Abstract
The microalgae Spirulina may be a popular dietary supplement rich in essential nutrients and vitamins, but oversight of the supplement industry, in general, remains limited, and increasing incidents of adulteration, misbranding, and undeclared ingredients together with misleading claims create potential risks. In response, this study characterized the elemental, amino acid and fatty acid content of commercially available Spirulina supplements in Slovenia using EDXRF, ICP-MS and GC-MS and compared the results with their nutritional declaration. The gathered data confirm that Spirulina supplements are a good source of calcium (0.15 to 29.5% of RDA), phosphorous (3.36–26.7% of RDA), potassium (0.5 to 7.69% of RDA) and selenium (0.01 to 38.6% of RDA) when consumed within recommended amounts. However, although iron contents were relatively high (7.64 to 316% of RDA), the actual bioavailability of iron was much lower since it was mainly present as the ferric cation. This study also confirms that pure Spirulina supplements are a good source of essential and non-essential amino acids, and ω-6 but not ω-3 polyunsaturated fatty acids. The presence of additives resulted in significant variation in nutrient content and, in some instances, lower product quality. Moreover, a high proportion (86.7%) of inappropriate declarations regarding the elemental content was observed. Overall, the study conclusions underline the need for a stricter control system for Spirulina-based supplements.
Keywords:
Spirulina; microalgae; cyanobacteria; elements; toxic elements; amino acids; fatty acids; authenticity; safety; quality 1. Introduction
The challenges associated with living sustainability, keeping food production costs down while meeting increasing food demand, has meant that sourcing alternative lipid, protein, pigment and polymer sources has become a global trend. In this context, microalgae rich in functional nutrients that positively affect human health is an excellent example of an alternative nutrient source [1,2,3,4,5]. Spirulina, or correctly Arthrospira spp., is one of the most important microalgal groups currently produced and contains macro- and micronutrients such as high-quality proteins, minerals, vitamins, fatty acids, polysaccharides and other bioactive compounds [6,7,8,9].
Spirulina is a multicellular filamentous cyanobacterium (blue-green microalgae) with nitrogen-fixing symbiotic bacteria. Its multicellular cylindrical trichomes are typically arranged along its entire length in a left-handed open helix, and its surface is without covering and smooth. It is a photosynthetic autotroph with phycocyanin as its primary photosynthetic pigment [10,11]. Spirulina is an excellent source of iron, calcium and phosphorous, pigments (carotenoids, c-phycocyanin, chlorophyll-a), and vitamins (vitamin E, vitamin B12). It is also a rich source of digestible proteins (up to 70% of its protein content), polysaccharides and lipids and has a well-balanced amino acid profile. It is also regarded as a good source of essential fatty acids, including ω-6 linoleic and γ-linolenic fatty acid, as well as ω-3 fatty acids such as α-linolenic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [9,12,13]. It is suggested that this high nutritional value could positively influence the treatment of several pathological conditions such as certain cancers, hepatotoxicity, cardiovascular disease and hyperlipidemia, among others [14,15,16,17]. The lack of cellulose in its cell wall and the absence of phytates and oxalates means that nutrient assimilation in the human gut is high, making it popular among consumers [18].
The Spirulina and Spirulina-based product market is expected to show continued and rapid growth until 2028 with a compound annual growth rate (CAGR) of 18.1%. Most microalgae-based commercial products are produced in Asia or Australia, while European companies account for approximately 5% of the global food/feed microalgae market [4]. The demand for Spirulina products is attributed to increasing health awareness and vegetarianism, malnutrition, dietary supplement intake, and the demand for natural colorings. At present, most production is directed towards food, supplements and nutraceuticals, which together account for 75% of the reported uses [4]. Spirulina supplements are segmented into powder, the most popular form, as well as flakes, capsules, tablets, frozen Spirulina and phycocyanin extract, and are promoted by producers and suppliers as a health food [19].
The cultivation and commercialization, such as processing and packaging methods and transport of Spirulina, can change its chemical composition, affecting its nutritional quality and toxicological properties [20]. Studies have shown the presence of cadmium, mercury, arsenic and lead in Spirulina products in anomalous quantities due to pesticide or fertilizer use adjacent to Spirulina cultivation areas [21]. Such contamination is of concern because dietary supplements are not strictly regulated or inspected to the same extent as other food products and pharmaceuticals. Further increased market demand and high cost and complexity of Spirulina culturing encourage the adulteration of these products with inferior cheaper materials/ingredients, e.g., flour and mung bean powder, resulting in economic losses and potentially putting consumers at risk [22,23].
Combatting adulteration, increasing consumer trust and guaranteeing the quality and safety of Spirulina products available on the market requires that products are regularly monitored [23,24,25]. This study focused on the characterization of commercial Spirulina supplements sold on the Slovenian market, including elemental, toxic elemental, amino acid, and fatty acid composition, and their compliance with their product declaration and identifying possible adulterations. Furthermore, the bioavailability of Fe was estimated for the first time.
2. Materials and Methods
2.1. Samples
Forty-six Spirulina dietary supplements were purchased from different health food stores/supermarkets and online stores in Slovenia (Table 1). The samples were collected over two months. Forty-four supplements (95.7%) were labelled as Spirulina spp., while two samples (4.35%) were mixed with other algae (Chlorella, Lithothamnium) and plant-based nutritional supplements, e.g., wheatgrass and barley grass. Among the Spirulina-only samples, 34 samples (73.9%) were labelled as pure Spirulina, while the remaining ten (21.7%) also contained additives. The samples were either fresh or in powder, tablet, or capsule form originating from Italy, Portugal, Japan, China, India, Taiwan, and Hawaii.

Table 1.
List of Spirulina supplements purchased from the Slovenian market, including their origin, product content and form as declared on the product label. The samples are clustered according to the declared country of origin.
2.2. Sample Preparation
Fresh samples were freeze-dried and ground to obtain a fine powder, the tablets were also finely ground, and the capsules were opened and the contents used for analysis. The samples were stored in plastic containers and kept refrigerated (4 °C) until analysis. Samples were analyzed in duplicate in small batches. All analyses were performed within one to three months after collection.
2.3. Macro-Elemental Analysis by X-ray Fluorescence Spectrometry
The macro-elemental composition (Si, P, S, Cl, K, Ca, Ti, Mn, Fe, Zn, Br, Rb and Sr) was determined non-destructively using Energy Dispersive X-Ray Fluorescence Spectrometry (EDXRF). Pellets (0.5–1.0 g) were prepared using a pellet die and a hydraulic press, and the disc radioisotope excitation sources Fe-55 (25 mCi, Eckert & Ziegler, Berlin, Germany) and Cd-109 (20 mCi, Eckert & Ziegler, Berlin, Germany) used for fluorescence excitation. Fluorescence was measured using the EDXRF spectrometer with an XR-100 SDD detector (Amptek, Bedford, MA, USA), a PX5 digital pulse processor (Amptek, Bedford, MA, USA), and a PC-based, multichannel analyzer software package (DPPMCA). In Fe-55 mode, the spectrometer was equipped with a vacuum chamber to measure light elements (Si, P, S, and Cl), and in Cd-109 mode, in the air for K, Ca, Ti, Mn, Fe, Zn, Br, Rb and Sr. The energy resolution was 125 eV at 5.9 keV. X-ray spectra were analyzed using AXIL Spectral Analysis software. Quantification was performed using the Quantitative Analysis of Environmental Samples (QAES) software developed in-house [26,27]. The estimated uncertainty budget of the EDXRF analysis was 11%. The method was validated by analyzing NIST 1547 (peach leaves) and NIST 1573a (tomato leaves).
