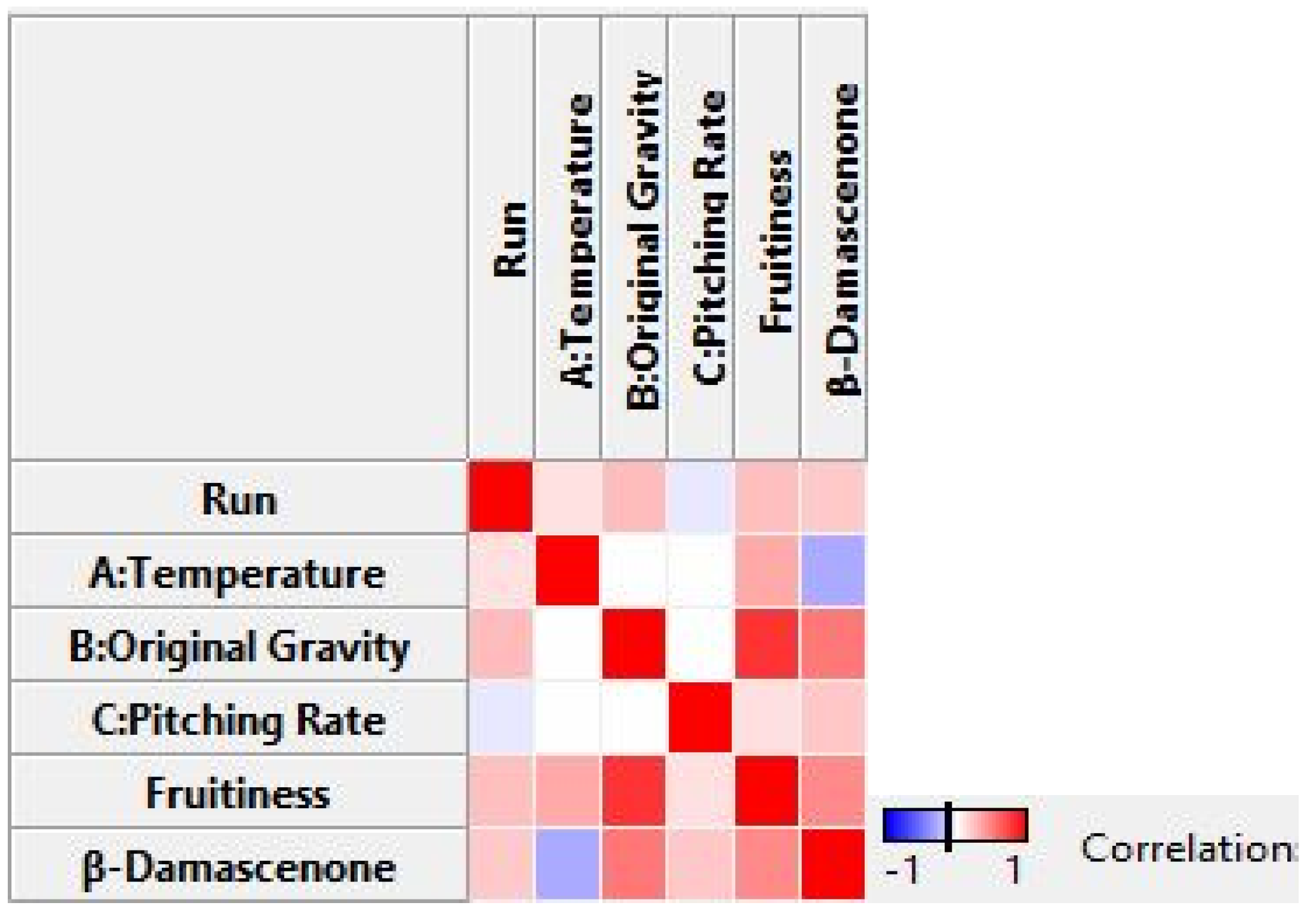
3.3. Optimization Using RSM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahuja, K.; Rawat, A. Market Size of Non-Alcoholic Beer Worldwide from 2016 to 2024 (in Billion U.S. Dollars). Available online: https://www.gminsights.com/industry-analysis/non-alcoholic-beer-market (accessed on 17 June 2021).
