A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Culture
2.2. Synthesis of TNP
2.3. Characterization of TNP
2.3.1. Particle Size, PDI and Encapsulation Efficiency of Nanoparticles
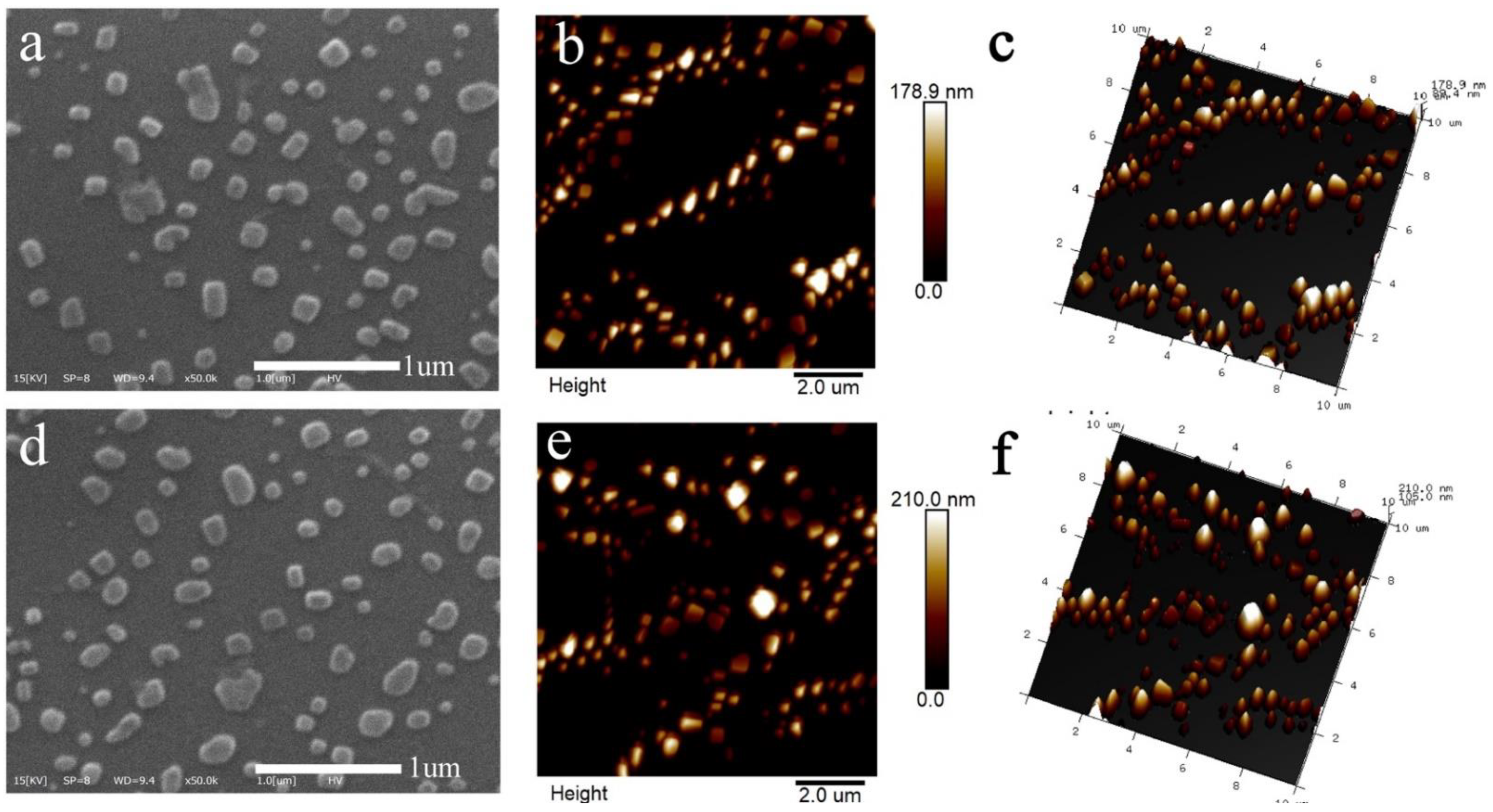
2.3.2. The Morphology of Nanoparticles
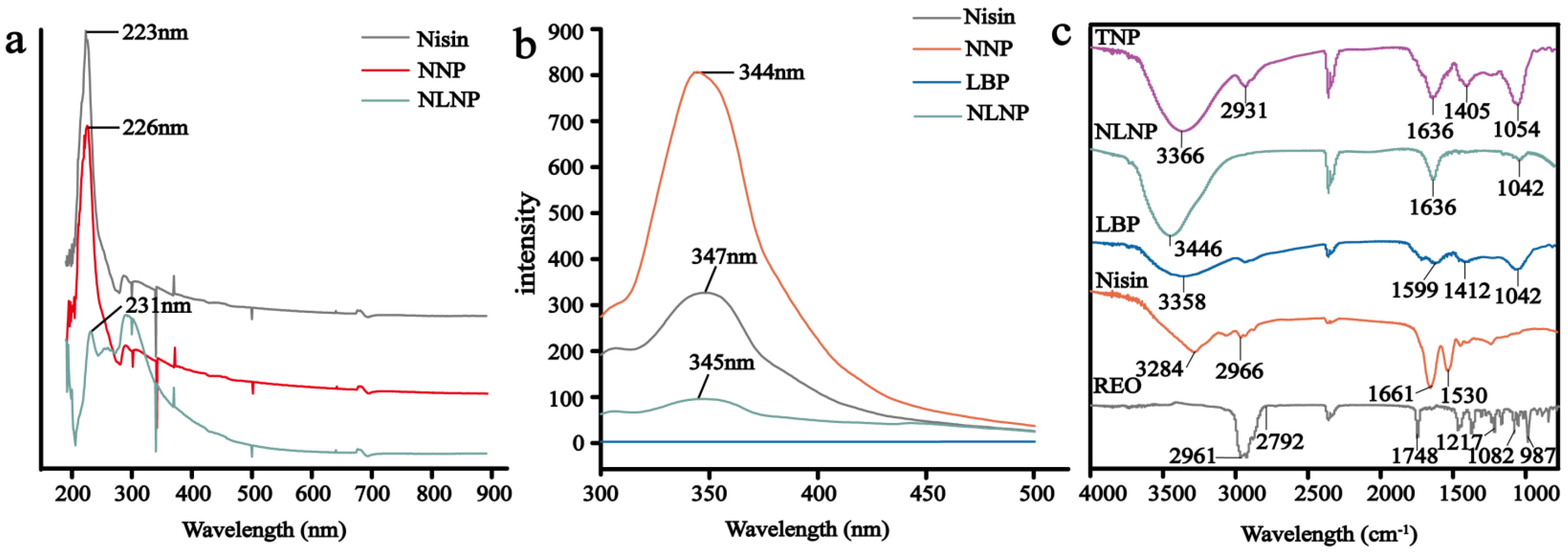