2.4. Iron Speciation by Fe K-Edge X-ray Absorption Near Edge Structure (XANES)
Powdered Spirulina samples were pressed into pellets (0.1–0.3 g), fixed on Teflon holders with Fe free scotch tape and mounted on an LN2 cooled stage. X-ray absorption spectra were obtained in fluorescence detection mode using an unfocused CLÆSS beamline at the ALBA synchrotron facility (ALBA, Barcelona, Spain). A pair of horizontal and vertical slits allowed the reduction of the beam size on the sample to about 5 mm × 1 mm, illuminating a major part of the pellet. A silicon (Si 111) double crystal monochromator was used with 1 eV resolution at the Fe K-edge (7112 eV). The samples were inserted between the first and the second ionization cell at 45° relative to the beam. An SDD fluorescence detector, positioned at 90° to the beam, was used to measure the intensity of the Fe-Kα fluorescence radiation. The fluorescence spectra were recorded as the ratio of the fluorescence detector signal and the signal of the incident photon beam from the first ionization chamber with an integration time of 4 s/step. The absorption spectra were measured within the interval −150 eV to 350 eV relative to the Fe K-edge. In the XANES region, equidistant energy steps of 0.2 eV were used and 1 eV steps elsewhere. Three replicates were measured to check scan reproducibility and improve the signal-to-noise ratio. No evidence of Fe K-edge shifts in consecutive scans of the samples was observed due to the absorbed dose of ionizing radiation. The monochromator’s exact energy was calibrated against a 5 μm thick Fe metal foil. The first inflection point in the XANES spectrum of Fe metal was at 7112 eV, while the absolute energy reproducibility was ± 0.03 eV or better. The Fe K-edge XANES spectra were analyzed using the IFEFFIT software package ATHENA [28].
The bioavailability of Fe was estimated by measuring the relative amounts of Fe2+ (ferrous iron) and Fe3+ (ferric iron). For this, we used the linear combination fit (LCF) method as described in [28,29]. The relative amounts of each Fe cation (Fe2+ and Fe3+) in the sample were determined based on a linear combination fit of the XANES spectrum of the sample, that of the Fe reference compound with known valence states of Fe.
2.5. Toxic Trace Element and Se Analysis by Inductively Coupled Plasma-Mass Spectrometry
Inductively coupled plasma-mass spectrometry (ICP-MS) was used to measure Se, As, Cd, Hg and Pb levels in commercial Spirulina samples. Powdered samples (0.05–0.1 g) were weighed into Teflon vials, followed by 2 mL of 65% HNO3 (Suprapur®, Merck, Darmstadt, Germany). Samples S10, S20, S31, S40, S41, S46 and S47 were prepared in duplicate. The samples were then digested in an UltraWave closed vessel microwave digestion system (Milestone, Sorisole (BG), Italy) at 1500 W and 100 bar maximum pressure. The temperature program was as follows: ramped to 240 °C in 20 min, held for 15 min and then cooled to 40 °C. The digests were then quantitatively transferred to 10 mL polyethylene graduated vials and filled to the mark with MilliQ water. The samples were then filtered through hydrophilic syringe filters (Millipore Millex-HV, Merck, Darmstadt, Germany) (0.45 μm) and diluted in a 1:10 ratio. The reference material BCR-414 (plankton trace elements) and blank samples (HNO3) were prepared similarly.
The samples were then analyzed using a triple quadrupole instrument ICP-QQQ (Agilent 8800, Santa Clara, CA, USA) in 1:10 dilution for Se, As, Cd and Hg and 1:100 dilution for Pb. A dilution of 1:100 was used for Hg in the reference material. Calibration curves were prepared for Se, As, Cd and Pb using MULTI XVI (ICP Multi-Element Standard Solution XVI CertiPUR®, Merck, Darmstadt, Germany) in 5% HNO3. The following concentrations were prepared: 0, 0.1, 0.5, 1, 5, 10, 50, 100, 250 and 1000 ng/mL. The Hg calibration curve was prepared using the NIST 3133 reference material (RM) in a 5% HNO3 solution at concentrations of 0, 0.1, 0.5, 1, 5 and 10 ng/mL.
2.6. Fatty Acid Analysis by Gas Chromatography-Mass Spectrometry Method
The analysis of fatty acids in Spirulina samples was determined using Gas Chromatography-Mass Spectrometry (GC-MS).
2.6.1. Fatty Acid Extraction and Esterification
Powdered Spirulina samples (150 mg) were weighed directly into screw-cap vials, and 500 μL of dichloromethane and 3 mL of 0.5 M sodium hydroxide in methanol were added for total lipid extraction. Samples were then purged with nitrogen and heated for 10 min at 90 °C. Once cool, 3 mL of BF3-MeOH was added to generate the fatty acids methyl esters (FAMEs) and purged with nitrogen. The samples were heated for 10 min at 90 °C. Once cool, the FAMEs were extracted using 1.5 mL of hexane. The hexane phase was transferred directly into a GC vial and stored at −20 °C. All the samples were prepared in triplicate.
2.6.2. FAME Analysis
Analysis was carried out using a 7890B GC and 5977A Series GC/MSD (Agilent, Santa Clara, CA, USA). Separation was achieved on a 30 m × 0.25 mm × 0.25 μm VF-WAXms capillary column (Agilent J&W, Santa Clara, CA, USA). The injection volume was 1 μL, with a split ratio of 10:1. The carrier gas was helium maintained at a 1.5 mL/min constant flow. The injector temperature was 280 °C and the detector temperature 350 °C. The temperature program was as follows: initial column temperature set at 50 °C for 1 min then programmed to 170 °C at 15 °C/min and held for 5 min, then from 170 °C to 200 °C at 3 °C/min, held 5 min, and from 200 °C to 230 °C at 5 °C/min, and held 17 min.
A standard Supelco 37 component FAME Mix in dichloromethane (Bellefonte, PA, USA) was used for identification and quantification. Compounds were identified based on a comparison of retention times with authentic compounds. With each set of samples, blank samples and the FAME Mix standard were analyzed to verify the stability of the analytical system. The results are expressed as the weight percent of an individual fatty acid to the total fatty acid (TFA) content calculated from the peak area using the appropriate correction factors [30].
2.7. Amino Acid Analysis by Gas Chromatography-Mass Spectrometry
The amino acid composition was determined using Gas Chromatography-Mass Spectrometry (GC-MS).
2.7.1. Liquid Phase Hydrolysis
The total amino acid extraction was based on hot protein hydrolysis and simultaneous free amino acid solubilization. Before the analysis, the hydrolysis micro-reaction vessels (5 mL, heavy-wall borosilicate glass, 20 mm × 65 mm, screw top, with a solid phenolic cap) were cleaned by pyrolysis at 500 °C for 6 h and left to cool overnight. The hydrolyzing agent (6N HCl) was prepared fresh from a 30% hydrochloric acid solution. The samples (15 mg) were weighed directly into the reaction vials and the hydrolyzing agent (1 mL) containing 4% thioglycolic acid, which acts as a reducing agent to prevent amino acid oxidation. After, 1% phenol was added to prevent halogenation of the tyrosine. Oxidation was prevented by purging the samples with N2 (5 min). The vials were then sealed and heated at 110 °C for 24 h.
2.7.2. Amino Acid Derivatization
For derivatization, a commercial EZ:faast Amino Acid Hydrolysate kit (Phenomenex, Torrance, CA, USA) was used. The procedure was as follows: 355 μL of the Na2CO3 solution was added to the hydrolysate sample (100 μL) to obtain a pH of 2–2.5. To this was added 20 μL of the norvaline internal standard solution (0.2 mM) and 100 μL 10% n-propanol. The samples were then extracted using solid-phase extraction and the amino acids eluted with 200 μL freshly prepared eluting medium (sodium hydroxide:n-propanol in 3-picoline, 3:2, v/v). Further, the amino acids were derivatized in a mixture of chloroform and propyl chloroformate (50 μL). The amino acids were then extracted into the organic chloroform layer by repeated emulsification and allowing the reactions to proceed for 1 min in between vortexing. Iso-octane (100 μL) was then added, and the mixture was emulsified for an additional 5 s and allowed to react for 1 min. The organic layer was then transferred into a GC vial and reduced to dryness (N2). The amino acid derivatives were then reconstituted in a solution (100 μL) of iso-octane:chloroform (80:20, v/v). All samples were prepared in duplicate.