- Bellut, K.; Arendt, E.K. Chance and Challenge: Non-Saccharomyces Yeasts in Nonalcoholic and Low Alcohol Beer Brewing—A Review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Gibson, B.; Krogerus, K.; Ekberg, J.; Mikkelson, A.; Pentikäinen, S.; Wilpola, A.; Vidgren, V. Non-conventional Yeast as a New Tool for Beer Flavour Modification. In Proceedings of the EBC Congress, Porto, Portugal, 24–28 May 2015. [Google Scholar]
- de Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Quek, A.Y.H. Evaluation of Beer Fermentation with a Novel Yeast Williopsis saturnus. Food Technol. Biotechnol. 2016, 54, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for new brewing yeasts in the non-Saccharomyces sector with Torulaspora delbrueckii as model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Methner, Y.; Hutzler, M.; Zarnkow, M.; Prowald, A.; Endres, F.; Jacob, F. Investigation of Non-Saccharomyces Yeast Strains for Their Suitability for the Production of Non-Alcoholic Beers with Novel Flavor Profiles. J. Am. Soc. Brew. Chem. 2022; Ahead-of-print. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; Atzler, J.J.; Hoehnel, A.; Lynch, K.M.; Arendt, E.K. Screening and Application of Cyberlindnera Yeasts to Produce a Fruity, Non-Alcoholic Beer. Fermentation 2019, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P. (Ed.) The Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123847072. [Google Scholar]
- Guido, L.F.; Carneiro, J.R.; Santos, J.R.; Almeida, P.J.; Rodrigues, J.A.; Barros, A.A. Simultaneous determination of E-2-nonenal and β-damascenone in beer by reversed-phase liquid chromatography with UV detection. J. Chrom. A 2004, 1032, 17–22. [Google Scholar] [CrossRef]
- Lee, P.-R.; Ong, Y.-L.; Yu, B.; Curran, P.; Liu, S.-Q. Evolution of volatile compounds in papaya wine fermented with three Williopsis saturnus yeasts. Int. J. Food Sci. Technol. 2010, 45, 2032–2041. [Google Scholar] [CrossRef]
- Schieberle, P. Primary odorants of pale lager beer. Eur. Food Res. Technol. 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Piornos, J.A.; Balagiannis, D.P.; Methven, L.; Koussissi, E.; Brouwer, E.; Parker, J.K. Elucidating the odor-active aroma compounds in alcohol-free beer and their contribution to the worty flavor. J. Agric. Food Chem. 2020, 68, 10088–10096. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database Version 1; Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany, 2022; Available online: https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database (accessed on 24 January 2022).