2.3.3. UV-Vis Absorption Spectrum
2.3.4. Fluorescence Spectroscopy Measurement
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Nanoparticles
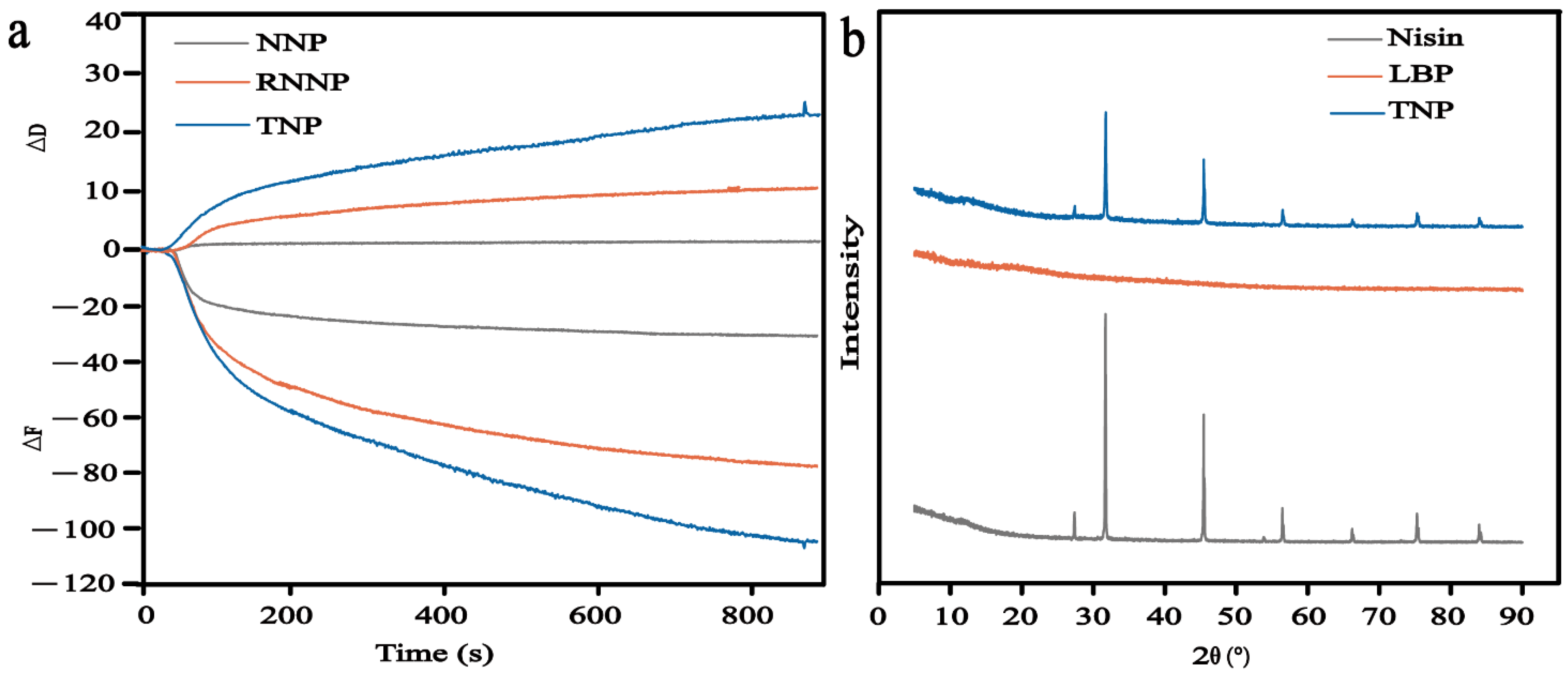
2.3.6. Quartz Crystal Microbalance (QCM)
2.3.7. X-ray Diffraction (XRD)
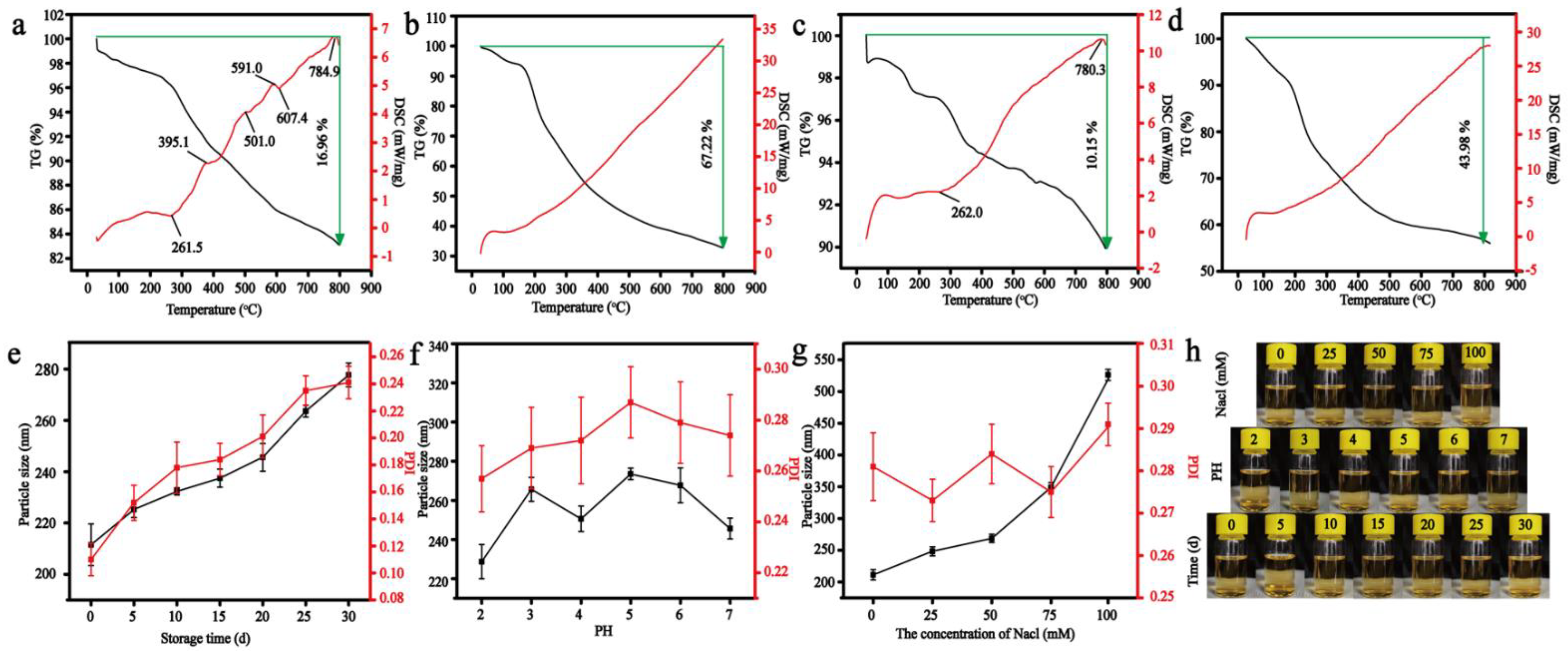