2.7.3. Gas Chromatography-Mass Spectrometry Method for Amino Acid Analysis
Amino acid analysis was performed using a 7890B GC and 5977A Series GC/MSD (Agilent, Santa Clara, CA, USA). Separation was achieved on a 10 m × 0.25 mm × 0.15 mm ZB-AAA GC column provided in the EZ:faast kit together with a FocusLiner®. The split ratio was 15:1, and the injector temperature was 250 °C. The injection volume was 1.5 μL. Helium was used as the carrier gas at a 1.5 mL/min flow rate. The temperature program was 110 °C to 320 °C at 30 °C/min. The detector temperature was set to 310 °C. Calibration curves were prepared for individual amino acids at concentrations of 50, 100 and 200 nmol/mL using the standard mixture (SD) provided. The amino acid standard mixture consisted of 200 nmoles/mL of each amino acid: alanine (ALA), glutamic acid (GLU), hydroxylysine (HLY), leucine (LEU), phenylalanine (PHE), threonine (THR), valine (VAL), aspartic acid (ASP), glycine (GLY), hydroxyproline (HYP), lysine (LYS), proline (PRO), tryptophan (TRP), cystine (C-C), histidine (HIS), isoleucine (ILE), methionine (Met), serine (SER) and tyrosine (TYR). Each calibrant was prepared in triplicate. From then on, the SD solutions were treated following the same procedure as the samples. Individual amino acids were identified by comparing peak retention times with known amino acids in the standard. The amino acid content results are expressed in mg/g of sample dry weight (dwt).
2.8. Statistical Analysis
Statistical analysis was performed using XLSTAT software (Addinsoft, Long Island, NY, USA, 2019). First, basic statistical methods were used for data analysis (median and quartiles, minimum, maximum, average). Principal component analysis (PCA) was applied further to identify characteristic parameters to discriminate samples based on their macro and trace-elemental composition, amino acid and fatty acid composition. The results are presented as biplots, simultaneous variables and as PCA plots.
3. Results and Discussion
3.1. Elemental Composition
The results are presented in Table 2. The elemental content in the supplements was as follows: Se < Rb < Br < Ti < Zn < Sr < Mn < Fe < Ca < Cl < Si < S < P < K. We specifically focused on elements Fe, Ca, K, Se and P since Spirulina is promoted as a rich source of these elements [13,15,31].

Table 2.
Macro-element composition of Spirulina supplements available on the Slovenian market.
Although the recommended daily intake (RDI) varies, the majority of the producers recommend 3–10 g of Spirulina supplement intake per day, regardless of the product form (powder, capsule or tablet). As well as the RDI, the data are further evaluated using Dietary Reference Values (DRV) such as Population Reference Intake (PRI), which represents the level of nutrient intake adequate for all people in a population group, or Adequate Intake (AI), which is used when a PRI cannot be determined. Where possible, the elemental composition was also evaluated in terms of Tolerable Upper Intake Level (UL), i.e., the maximum daily intake of a nutrient from all sources unlikely to pose adverse health effects on humans [32]. The data are presented as the median value and interquartile range (IR, in parentheses).
Given the recommended daily dose, the minimal intake of Ca is 1.38 to 4.60 mg Ca/day, the maximal Ca intake is 84.0 to 280 mg Ca/day, and a median value of 4.38 (2.92–7.8)–14.6 (9.72–26.0) mg Ca/day. The PRI for Ca is 950 mg/day [33]. Therefore, the values are within the recommended PRI values of 0.15% to 29.5%. This variability can be attributed to the differences in supplement formulation and the presence of additives. For example, values were found in S19, which contains calcium carbonate, and S1, which contains calcium carbonate in the form of edible scallop shell powder [34]. The lowest values were in S41, S42 and S46. The highest amount of Ca was in S18, a mixed sample containing Spirulina, Chlorella and Lithothamnium algae (191–635 mg/day for 3 g to 10 g of supplement/day, respectively). This value is likely due to Lithothamnium calcareum (L. calcareum), a seaweed that crystallizes calcium carbonate in its cell walls [35]. However, the UL of 2500 mg/day is unlikely to be exceeded by including Spirulina supplements in the diet [36].
The minimal and maximal P intake based on RDI was 18.5–61.6 and 44.1–147 mg/day, respectively. The median value was 32.7 (30.3–35.9)–109 (101–120) mg P/day. The P content represents 3.36–26.7% of the AI (550 mg/day) [33]. The lowest value among all samples was in S9, a mixed sample containing wheatgrass, barley grass, Chlorella and Spirulina, i.e., 15.2 (for 3 g of supplement/day)–50.6 mg/day (for 10 g of supplement/day). This most likely results from the lower amount of Spirulina and Chlorella algae in the supplement, especially since Spirulina and Chlorella typically contain much higher phosphorous content than cereal grasses [13,15,37]. Like Ca, if the Spirulina supplement consumption remains within the RDI, the UL determined for P (3000 mg/day) [36] is unlikely to be exceeded.
The minimal intake of K varies from 17.5 to 58.3 mg/day and maximal from 80.7 to 269 mg/day, with a medium intake of 45.6 (42.8–50.0)–152 (143–167) mg/day. This amount would account for 0.5–7.69% of the AI value (3500 mg/day) for an adult [33]. Three of the highest K values were in S5, S44 and S46, where S44 and S46 originate from Italy. The lowest values were measured in S37 and S22, which contained additives and did not declare an origin. No UL was set for K consumption.
The daily intake of Fe (mg Fe/day) was from 0.84–2.81 (minimal value) and 10.4–34.8 (maximal value) with a median of 2.07 (1.47–3.40)–6.89 (4.90–11.3). Such amounts account for between 5.25 to 218% of the PRI for females (16 mg/day) and 7.64 to 316% for males (11 mg/day) [33]. The high deviation observed among the samples is due to the high amount of Fe in S4 and S26 from Hawaii and S2 with no declared origin. High Fe values likely result from a high concentration of Fe in the growth medium. The Fe content in the Spirulina microalgae has been proven to reflect that in the growth medium [38,39].
Iron bioavailability in Spirulina was determined by analyzing the relative ferrous and ferric iron amounts using XANES analysis. The results are presented in Table 3.

Table 3.
Relative amounts of Fe3+ and Fe2+ cations as determined by LCF analysis of Fe K-edge XANES spectra of the Spirulina samples.
The results show that most iron (82–92%) is present as Fe3+, which means that the bioavailability of Fe from the supplements is low, as only a small amount of iron is available in a more bioavailable ferrous form, Fe2+ [40,41,42]. This finding also means that promoting Spirulina as a rich source of dietary iron should be reconsidered.
The minimal and maximal Se intake was 0.01 to 0.04 and 8.10 to 27.0 μg/day, respectively, with the median being 0.30 (0.20–0.80)–1.01 (0.66–2.67) μg Se/day. The amounts of Se also varied significantly, but S14, S15 and S32 stand out due to their high Se value. The former two are from Taiwan, and the latter is from China. High Se values in these samples are believed to be due to higher amounts of Se in the growth medium. However, Se from these types of samples has been shown to have a lower bioavailability than classical sources such as selenomethionine and inorganic Se salts. In addition, Se from Spirulina is metabolized differently due to its chemical form [43]. The lowest Se values were determined in pure Spirulina samples from Italy (S44 and S46). The Se accounts for 0.01% to 38.6% of the recommended AI of 70 μg/day [33]. The UL for Se is 300 μg/day [36] but is unlikely to be exceeded by adding daily Spirulina supplements to a regular diet.
Determined values of all elements are similar to those previously reported in the literature [13,15,20,44]. However, the significant variability observed in the amounts of certain elements is likely related to the growth medium used and intentional enrichment. It has been shown that micronutrients in the growth medium significantly improve uptake and accumulation of the macro- and micronutrients [39,43,45]. The growth medium pH can also affect Spirulina mineral assimilation, i.e., metal ion assimilation increases at higher pH [46]. According to the data, Spirulina food supplements are a good source of iron, calcium and phosphorous and can provide substantial amounts of potassium and selenium. However, the intake of specific nutrients is product dependent and depends on the amount of supplement consumed, since the recommended daily consumption values differ among producers.