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Schneiderbanger, H.; Haselbeck, K.; Zarnkow, M.; Hutzler, M. Optimization of Beer Fermentation with a Novel Brewing Strain Torulaspora delbrueckii Using Response Surface Methodology. TQ 2017, 54, 23–33. [Google Scholar] [CrossRef]
- Methner, Y.; Hutzler, M.; Matoulková, D.; Jacob, F.; Michel, M. Screening for the Brewing Ability of Different Non-Saccharomyces Yeasts. Fermentation 2019, 5, 101. [Google Scholar] [CrossRef] [Green Version]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Saison, D.; de Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Gijs, L.; Chevance, F.; Jerkovic, V.; Collin, S. How low pH can intensify beta-damascenone and dimethyl trisulfide production through beer aging. J. Agric. Food Chem. 2002, 50, 5612–5616. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.-P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Puerari, C.; Strejc, J.; Souza, A.C.; Karabín, M.; Schwan, R.F.; Brányik, T. Optimization of alcohol-free beer production by lager and cachaça yeast strains using response surface methodology. J. Instig. Brew. 2016, 122, 69–75. [Google Scholar] [CrossRef] [Green Version]
- de Schutter, D.P.; Saison, D.; Delvaux, F.; Derdelinckx, G.; Rock, J.-M.; Neven, H.; Delvaux, F.R. Release and evaporation of volatiles during boiling of unhopped wort. J. Agric. Food Chem. 2008, 56, 5172–5180. [Google Scholar] [CrossRef]
- Siefker, J.A.; Pollock, G.E. Melanoidins in the Brewing Processes. I. Formation of Aldehydes during Wort Boiling. Proc. Annu. Meet.—Am. Soc. Brew. Chem. 1956, 14, 5–12. Available online: https://www.tandfonline.com/doi/abs/10.1080/00960845.1956.12006472?journalCode=ujbc19 (accessed on 17 June 2021). [CrossRef]
- Gernat, D.C.; Brouwer, E.; Ottens, M. Aldehydes as Wort Off-Flavours in Alcohol-Free Beers—Origin and Control. Food Bioprocess Technol. 2020, 13, 195–216. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Phylogenetic relationships among yeasts of the ? complex? determined from multigene sequence analyses. FEMS Yeast Res. 2003, 3, 417–432. [Google Scholar] [CrossRef] [Green Version]
- van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Sen, A.; Laskawy, G.; Schieberle, P.; Grosch, W. Quantitative determination of beta-damascenone in foods using a stable isotope dilution assay. J. Agric. Food Chem. 1991, 39, 757–759. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Bemelmans, J.M.H. Review of isolation and concentration techniques. Prog. Flavour Res. 1979, 8, 79–98. [Google Scholar]
- Hildebrandt, G.; Schneider-Haeder, B. Sensorische Analyse: Methodenüberblick und Einsatzbereiche—DLG-Sensorik; DLG e.V.: Frankfurt am Main, Germany, 2016; Available online: www.dlg.org (accessed on 29 November 2021).