2.4. Stability of Nanoparticles
2.5. Antibacterial Effect of Nanoparticles
2.6. Antioxidant Effect of Nanoparticles
2.6.1. DPPH Scavenging Activity
2.6.2. Antioxidant Effect on Beef
2.7. The Application of Nanoparticles against S. aureus and E. coli O157:H7 on Beef
2.8. Characterization of Coated Beef
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.1.1. Particle Size, PDI and Encapsulation Efficiency of Nanoparticles
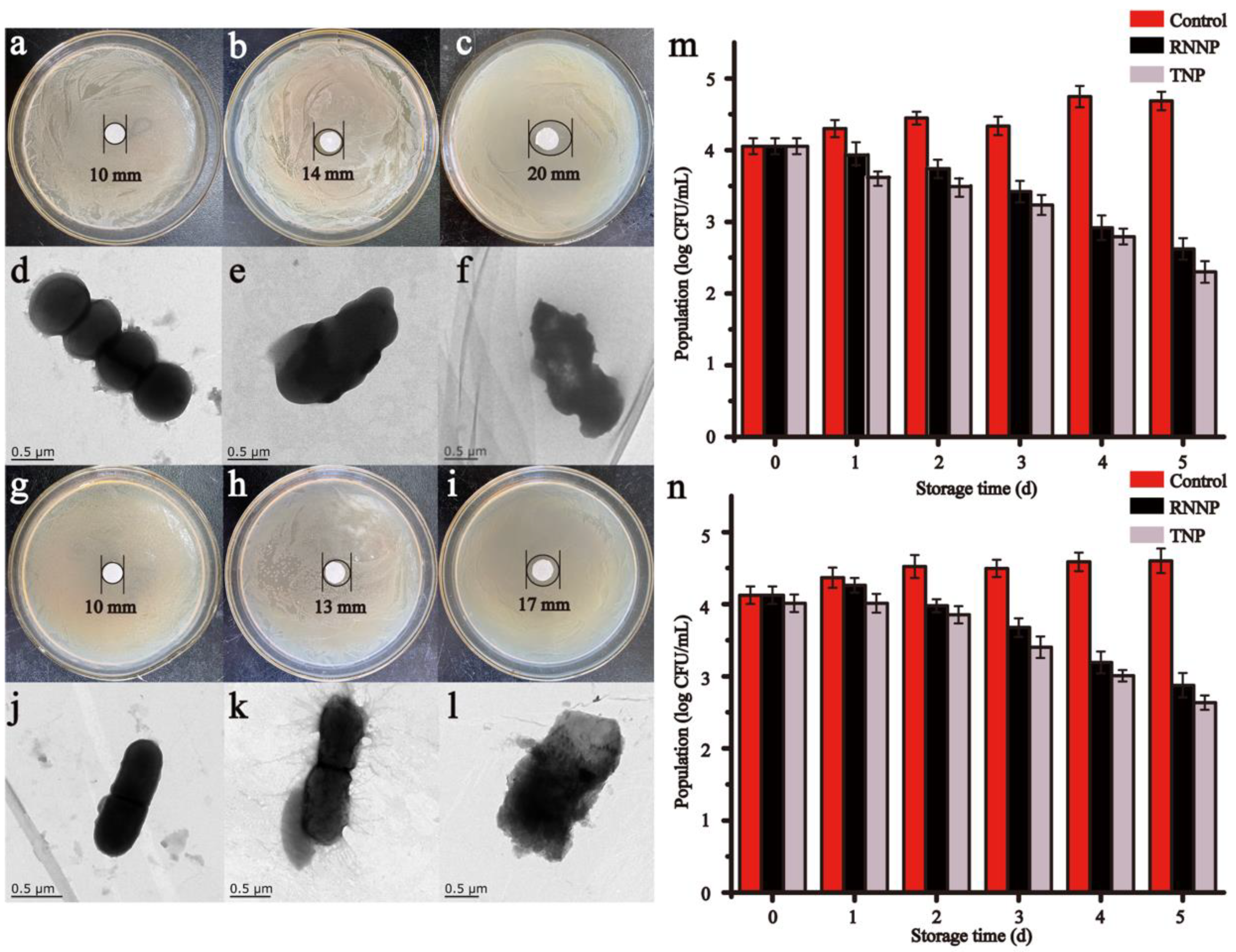
3.1.2. Morphology Analysis of Nanoparticles
3.1.3. UV-Vis Spectral Analysis
3.1.4. Fluorescence Spectroscopy Analysis