The high iron content in Spirulina supplements is significant for those who consume, for example, fewer foods of animal origin and therefore have a lower iron intake in their diet. In addition to containing high amounts of iron, Spirulina also does not contain phytates or oxalates that would cause iron chelation, making Spirulina iron highly available for absorption in the human intestine [15,47]. However, as this study has shown, more research is needed to assess the actual iron bioavailability from Spirulina due to the predominance of the ferric (Fe3+) iron form.
Compliance with Their Nutrient Declaration
Measured elemental values were compared to the values declared on the products. Table 4 lists the 15 products that provided information on the content of Fe, Mn, Ca, Zn and P together with the degree of deviation (%) from the declared values. Iron had the most declarations (32.6% of the products), followed by Mn (13.0%), Ca and Zn (8.70%), P (4.3%) and finally K and Se (2.17%). The maximum permissible deviation of mineral content in food supplements is from −20% to 45% [48]. An excessive negative deviation from the declared Fe content was found in S5, S24 and S28 and a positive deviation in S4, S8, S10, S13, S14, S15, S17 and S36. The Mn content deviated positively in S4 and negatively in S12, S13 and S17, while S28 had an insufficient Ca content. The Zn content was low in S17, S23 and S24, while the P content was high in S12. In the case of K, the measured content in S17 was within the declared limits (+10.7%), while Se was much lower (−95.8%) than the declared value. However, several producers state that mineral levels can deviate due to seasonal fluctuations. In addition, even though some values are high, they remain below the UL and are unlikely to pose a risk to human health. However, the proportion of inappropriate declarations (86.7%) is a cause for concern and could undermine consumer confidence, and supports the need for regular monitoring and improved quality control.

Table 4.
Compliance with Spirulina declared nutrient values (% deviation).
3.2. Toxic Trace Element Content
The content of Cd, Hg and Pb was evaluated according to the maximum allowed European Commission levels [49]. In contrast, the As content was evaluated according to benchmark dose lower confidence limit (BMDL01) for cancers of the lung, skin and bladder, and skin lesions determined by The Scientific Panel on Contaminants in the Food Chain (CONTAM) [50]. Toxic trace element content and their maximum allowed values are presented in Table 5.

Table 5.
Toxic trace element content in Spirulina supplements.
The maximum measured values of Cd and Pb were 226 μg/kg dwt and 1320 μg/kg dwt, respectively, and did not exceed the maximum allowed value in food supplements. The daily BMDL01 values were calculated based on the average male (87 kg) and female (68 kg) body weight in the Slovenian population [51]. The BMDL01 values for As range between 0.3 and 8 μg/kg b.w. per day. Based on an RDI of 3–10 g/day, the maximum daily intake from consuming Spirulina was 8.11–27.0 μg/day and did not exceed the upper BMDL01 value for all of the supplements tested. In the case of Hg, S39 exceeded the maximum value of 100 μg/kg dwt by 1% (101 μg/kg dwt). All others were below the maximum allowed Hg value in food supplements. The samples’ median value (interquartile range) was 5.57 (3.57–7.63) μg/kg dwt. The lowest values of As, Cd and Pb were found in S46 produced in Italy, while the lowest Hg value was measured in S18, a mixed sample containing Spirulina, Chlorella and Lithothamnium algae. The measured values of elements Cd, Hg and As are comparable to literature values [6,21,52,53]. Pb values are also consistent with those determined by Al-Homaidan [53] but are lower than in commercial samples tested by Hsu et al. and Campanella et al., which ranged between 5600–15,200 μg/kg dwt, and 30.8% of the Spirulina samples tested by Rzymski et al., which ranged from 3500 μg/kg dwt to 5000 μg/kg dwt. The authors attributed the high values to local contamination, greater propensity toward Pb assimilation by microalgae and natural background levels [6,52,54,55].
Based on the data from the present study, Spirulina supplements do not contribute significantly to the intake of toxic trace elements and do not pose a serious risk. Nevertheless, Spirulina is an effective accumulator of trace elements which is an advantage when it accumulates elements essential for human health but a disadvantage for toxic elements [21]. Spirulina is also a potential source of Pb, Hg, Cd, and As in open production systems, where pedoclimatic conditions and agricultural practices could contribute to their presence in higher values and again supports the need for regular monitoring [56,57,58]. In contrast, this is not an issue when grown in controlled (closed) environments [21].
3.3. Amino Acid Content
Amino acids asparagine (ASN) and glutamine (GLN) were quantitatively converted to aspartate (ASP) and glutamate (GLU) during acid hydrolysis. Tryptophan (TRP) was lost during acid hydrolysis, and arginine (ARG) and cysteine (CYS) were not included in the GC-MS EZ:faast kit due to their thermal instability [59,60]. Again, the results (Table 6) are presented according to the RDI of 3–10 g. The measured values are compared to the daily recommended amounts [61] for the average male (87 kg) and female (68 kg) in Slovenia [51].

Table 6.
Total amino acid content (mg/day) in Spirulina supplements, presented for minimal (3 g) and maximal (10 g) recommended daily intake.
The amino acid values determined in this study are presented in Table S1 and are within the literature values, except for VAL and HIS, where the measured values were higher [6,62,63]. The VAL content ranged from 50.8–123 mg/g dwt in this study, while the previously reported values range from 35.8–60 mg/g dwt. The HIS content varied from 16.3–22.2 mg/g dwt, compared to 6.00–11.9 mg/g dwt reported in the literature (Figure 1; Table S1). The lowest content of all amino acids except MET was determined in S22, closely followed by S19 and S37. These samples contain Spirulina and excipients, especially S22 and S19, which have the most declared excipients among all the samples. It is known that a high excipient content affects the amino acid content due to the reduced amount of Spirulina [6]. Valine, ILE and HIS were present in the lowest amounts in all samples. The sample with the second-lowest content of all other analyzed amino acids was S9—a mixed sample of wheatgrass, barley grass, Spirulina and Chlorella. This result could also be due to the low microalgae content in the supplement, as cereal grasses are not as rich in protein as Spirulina and Chlorella [64,65]. These results suggest that, combined with elemental composition, the amino acid content could distinguish authentic samples from adulterated ones. The highest values of ALA, GLY, VAL, LEU, ILE, THR, SER, ASP and TYR were found in S36 and S40, PRO and LYS were highest in S6, PHE in S8, HIS in S28, MET in S39 and GLU in S46—all were declared as pure Spirulina products.
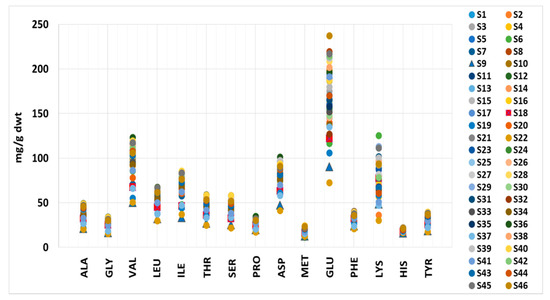
Figure 1.
The amino acid content range in Spirulina supplements.
In addition to product composition, other factors affecting the amino acid content have been reported. For example, cells grown under stress conditions, including salinity stress, have a lower capacity for protein synthesis, which is seen in the lower protein content found in biomass grown in salinated water [66,67]. Differences also occur due to different cultivation times, light intensities, temperatures, and the growth medium’s nutrient composition. For example, Spirulina grown in a urea growth medium has a higher amino acid content than others [62]. The drying processes used can also affect the amount of protein and, consequently, amino acid content. The highest protein losses are connected to convective and infrared drying in spreading cylinders, while freeze-drying gives the highest protein recoveries. In addition, thin layer drying results in higher protein recoveries than cylinder drying [68]. Drying at low temperatures (40–50 °C) does not affect the products’ nutritional quality compared to the fresh Spirulina [69].