- Narziss, L.; Back, W.; Gastl, M.; Zarnkow, M. Abriss der Bierbrauerei, 8th ed.; John Wiley & Sons Incorporated: Newark, NJ, USA, 2017; ISBN 9783527340361. [Google Scholar]
- Leskošek, I.; Stojanović, M. A possible application of ale brewery strains of Saccharomyces cerevisiae in lager beer production. World J. Microbiol. Biotechnol. 1993, 9, 70–72. [Google Scholar] [CrossRef]
- KRUGER, L. Yeast metabolism and its effect on flavour: Part. Brew. Guard. 1998, 127, 27–30. [Google Scholar]
- Back, W.; Bohak, I. Ausgewählte Kapitel der Brauereitechnologie; 2. aktualisierte Aufl.; Carl: Nürnberg, Germany, 2008; ISBN 9783418008028. [Google Scholar]
- Meilgaard, M.C. Flavor chemistry of beer. II. Flavor and threshold of 239 aroma volatiles. Tech. Quart. Master. Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Sannino, C.; Mezzasoma, A.; Buzzini, P.; Turchetti, B. Non-conventional Yeasts for Producing Alternative Beers. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 361–388. ISBN 978-3-030-21109-7. [Google Scholar]
- Collin, S.; Derdelinckx, G.; Dufour, J.-P. Relationships between the chemical composition and sensory evaluation of lager beers. Food Qual. Prefer. 1994, 5, 145–149. [Google Scholar] [CrossRef]
- Fritsch, H.T.; Schieberle, P. Identification based on quantitative measurements and aroma recombination of the character impact odorants in a Bavarian Pilsner-type beer. J. Agric. Food Chem. 2005, 53, 7544–7551. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer volatile compounds and their application to low-malt beer fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The chemical profiling of fatty acids during the brewing process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, D.B.; Jones, R.D.; Barrbett, P.A.; Kavanagh, T.E.; Clarke, B.J. Methods for the analysis of C4 to C10 fatty acids in beer, wort and carbohydrate syrups. J. Inst. Brew. 1986, 92, 181–184. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. 125th Anniversary Review: Diacetyl and its control during brewery fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Kanauchi, M. Enzymology of Vicinal Diketone Reduction in Brewer’s Yeast. J. Inst. Brew. 2004, 110, 83–93. [Google Scholar] [CrossRef]
- Kishimoto, T.; Wanikawa, A.; Kagami, N.; Kawatsura, K. Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar-sorptive extraction method with GC-MS. J. Agric. Food Chem. 2005, 53, 4701–4707. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.d.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, D.; Li, g.; Hao, J.; Jiang, W.; Liu, Z.; Qin, Q. Flavor Contribution of Esters in Lager Beers and an Analysis of Their Flavor Thresholds. J. Am. Soc. Brew. Chem. 2017, 75, 201–206. [Google Scholar] [CrossRef]
- Rettberg, N.; Biendl, M.; Garbe, L.-A. Hop Aroma and Hoppy Beer Flavor: Chemical Backgrounds and Analytical Tools—A Review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Engan, S. Organoleptic Threshold Values of Some Alcohols and Esters in Beer. J. Inst. Brew. 1972, 78, 33–36. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Delvaux, F.R. Esters in beer-part 1: The fermentation process: More than ethanol formation. Cerevisia 2003, 28, 41–49. [Google Scholar]
- Schieberle, P.; Komarek, D. Changes in Key Aroma Compounds during Natural Beer Aging. In Freshness and Shelf Life of Foods; Cadwallader, K.R., Weenen, H., Eds.; American Chemical Society: Washington, DC, USA, 2003; pp. 70–79. ISBN 9780841238015. [Google Scholar]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef] [PubMed]
- Kotseridis, Y.; Baumes, R.; Skouroumounis, G.K. Synthesis of labelled [2H4] β-damascenone, [2H2] 2-methoxy-3-isobutylpyrazine, [2H3] α-ionone, and [2H3] β-ionone, for quantification in grapes, juices and wines. J. Chrom. A 1998, 824, 71–78. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two bavarian wheat beers by means of the sensomics approach. J. Agric. Food Chem. 2013, 61, 11303–11311. [Google Scholar] [CrossRef]
- Chevance, F.; Guyot-Declerck, C.; Dupont, J.; Collin, S. Investigation of the beta-damascenone level in fresh and aged commercial beers. J. Agric. Food Chem. 2002, 50, 3818–3821. [Google Scholar] [CrossRef]
- Lloyd, N.D.R.; Capone, D.L.; Ugliano, M.; Taylor, D.K.; Skouroumounis, G.K.; Sefton, M.A.; Elsey, G.M. Formation of Damascenone under both commercial and model fermentation conditions. J. Agric. Food Chem. 2011, 59, 1338–1343. [Google Scholar] [CrossRef]
- de Schutter, D.P.; Saison, D.; Delvaux, F.; Derdelinckx, G.; Rock, J.-M.; Neven, H.; Delvaux, F.R. Characterization of volatiles in unhopped wort. J. Agric. Food Chem. 2008, 56, 246–254. [Google Scholar] [CrossRef]

| Run | Factor 1 A: Temperature (°C) | Factor 2 B: Original Gravity (°P) | Factor 3 C: Pitching Rate (×106 cells/mL) |
|---|---|---|---|
| 1 | 20 | 7 | 15 |
| 2 | 28 | 9 | 15 |
| 3 * | 20 | 9 | 15 |
| 4 | 12 | 7 | 25 |
| 5 | 12 | 9 | 15 |
| 6 | 20 | 11 | 15 |
| 7 * | 20 | 9 | 15 |
| 8 * | 20 | 9 | 15 |
| 9 | 28 | 7 | 5 |
| 10 * | 20 | 9 | 15 |
| 11 | 20 | 9 | 25 |
| 12 | 12 | 7 | 5 |
| 13 | 28 | 11 | 25 |
| 14 | 12 | 11 | 5 |
| 15 | 12 | 11 | 25 |
| 16 | 20 | 9 | 5 |
| 17 | 28 | 11 | 5 |
| 18 | 28 | 7 | 25 |
| 19 * | 20 | 9 | 15 |
| 20 * | 20 | 9 | 15 |
| Analysis | Method | Device |
|---|---|---|
| Original gravity, ethanol content, apparent attenuation | MEBAK WBBM 2.9.6.3 | Bending vibration and NIR spectroscopy, Alcolyzer Plus with DMA 5000 X sample 122 (Anton-Paar GmbH, Ostfildern, Germany) |
| pH value | MEBAK WBBM 2.13 | pH meter with pH electrode, ProfiLine pH3210 pH meter (Xylem Inc., New York, NY, USA) |
| (Methylsulfanyl) methane (dimethyl sulfide) | MEBAK WBBM 2.23.1.1 | GC-FID Clarus 580 (Perkin Elmer, Waltham, MA, USA), Column: 50 m × 0.32 mm Phenomenex FFAP, 0.25 μm |