This study shows that the Spirulina supplements contained all the essential and non-essential amino acids measured. Coverage of daily requirements for adults [61] based on median values (%) was: TYR (males (M): 4.79–16.0, females (F): 6.13–20.4), LEU (M: 4.99–16.6, F: 6.39–21.3), PHE (M: 4.99–16.6, F: 6.38–21.3), HIS (M: 6.75–22.5, F: 8.63–28.8), MET (M: 7.06–23.5, F: 9.03–30.1), LYS (M: 8.85–29.5, F: 11.3–37.8), THR (M: 11.4–38.1, F: 14.6–48.7), ILE (M: 12.6–42.1, F: 16.2–53.9) and VAL (M: 13.6–45.2, F: 17.4–57.8). According to these results, the tested Spirulina supplements are a good source of essential and non-essential amino acids, as shown by other authors analyzing commercial Spirulina products [9,63,66]. However, despite that S22, S19 and S37 stand out because of their low amino acid content, overall, Spirulina food supplements would be reasonable when choosing high amino acid content products.
3.4. Fatty Acid Content
The amounts of individual fatty acids (FA) are presented in Table 7 and Table S2. The results in Table 7 are presented as the median value (interquartile range in parentheses) of weight percent of individual fatty acids of total fatty acids (TFA). The most abundant FA was palmitic acid (16:0) followed by linoleic (C18:2 ω-6c), γ-linolenic (C18:3 ω-6), palmitoleic (C16:1 ω-7), stearic (C18:0) and oleic/elaidic acid (C18:1 ω-9c/9t). The amounts of fatty acids agree with published data [6,70], although levels of γ-linolenic, linoleic, palmitoleic and stearic acid vary [71,72,73,74]. These differences could be due to higher or lower content of saturated/unsaturated FA in the algal cells, which depends on metabolic needs, such as membrane fluidity, which depends on the growth conditions [75]. It is known that certain microalgae, including Spirulina, regulate their lipid composition at low temperatures to achieve better membrane fluidity, which results in increased levels of unsaturated FA.

Table 7.
Fatty acid content (% of total fatty acid content) in Spirulina supplements.
High temperatures during growth also favor the formation of saturated FA [70,76,77], and the formation of certain fatty acids is more intensive at specific temperatures for certain strains, i.e., the highest γ-linolenic FA production in Spirulina maxima is between 35 and 40 °C. In contrast, in Spirulina platensis, the production of γ-linolenic is higher at 30 °C [78]. Culture age and growth medium salinity are also important, and prolonged exposure to high salinity stress results in higher values of γ-linolenic acid in different Spirulina strains [72]. As with amino acids, the drying process can affect lipid yield and total fatty acid content. Namely, the maximum monounsaturated and polyunsaturated FA levels are obtained in the freeze-dried samples, while the sun-dried samples contain higher amounts of saturated acids. In addition, freeze drying provides better nutrient preservation than other dehydration methods such as sun drying, oven drying or spray drying [79].
In most samples, only small amounts or no ω-3 fatty acids were found, making commercial Spirulina samples a good source of ω-6 (especially LA and GLA), but not ω-3 fatty acids, which was also confirmed by other studies [6,70,74,80].
Like elemental and amino acid composition, certain samples stood out regarding TFA content (Figure 2). According to median and associated IR values, anomalously high levels of C15:0 (0.22% of TFA), C16:2 ω-6 (9.71% of TFA), C16:2 ω-4 (9.38% of TFA), C16:3 ω-3 (9.58% of TFA) and C18:3 ω-3 (18.4% of TFA) were found in S6. Additionally, in the same sample, anomalously low levels of C16:0 (18.4% of TFA), C17:1 (< LOD of TFA) and C18:3 ω-6 (< LOD of TFA) were observed, suggesting possible adulteration. S19 contained unusually high levels of C18:0 (25.8% of TFA), and low values of C17:1 (< LOD of TFA), C18:2 ω-6c (12.1% of TFA) and C18:3 ω-6c (9.72% of TFA); likewise, S37 had lower amounts of C18:2 ω-6c (11.1% of TFA) and a marked deviation in its C22:0 content (30.2% of TFA). The presence of various excipients could explain these differences. However, for S37, there remains the possibility of adulteration since it contains high levels of C22:0 FA, which was otherwise detected only in S6 (0.27% of TFA, possibly adulterated), and in the mixed sample S9 (0.59% of TFA), which contains wheatgrass, barley grass, Spirulina and Chlorella. Sample S9 also stands out due to its high content of C14:0 (0.84% of TFA), C18:3 ω-3 (22.8% of TFA), C16:2 ω-6 (5.52% of TFA), and C16:3 ω-3 (5.27% of TFA) and low content of C16:1 ω-7 (2.75% of TFA), C16:0 (24.6% of TFA) and C18:3 ω-6 (5.69% of TFA).
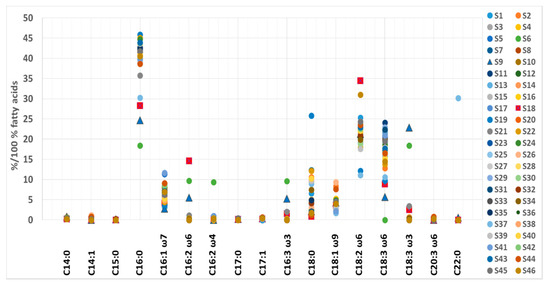
Figure 2.
The fatty acid content range in Spirulina-based supplements.
In addition, S18 differed from supplements containing only Spirulina. This sample contains high amounts of C16:2 ω-6 (14.6% of TFA), C18:2 ω-6c (34.4% of TFA) and C18:3 ω-3 (2.52% of TFA). In contrast, the amounts of C16:0 (28.3% of TFA), C18:0 (0.88% of TFA) and C18:3 ω-6 (8.84% of TFA) were exceptionally low. Higher levels of C18:3 ω-3 (ALA, α-linolenic acid) in mixed samples likely result from the presence of Chlorella, which is known to contain large amounts of this FA. Alternatively, low GLA and palmitic acid levels point to a low Spirulina content in S9 and S18, as their high content is typical for pure Spirulina products [74]. The fatty acid distribution (% of TFA) in the analyzed samples is presented in Figure 2.
The data make it possible to distinguish between mixed samples and samples containing pure Spirulina based on the fatty acid composition. Such knowledge could help separate authentic from adulterated samples. In addition, the Spirulina supplements tested proved to be a good source of ω-6, but not ω-3 polyunsaturated fatty acids. However, since the FA content varied in samples, it would be sensible to choose pure Spirulina products for consumption with no added excipients from a trusted producer to guarantee optimal FA composition.
3.5. Principal Component Analysis (PCA) of Spirulina Samples from Slovenian Market Analysis Results
The data set of 46 Spirulina samples and 43 analyzed parameters (macro- and trace-elemental, amino acid and fatty acid composition data) was analyzed by PCA to identify the trends and examine the distribution of variables in the investigated samples (Figure 3 and Figure 4). PC1 explained 33.0% and PC2 13.5% of the total variance. Four groups of samples can be identified (Figure 3), each represented by different variables. Most parameter vectors are directed towards the upper right quadrant of Figure 4. The positive trend is due to an increased amino acid composition as well as C16:0, C16:1 ω-7, C18:2 ω-6c and C18:3 ω-6 fatty acids. The blue group (Figure 3) has the highest content of these compounds, followed by the yellow group, while the red group has the lowest amount. Finally, ungrouped samples (S6, S9, S18, S19, S22 and S37), which have the lowest content of selected amino acids and fatty acids, are located on the left-hand side of the graph. A similar positive trend for elements Cl, Ti, Fe, Zn, Mn, Rb and Br and fatty acids C14:1 and C18:1 ω-9c/C18:1 ω-9t is observed in the upper part of the second PCA graph (Figure 4). The samples from Hawaii (orange group) contained the highest amounts of these elements and fatty acids. Interestingly, the undeclared sample S2 falls in the same group, indicating that this Spirulina supplement might originate from Hawaii. Alternatively, ungrouped samples to the left of the vertical line of Figure 3 (S6, S9, S18, S19, S22 and S37) show a positive Ca, Sr and Cd and C14:0, C18:0 and C16:2 ω-4 trend (Figure 4).