| Fatty acid esters, fatty acids, 2-phenylethan-1-ol | MEBAK WBBM 2.23.6 | GC-FID Clarus 580 (Perkin Elmer, Waltham, MA, USA), Column: 50 m × 0.32 mm Phenomenex FFAP, 0.25 μm |
| Acetaldehyde, ethyl acetate, isoamyl acetate, higher alcohols (propan-1-ol (n-propanol), 2-methylpropan-1-ol (isobutanol), amyl alcohols), ethyl methanoate (ethyl formate), ethyl propanoate (ethyl propionate) | MEBAK WBBM 2.21.1 | GC-FID Clarus 580, Turbo Matrix 40, Head Space (Perkin Elmer, Waltham, MA, USA), Column: INNOWAX cross-linked polyethylene glycol, 60 m × 0.32 mm × 0.5 μm |
| Diacetyl, pentane-2,3-dione | MEBAK WBBM 2.21.5.1 | GC-FID Clarus 580, Turbo Matrix 40, Head Space (Perkin Elmer, Waltham, MA, USA), Column: INNOWAX cross-linked polyethylene glycol, 60 m × 0.32 mm × 0.5 μm |
| Response | Unit | Minimum | Maximum | Model | p-Value | LOF p-Value |
|---|---|---|---|---|---|---|
| ∑ Esters | mg/L | 2.1 | 3.6 | Quadratic | 0.0012 | 0.1928 |
| Isoamyl acetate | mg/L | 0.7 | 1.9 | Linear | 0.0066 | 0.0065 * |
| Isovaleric acid | mg/L | 0.53 | 1.30 | Quadratic | <0.0001 | 0.8221 |
| Diacetyl | mg/L | 0.06 | 0.19 | Linear | 0.0005 | 0.4825 |
| (E)-β-damascenone | µg/L | 0.873 | 1.57 | Linear | 0.0058 | <0.0001 * |
| Ethanol | % (v/v) | 0.11 | 0.63 | 2FI 1 | <0.0001 | 0.0210 * |
| Fruitiness | % | 50 | 77 | Linear | <0.0001 | <0.0001 * |
| Sum DLG | points | 3.93 | 4.70 | Quadratic | 0.0002 | <0.0001 * |
| Run | Original Gravity (°P) | Ethanol (% v/v) | Apparent Attenuation (%) | pH Value Beer |
|---|---|---|---|---|
| 1 | 6.90 | 0.26 | 7.6 | 4.83 |
| 2 | 8.90 | 0.48 | 7.6 | 4.76 |
| 3 * | 8.94 | 0.29 | 6.4 | 4.86 |
| 4 | 6.88 | 0.18 | 5.2 | 4.93 |
| 5 | 8.88 | 0.15 | 3.3 | 5.00 |
| 6 | 10.84 | 0.29 | 5.1 | 4.91 |
| 7 * | 8.95 | 0.27 | 5.8 | 4.87 |
| 8 * | 8.89 | 0.26 | 5.7 | 4.88 |
| 9 | 6.89 | 0.19 | 5.5 | 4.89 |
| 10 * | 8.86 | 0.28 | 6.2 | 4.91 |
| 11 | 8.92 | 0.32 | 6.9 | 4.86 |
| 12 | 6.89 | 0.11 | 3.3 | 5.01 |
| 13 | 10.81 | 0.63 | 8.8 | 4.88 |
| 14 | 10.87 | 0.14 | 2.5 | 5.10 |
| 15 | 10.79 | 0.20 | 3.6 | 4.99 |
| 16 | 8.90 | 0.16 | 3.4 | 5.00 |
| 17 | 10.79 | 0.48 | 8.5 | 4.75 |
| 18 | 6.88 | 0.35 | 10.1 | 4.74 |
| 19 * | 8.90 | 0.25 | 5.5 | 4.89 |
| 20 * | 8.87 | 0.29 | 6.4 | 4.85 |
| Run | 1 | 2 | 3 * | 4 | 5 | 6 | 7 * | 8 * | 9 | 10 * | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 * | 20 * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esters (mg/L) | Ethyl acetate | 1.3 | 1.2 | 1.2 | 1.0 | 0.93 | 1.3 | 1.1 | 1.2 | 0.96 | 1.3 | 1.2 | 0.83 | 1.5 | 0.79 | 1.1 | 1.3 | 1.0 | 1.1 | 1.1 | 1.2 |
| Isoamyl acetate | 1.4 | 0.8 | 1.1 | 0.8 | 0.7 | 1.0 | 1.0 | 1.2 | 1.9 | 1.2 | 1.3 | 1.3 | 0.8 | 1.2 | 0.7 | 2.7 | 1.3 | 0.8 | 1.1 | 1.1 | |
| 2-Phenylethyl acetate | 0.73 | 0.36 | 0.62 | 0.50 | 0.38 | 0.62 | 0.65 | 0.59 | 0.67 | 0.57 | 0.70 | 0.58 | 0.39 | 0.49 | 0.42 | 1.1 | 0.43 | 0.45 | 0.62 | 0.81 | |