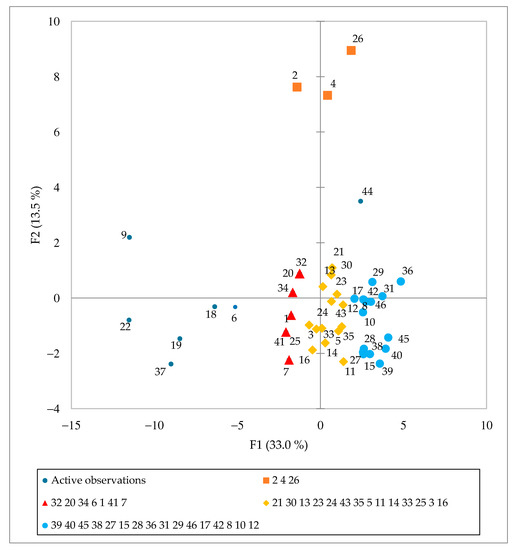
Figure 3.
Principal Component Analysis score plot of Spirulina dietary supplements (n = 47) available in Slovenia.
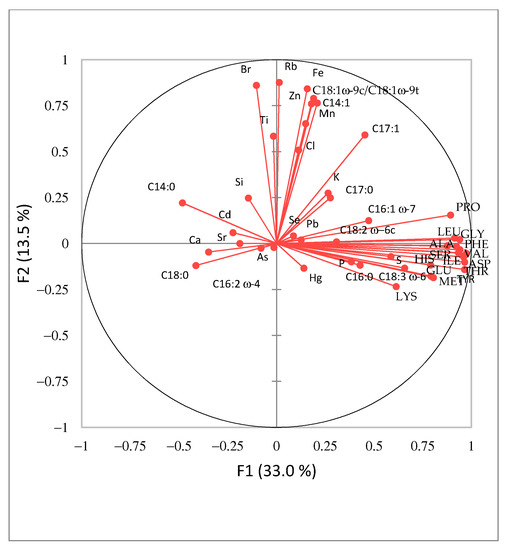
Figure 4.
Principle Component Analysis variable loading plot for Spirulina dietary supplements (n = 47) available in Slovenia.
Samples S9 and S18 were expected to stand out since they contain other plant or algae material, and their parameters were expected to differ from the samples containing pure Spirulina. In addition, S19 and S22 contain various excipients, affecting the nutrient composition. In contrast, close inspection of the data for S6 and S37 suggests possible adulteration since S6 is declared as pure Spirulina and S37 contains excipients, which did not affect our results. Overall statistical evaluation of the results supports our previous observations.
4. Conclusions
This study finds that when consumed in recommended amounts, the analyzed Spirulina supplements are a good source of calcium, phosphorous, potassium, and selenium, while toxic trace elements do not represent a serious health risk. They are also a good source of essential and non-essential amino acids as well as ω-6 polyunsaturated fatty acids. The study data also show that Spirulina contains low amounts of ω-3 polyunsaturated fatty acids, and although they contain high amounts of Fe, since it is mainly present as Fe3+, the Fe is less bioavailable.
Therefore, a well-thought-out selection of Spirulina supplements would be advised and choosing pure Spirulina supplements is advisable, especially since pure Spirulina samples have a higher amino acid, γ-linolenic and linoleic fatty acid, P and Se content. Additionally, as different Spirulina products might have a different nutrient composition, the products should be chosen according to the specific nutrient needs of the individual. Supplements from Hawaii are rich in Fe, Zn, Mn, Cl, Ti, Rb and Br.
Notably, a high proportion (86.7%) of inappropriate declarations was found among the analyzed samples regarding the content of Fe, Mn, Ca, Zn, P, K and Se, which is a cause for concern. Deviations of more than 45% over the declared value could pose a risk to human health through excessive elemental intake. Such deviations can also undermine consumer confidence. Fortunately, in this case, UL levels were not exceeded.
This study also showed how adding algal or plant material supplements alters the elemental, amino acid and fatty acid composition. Such data could distinguish mixed products from those containing only Spirulina and therefore be valuable in authenticity studies. Multivariate analysis was able to discern these products from those containing only Spirulina. In addition, the amino acid, fatty acid and mineral composition data suggest that at least two samples were adulterated, since such differences in composition compared to pure Spirulina could not be explained by the addition of excipients.
Overall, Spirulina is a good source of nutrients. However, in the case of dietary supplements, regular monitoring and inspection are advised to identify adulterations and deviations from the declared content. In this way, potential hazards for consumer health can be avoided.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods11060849/s1, Table S1: Amino acid composition of Spirulina supplements available on the Slovenian market (mg/g dwt), Table S2: Fatty acid composition of Spirulina supplements available on the Slovenian market (% of total fatty acid content).
Author Contributions
Conceptualization, J.M.R. and N.O.; methodology, J.M.R., M.J.H., M.N., K.V.M. and I.A.; validation, J.M.R., M.J.H., M.N., K.V.M. and I.A.; formal analysis, J.M.R., M.J.H., M.N. and K.V.M.; investigation, J.M.R., K.V.M., I.A. and N.O.; data curation, J.M.R., M.J.H., M.N., K.V.M. and I.A.; writing—original draft preparation, J.M.R.; writing—review and editing, N.O., M.N. and K.V.M.; visualization, J.M.R.; supervision, N.O.; funding acquisition, N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (Young Researcher’s program, grant number 1000-17-0106, research programs No. P1-0143, P1-0112 and research project No. J4-1773).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Acknowledgments
Synchrotron ALBA (Barcelona) is acknowledged for the provision of beamtime (No. 2017082304). Anja Kavčič and Wojciech Olszewski are acknowledged for help with the measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. The Use of Saline Waters for Crop Production; FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; ISBN 92-5-103237-8. [Google Scholar]
- Food and Agriculture Organization of the United Nations. High Level Expert Forum-How to Feed the World in 2050; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; p. 35. [Google Scholar]
- Gardner, G.T.; Halweil, B. Underfed and Overfed: The Global Epidemic of Malnutrition; Peterson, J.A., Ed.; Worldwatch Paper; Worldwatch Institute: Washington, DC, USA, 2000; ISBN 978-1-878071-52-1. [Google Scholar]
- Sotiroudis, T.; Sotiroudis, G. Health Aspects of Spirulina (Arthrospira) Microalga Food Supplement. J. Serb. Chem. Soc. 2013, 78, 395–405. [Google Scholar] [CrossRef]
- Rogers, J.N.; Rosenberg, J.N.; Guzman, B.J.; Oh, V.H.; Mimbela, L.E.; Ghassemi, A.; Betenbaugh, M.J.; Oyler, G.A.; Donohue, M.D. A Critical Analysis of Paddlewheel-Driven Raceway Ponds for Algal Biofuel Production at Commercial Scales. Algal Res. 2014, 4, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Campanella, L.; Crescentini, G.; Avino, P. Chemical Composition and Nutritional Evaluation of Some Natural and Commercial Food Products Based on Spirulina. Analusis 1999, 27, 533–540. [Google Scholar] [CrossRef]
- Chu, W.-L.; Lim, Y.-W.; Radhakrishnan, A.K.; Lim, P.-E. Protective Effect of Aqueous Extract from Spirulina Platensis against Cell Death Induced by Free Radicals. BMC Complement. Altern. Med. 2010, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Baky, H.H.A.; El-Baz, F.K.; El-Baroty, G.S. Characterization of Nutraceutical Compounds in Blue Green Alga Spirulina Maxima. J. Med. Plant Res. 2008, 2, 292–300. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V. Quantification of Phytochemicals from Commercial Spirulina Products and Their Antioxidant Activities. Evid.-Based Complement. Altern. Med. 2016, 2016, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capelli, B.; Cysewski, G. Potential Health Benefits of Spirulina Microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Ali, S.K.; Saleh, A.M. Spirulina-an Overview. Int. J. Pharm. Pharm. Sci. 2012, 4, 9–15. [Google Scholar]
- Tokuşoglu, Ö.; Ünal, M.K. Biomass Nutrient Profiles of Three Microalgae: Spirulina Platensis, Chlorella Vulgaris, and Isochrisis Galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Babadzhanov, A.S.; Abdusamatova, N.; Yusupova, F.M.; Faizullaeva, N.; Mezhlumyan, L.G.; Malikova, M.K. Chemical Composition of Spirulina Platensis Cultivated in Uzbekistan. Chem. Nat. Compd. 2004, 40, 276–279. [Google Scholar] [CrossRef]
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Gad, A.S.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Hassan, N.S.; Abdel-Wahhab, M.A. Antioxidant Activity and Hepatoprotective Effects of Whey Protein and Spirulina in Rats. Nutrition 2011, 27, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Czerwonka, A.; Kaławaj, K.; Sławińska-Brych, A.; Lemieszek, M.K.; Bartnik, M.; Wojtanowski, K.K.; Zdzisińska, B.; Rzeski, W. Anticancer Effect of the Water Extract of a Commercial Spirulina (Arthrospira Platensis) Product on the Human Lung Cancer A549 Cell Line. Biomed. Pharmacother. 2018, 106, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Otsuka, A.; Hirahashi, T.; Kato, T. A Novel Protein C-Phycocyanin Plays a Crucial Role in the Hypocholesterolemic Action of Spirulina Platensis Concentrate in Rats. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Nutritional and Toxicological Aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Meticulous Research® Spirulina Market by Distribution Channel (Consumer Channel, Business Channel), Product Type (Powder, Tablets, Capsules, Flakes, Phycocyanin Extract), Application (Nutraceuticals, Food and Beverages, Agriculture, Animal Feed)-Global Forecast to 2028. Available online: https://www.meticulousresearch.com/product/spirulina-market-5070#description (accessed on 1 March 2022).
- Kejžar, J.; Jagodic Hudobivnik, M.; Nečemer, M.; Ogrinc, N.; Masten Rutar, J.; Poklar Ulrih, N. Characterization of Algae Dietary Supplements Using Antioxidative Potential, Elemental Composition, and Stable Isotopes Approach. Front. Nutr. 2021, 7, 618503. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A. Heavy Metal Analysis in Commercial Spirulina Products for Human Consumption. Saudi J. Biol. Sci. 2013, 20, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo-Velázquez, T.; Osorio-Revilla, G.; Zuñiga-de Loa, M.; Rivera-Espinoza, Y. Application of FTIR-HATR Spectroscopy and Multivariate Analysis to the Quantification of Adulterants in Mexican Honeys. Food Res. Int. 2009, 42, 313–318. [Google Scholar] [CrossRef]
- Wu, D.; Nie, P.; Cuello, J.; He, Y.; Wang, Z.; Wu, H. Application of Visible and Near Infrared Spectroscopy for Rapid and Non-Invasive Quantification of Common Adulterants in Spirulina Powder. J. Food Eng. 2011, 102, 278–286. [Google Scholar] [CrossRef]
- De Carvalho, L.M.D.; Cohen, P.A.; Silva, C.V.; Moreira, A.P.L.; Falcão, T.M.; Dal Molin, T.R.; Zemolin, G.; Martini, M. A New Approach to Determining Pharmacologic Adulteration of Herbal Weight Loss Products. Food Addit. Contam. Part A 2012, 29, 1661–1667. [Google Scholar] [CrossRef]
- Moreira, A.P.L.; Gobo, L.A.; Viana, C.; de Carvalho, L.M. Simultaneous Analysis of Antihypertensive Drugs and Diuretics as Adulterants in Herbal-Based Products by Ultra-High Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Anal. Methods 2016, 8, 1881–1888. [Google Scholar] [CrossRef]
- Nečemer, M.; Kump, P.; Vogel-Mikuš, K. Use of X-Ray fluorescence-based analytical techniques in phytoremediation. In Handbook of Phytoremediation; Golubev, I.A., Ed.; Environmental Science, Engineering and Technology; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 331–358. ISBN 978-1-61728-753-4. [Google Scholar]
- Nečemer, M.; Kump, P.; Ščančar, J.; Jaćimović, R.; Simčič, J.; Pelicon, P.; Budnar, M.; Jeran, Z.; Pongrac, P.; Regvar, M.; et al. Application of X-ray Fluorescence Analytical Techniques in Phytoremediation and Plant Biology Studies. Spectrochim. Acta B At. Spectrosc. 2008, 63, 1240–1247. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-Ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pongrac, P.; Arčon, I.; Castillo-Michel, H.; Vogel-Mikuš, K. Mineral Element Composition in Grain of Awned and Awnletted Wheat (Triticum Aestivum L.) Cultivars: Tissue-Specific Iron Speciation and Phytate and Non-Phytate Ligand Ratio. Plants 2020, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; The Oily Press: Cambridge, UK, 2010; ISBN 978-0-85709-786-6. [Google Scholar]
- Paniagua-Michel, J. Chapter 16-Microalgal Nutraceuticals. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 255–267. ISBN 978-0-12-800776-1. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Principles for Deriving and Applying Dietary Reference Values. EFSA J. 2010, 8, 1–30. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Dietary Reference Values for Nutrients. Summary Report. EFSA Supporting Publ. 2019, 14, e15121. [Google Scholar] [CrossRef] [Green Version]
- Sawai, J. Antimicrobial Characteristics of Heated Scallop Shell Powder and Its Application. Biocontrol Sci. 2011, 16, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, R.P.; Kawai, G.S.D.; de Andrade, F.R.D.; Bezzon, V.D.N.; Ferraz, H.G. Characterisation and Traceability of Calcium Carbonate from the Seaweed Lithothamnium Calcareum. Solids 2021, 2, 192–211. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Tolerable Upper Intake Levels for Vitamins and Minerals, 1st ed.; European Food Safety Authority: Parma, Italy, 2006; ISBN 92-9199-014-0.
- Qamar, A.; Saeed, F.; Tahir-Nadeem, M.; Hussain, A.I.; Niaz, B.; Ullah Khan, A.; Afzaal, M.; Badar Ul Ain, H.; Imran, M. Exploring the Phytochemical Profile of Green Grasses with Special Reference to Antioxidant Properties. Int. J. Food Prop. 2018, 21, 2566–2577. [Google Scholar] [CrossRef] [Green Version]
- Puyfoulhoux, G.; Rouanet, J.-M.; Besançon, P.; Baroux, B.; Baccou, J.-C.; Caporiccio, B. Iron Availability from Iron-Fortified Spirulina by an in Vitro Digestion/Caco-2 Cell Culture Model. J. Agric. Food Chem. 2001, 49, 1625–1629. [Google Scholar] [CrossRef]
- Sukumaran, P.; Dahlan, F.L.; Omar, H.; Ismail, A. Macro- and Micronutrients Status in Arthrospira Platensis Grown in Freshwater and Brackish Water Medium. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 384–391. [Google Scholar]
- Scheers, N.; Andlid, T.; Alminger, M.; Sandberg, A.-S. Determination of Fe2+ and Fe3+ in Aqueous Solutions Containing Food Chelators by Differential Pulse Anodic Stripping Voltammetry. Electroanalysis 2010, 22, 1090–1096. [Google Scholar] [CrossRef]
- Chidambaram, M.V.; Reddy, M.B.; Thompson, J.L.; Bates, G.W. In Vitro Studies of Iron Bioavailability. Probing the Concentration and Oxidation-Reduction Reactivity of Pinto Bean Iron with Ferrous Chrornogens. Biol. Trace. Elem. Res. 1989, 19, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J.N. Vitamin and Mineral Safety, 3rd ed.; MacKay, D., Ed.; Council for Responsible Nutrition (CRN): Washington, DC, USA, 2014. [Google Scholar]
- Cases, J.; Vacchina, V.; Napolitano, A.; Caporiccio, B.; Besançon, P.; Lobinski, R.; Rouanet, J.-M. Selenium from Selenium-Rich Spirulina Is Less Bioavailable than Selenium from Sodium Selenite and Selenomethionine in Selenium-Deficient Rats. J. Nutr. 2001, 131, 2343–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falquet, J. The Nutritional Aspects of Spirulina. Antenna Technologies. Available online: https://iceagefarmer.com/docs/Crop%20Focus/Spirulina/AspectNut_UK.pdf (accessed on 3 October 2021).