| Isobutyl acetate | 0.01 | 0.01 | 0.01 | <LOQ | <LOQ | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | <LOQ | 0.01 | 0.01 | <LOQ | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | |
| Ethyl butanoate | <LOQ | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| Ethyl hexanoate | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.02 | 0.03 | 0.01 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Ethyl octanoate | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.01 | <LOQ | <LOQ | <LOQ | 0.01 | 0.01 | <LOQ | <LOQ | <LOQ | |
| Organic acids (mg/L) | Isovaleric acid | 1.2 | 1.2 | 1.1 | 0.76 | 0.61 | 1.1 | 1.1 | 1.1 | 0.74 | 1.0 | 1.1 | 0.54 | 1.2 | 0.53 | 0.62 | 0.83 | 0.86 | 1.2 | 1.1 | 1.3 |
| Higher alcohols (mg/L) | n-Propanol | 1.8 | 3.1 | 2.0 | 1.0 | 1.1 | 1.9 | 1.5 | 1.8 | 2.0 | 2.1 | 2.0 | 0.9 | 3.7 | 1.0 | 1.1 | 1.1 | 2.4 | 2.4 | 1.6 | 2.1 |
| Isobutyl alcohol | 7.3 | 11.1 | 6.8 | 2.8 | 2.4 | 6.3 | 5.7 | 5.9 | 9.6 | 7.4 | 7.2 | 1.8 | 13.2 | 1.9 | 2.8 | 5.4 | 9.7 | 10.5 | 6.1 | 6.9 | |
| Isoamyl alcohols | 15.9 | 18.6 | 15.6 | 10.3 | 8.8 | 16.2 | 13.4 | 14.7 | 13.0 | 16.1 | 19.6 | 5.4 | 23.4 | 5.9 | 9.7 | 9.1 | 12.8 | 18.4 | 14.5 | 16.0 | |
| Aldehydes (mg/L) | Acetaldehyde | 1.4 | 1.7 | 1.7 | 1.6 | 1.2 | 1.2 | 1.3 | 1.6 | 1.8 | 1.8 | 1.7 | 1.3 | 2.1 | 1.2 | 1.4 | 1.5 | 1.7 | 1.8 | 1.3 | 1.6 |
| Ketones (mg/L) | Diacetyl | 0.11 | 0.29 | 0.14 | 0.12 | 0.10 | 0.10 | 0.11 | 0.10 | 0.15 | 0.13 | 0.10 | 0.06 | 0.19 | 0.08 | 0.12 | 0.08 | 0.15 | 0.16 | 0.10 | 0.14 |
| Sulfide (mg/L) | Dimethyl sulfide | 0.016 | 0.022 | 0.024 | 0.015 | 0.023 | 0.028 | 0.022 | 0.021 | 0.017 | 0.022 | 0.024 | 0.020 | 0.029 | 0.029 | 0.030 | 0.021 | 0.031 | 0.019 | 0.023 | 0.022 |
| Run | Conc. (E)-β-Damascenone 1 (μg/L) | Range (E)-β-Damascenone (μg/L) |
|---|---|---|
| 1 | 0.873 | 0.787–0.941 |
| 2 | 1.01 | 0.967–1.06 |
| 3 * | 1.43 | 1.37–1.48 |
| 4 | 1.13 | 1.09–1.18 |
| 5 | 1.27 | 1.21–1.34 |
| 6 | 1.54 | 1.50–1.59 |
| 9 | 0.978 | 0.943–1.04 |
| 11 | 1.46 | 1.35–1.52 |
| 12 | 0.999 | 0.934–1.03 |
| 13 | 1.07 | 0.971–1.13 |
| 14 | 1.25 | 1.17–1.34 |
| 15 | 1.57 | 1.53–1.61 |
| 16 | 1.29 | 1.25–1.33 |
| 17 | 1.15 | 1.07–1.22 |
| 18 | 1.05 | 1.01–1.08 |
| Run | Sum DLG | Fruitiness (%) |
|---|---|---|
| 1 | 4.25 | 53 |
| 2 | 4.60 | 68 |
| CS * | 4.70 | 68 |
| 4 | 4.23 | 50 |
| 5 | 4.60 | 65 |
| 6 | 4.50 | 68 |
| 9 | 4.32 | 57 |
| 11 | 4.63 | 62 |
| 12 | 3.93 | 38 |
| 13 | 4.65 | 77 |
| 14 | 4.67 | 75 |
| 15 | 4.70 | 68 |
| 16 | 4.48 | 62 |
| 17 | 4.60 | 73 |
| 18 | 4.40 | 63 |
| Run | OAV 1 | ||||||
|---|---|---|---|---|---|---|---|
| (E)-β-Damascenone | Isoamyl Acetate | Ethyl Hexanoate | Ethyl Ocanoate | Ethyl Decanoate | 2-Phenylethyl Acetate | Acetaldehyde | |
| 1 | 150 | 190 | 17 | <1 | <1 | 2.0 | 88 |
| 2 | 170 | 110 | 17 | <1 | <1 | 1.0 | 110 |