- Frontasyeva, M.V.; Pavlov, S.S.; Mosulishvili, L.; Kirkesali, E.; Ginturi, E.; Kuchava, N. Accumulation of Trace Elements by Biological Matrice of Spirulina Platensis. Ecol. Chem. Eng. S 2009, 16, 277–285. [Google Scholar]
- Dmytryk, A.; Saeid, A.; Chojnacka, K. Biosorption of Microelements by Spirulina: Towards Technology of Mineral Feed Supplements. Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Guidance Document for Competent Authorities for the Control of Compliance with EU Legislation on: Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 and Council Directive 90/496/EEC of 24 September 1990 on Nutrition Labelling of Foodstuffs and Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements with Regard to the Setting of Tolerances for Nutrient Values Declared on a Label; European Commission: Brussels, Belgium, 2012; p. 15. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Last Amended by Commission Regulation (EU) 2021/1323 of 10 August 2021); European Commission: Brussels, Belgium, 2021; Volume 364, p. 33. [Google Scholar]
- European Commission. Commission Regulation (EU) 2015/1006 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs; European Commission: Brussels, Belgium, 2015; Volume 161, pp. 14–16. [Google Scholar]
- SURS. Statistical Office of Republic of Slovenia. Available online: https://www.stat.si/StatWeb/en (accessed on 6 October 2021).
- Hsu, Y.-M.; Hwang, J.-M.; Yeh, T.-R. Inorganic Elements Determination for Algae/Spirulina Food Marketed in Taiwan. J. Food Drug. Anal. 2001, 9, 178–182. [Google Scholar] [CrossRef]
- Al-Homaidan, A. Heavy Metal Levels in Saudi Arabian Spirulina. Pak. J. Biol. Sci. 2006, 9, 2693–2695. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and Toxic Elements in Commercial Microalgal Food Supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Niedzielski, P.; Karczewski, J.; Poniedziałek, B. Biosorption of Toxic Metals Using Freely Suspended Microcystis Aeruginosa Biomass. Cent. Eur. J. Chem. 2014, 12, 1232–1238. [Google Scholar] [CrossRef]
- Kuhnlein, H.V.; Chan, H.M. Environment and Contaminants in Traditional Food Systems of Northern Indigenous Peoples. Annu. Rev. Nutr. 2000, 20, 595–626. [Google Scholar] [CrossRef] [PubMed]
- Kotangele, L.R.; Sarkar, R.; Krishnamoorthi, K.P. Toxicity of Mercury and Zinc to Spirulina Platensis. Indian J. Environ. Health 1984, 26, 41–46. [Google Scholar]
- Pande, A.S.; Sarkar, R.; Krishnamoorthi, K.P. Toxicity of Copper Sulphate to the Alga Spirulina Platensis and the Ciliate Tetrahymena Pyriformis. Indian J. Exp. Biol. 1981, 19, 500–502. [Google Scholar]
- Badawy, A.A.-B.; Morgan, C.J.; Turner, J.A. Application of the Phenomenex EZ:FaastTM Amino Acid Analysis Kit for Rapid Gas-Chromatographic Determination of Concentrations of Plasma Tryptophan and Its Brain Uptake Competitors. Amino Acids 2008, 34, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated GC–MS Analysis of Free Amino Acids in Biological Fluids. J. Chromatogr. B 2008, 870, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2007; p. 265.
- Dewi, E.N.; Amalia, U.; Mel, M. The Effect of Different Treatments to the Amino Acid Contents of Micro Algae Spirulina sp. Aquat. Procedia 2016, 7, 59–65. [Google Scholar] [CrossRef]
- Bashir, S.; Sharif, M.K.; Butt, M.S.; Shahid, M. Functional Properties and Amino Acid Profile of Spirulina Platensis Protein Isolates. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2016, 59, 12–19. [Google Scholar] [CrossRef]
- Ghumman, A.; Singh, N.; Kaur, A. Chemical, Nutritional and Phenolic Composition of Wheatgrass and Pulse Shoots. Int. J. Food Sci. Technol. 2017, 52, 2191–2200. [Google Scholar] [CrossRef]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High Variability in Nutritional Value and Safety of Commercially Available Chlorella and Spirulina Biomass Indicates the Need for Smart Production Strategies. Bioresour. Technol. 2019, 275, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.B.; Sant’Anna, E.S. Cultivation of Arthrospira (Spirulina) 11 Platensis in Desalinator Wastewater and Salinated Synthetic Medium: Protein Content and Amino-Acid Profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.-T.; Vonshak, A. Adaptation of Spirulina Platensis to Salinity-Stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 113–118. [Google Scholar] [CrossRef]
- Desmorieux, H.; Hernandez, F. Biochemical and Physical Criteria of Spirulina after Different Drying Processes. In Proceedings of the 14th International Drying Symposium, Sao Paulo, Brazil, 22–25 August 2004; Volume B, pp. 900–907. [Google Scholar]
- Agustini, T.W.; Suzery, M.; Sutrisnanto, D.; Ma’ruf, W.F. Hadiyanto Comparative Study of Bioactive Substances Extracted from Fresh and Dried Spirulina sp. Procedia Environ. Sci. 2015, 23, 282–289. [Google Scholar] [CrossRef] [Green Version]
- de Jesus, C.S.; da Silva Uebel, L.; Costa, S.S.; Miranda, A.L.; de Morais, E.G.; de Morais, M.G.; Costa, J.A.V.; Nunes, I.L.; de Souza Ferreira, E.; Druzian, J.I. Outdoor Pilot-Scale Cultivation of Spirulina Sp. LEB-18 in Different Geographic Locations for Evaluating Its Growth and Chemical Composition. Bioresour. Technol. 2018, 256, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Diraman, H.; Koru, E.; Dibeklioglu, H. Fatty Acid Profile of Spirulina Platensis Used as a Food Supplement. Isr. J. Aquac. 2009, 61, 134–142. [Google Scholar]
- Bhakar, R.; Kumar, R.; Pabbi, S. Total Lipids and Fatty Acid Profile of Different Spirulina Strains as Affected by Salinity and Incubation Time. Int. J. Plant Res. 2013, 26, 148–154. [Google Scholar] [CrossRef]
- Cohen, Z.; Vonshak, A.; Richmond, A. Fatty Acid Composition of Spirulina Strains Grown under Various Environmental Conditions. Phytochemistry 1987, 26, 2255–2258. [Google Scholar] [CrossRef]
- Ötleş, S.; Pire, R. Fatty Acid Composition of Chlorella and Spirulina Microalgae Species. J. AOAC Int. 2001, 84, 1708–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carcea, M.; Sorto, M.; Batello, C.; Narducci, V.; Aguzzi, A.; Azzini, E.; Fantauzzi, P.; Finotti, E.; Gabrielli, P.; Galli, V.; et al. Nutritional Characterization of Traditional and Improved Dihé, Alimentary Blue-Green Algae from the Lake Chad Region in Africa. LWT Food Sci. Technol. 2015, 62, 753–763. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Hallenbeck, P.C.; Leite, G.B.; Paranjape, K.; Huo, D.-Q. Growth and Lipid Accumulation of Indigenous Algal Strains under Photoautotrophic and Mixotrophic Modes at Low Temperature. Algal Res. 2016, 16, 195–200. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Lambrinidis, G.; Parry, D.L. Effect of Temperature on Growth, Chemical Composition and Fatty Acid Composition of Tropical Australian Microalgae Grown in Batch Cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- de Oliveira, M.A.C.L.; Monteiro, M.P.C.; Robbs, P.G.; Leite, S.G.F. Growth and Chemical Composition of Spirulina Maxima and Spirulina Platensis Biomass at Different Temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Shekarabi, S.P.H.; Mehrgan, M.S.; Razi, N.; Sabzi, S. Biochemical Composition and Fatty Acid Profile of the Marine Microalga Isochrysis Galbana Dried with Different Methods. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 521–524. [Google Scholar] [CrossRef]
- Sharoba, A.M. Nutritional Value of Spirulina and Its Use in the Preparation of Some Complementary Baby Food Formulas. J. Dairy Sci. 2014, 5, 517–538. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).