| CS * | 240 | 150 | 17 | <1 | <1 | 1.8 | 97 |
| 4 | 190 | 110 | 25 | <1 | <1 | 1.4 | 100 |
| 5 | 210 | 97 | 25 | <1 | <1 | 1.1 | 75 |
| 6 | 260 | 140 | 17 | <1 | <1 | 1.7 | 75 |
| 9 | 160 | 260 | 25 | <1 | <1 | 1.9 | 110 |
| 11 | 240 | 180 | 17 | <1 | <1 | 1.9 | 110 |
| 12 | 170 | 180 | 25 | 1.1 | <1 | 1.6 | 81 |
| 13 | 180 | 110 | 8.3 | <1 | <1 | 1.1 | 130 |
| 14 | 210 | 170 | 25 | <1 | <1 | 1.4 | 75 |
| 15 | 260 | 97 | 25 | <1 | <1 | 1.2 | 88 |
| 16 | 220 | 380 | 17 | 1.1 | <1 | 3.1 | 94 |
| 17 | 190 | 180 | 17 | 1.1 | <1 | 1.2 | 110 |
| 18 | 180 | 110 | 17 | <1 | <1 | 1.3 | 110 |
| Response | Unit | Predicted Mean | Observed Mean | Standard Deviation | 95% PI Low | 95% PI High |
|---|---|---|---|---|---|---|
| ∑ Esters | mg/L | 3.2 | 6.1 | 0.18 | 2.73 | 3.57 |
| Isoamyl acetate * | mg/L | 1.2 | 3.8 | 0.22 | 0.88 | 1.59 |
| Isovaleric acid | mg/L | 0.73 | 0.53 | 0.08 | 0.56 | 0.90 |
| Diacetyl | mg/L | 0.09 | 0.08 | 0.02 | 0.06 | 0.12 |
| (E)-β-damascenone * | µg/L | 1.30 | 1.41 | 0.14 | 1.07 | 1.54 |
| Ethanol * | % (v/v) | 0.19 | 0.19 | 0.04 | 0.13 | 0.26 |
| Fruitiness * | % | 71 | 87 | 3.67 | 64 | 76 |
| Sum DLG * | points | 4.62 | 4.61 | 0.08 | 4.45 | 4.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Methner, Y.; Dancker, P.; Maier, R.; Latorre, M.; Hutzler, M.; Zarnkow, M.; Steinhaus, M.; Libkind, D.; Frank, S.; Jacob, F. Influence of Varying Fermentation Parameters of the Yeast Strain Cyberlindnera saturnus on the Concentrations of Selected Flavor Components in Non-Alcoholic Beer Focusing on (E)-β-Damascenone. Foods 2022, 11, 1038. https://doi.org/10.3390/foods11071038
Methner Y, Dancker P, Maier R, Latorre M, Hutzler M, Zarnkow M, Steinhaus M, Libkind D, Frank S, Jacob F. Influence of Varying Fermentation Parameters of the Yeast Strain Cyberlindnera saturnus on the Concentrations of Selected Flavor Components in Non-Alcoholic Beer Focusing on (E)-β-Damascenone. Foods. 2022; 11(7):1038. https://doi.org/10.3390/foods11071038
Chicago/Turabian StyleMethner, Yvonne, Philipp Dancker, Robin Maier, Mailen Latorre, Mathias Hutzler, Martin Zarnkow, Martin Steinhaus, Diego Libkind, Stephanie Frank, and Fritz Jacob. 2022. "Influence of Varying Fermentation Parameters of the Yeast Strain Cyberlindnera saturnus on the Concentrations of Selected Flavor Components in Non-Alcoholic Beer Focusing on (E)-β-Damascenone" Foods 11, no. 7: 1038. https://doi.org/10.3390/foods11071038
APA StyleMethner, Y., Dancker, P., Maier, R., Latorre, M., Hutzler, M., Zarnkow, M., Steinhaus, M., Libkind, D., Frank, S., & Jacob, F. (2022). Influence of Varying Fermentation Parameters of the Yeast Strain Cyberlindnera saturnus on the Concentrations of Selected Flavor Components in Non-Alcoholic Beer Focusing on (E)-β-Damascenone. Foods, 11(7), 1038. https://doi.org/10.3390/foods11071038