3.1.5. Infrared Spectroscopic Analysis of Nanoparticles
3.1.6. QCM
3.1.7. XRD
3.2. The Stability of Nanoparticles
3.3. Antimicrobial Effect of Nanoparticles
3.4. Antioxidant Effect of Nanoparticles
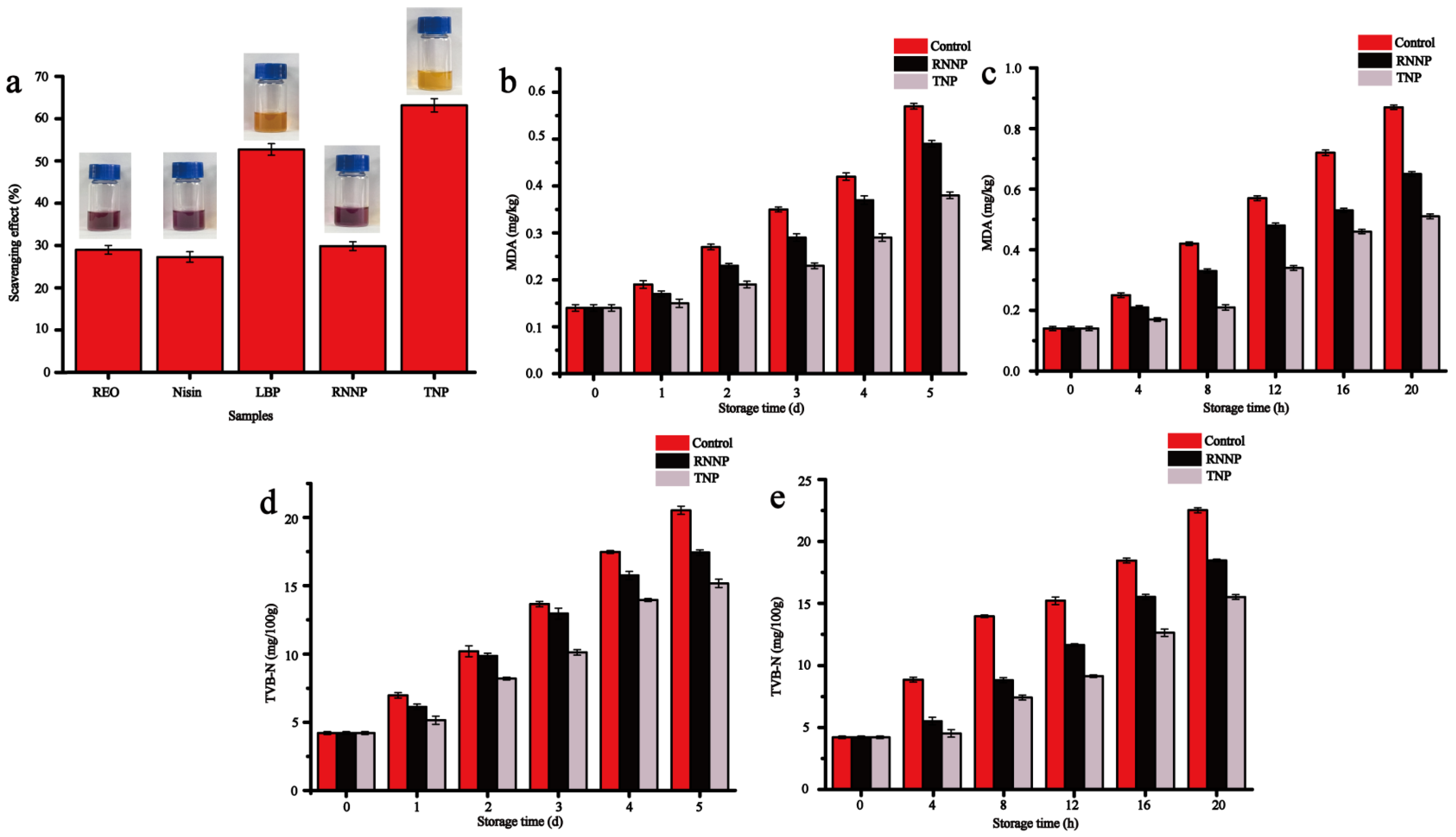
3.4.1. DPPH Scavenging Ability
3.4.2. Antioxidant Effect on Beef
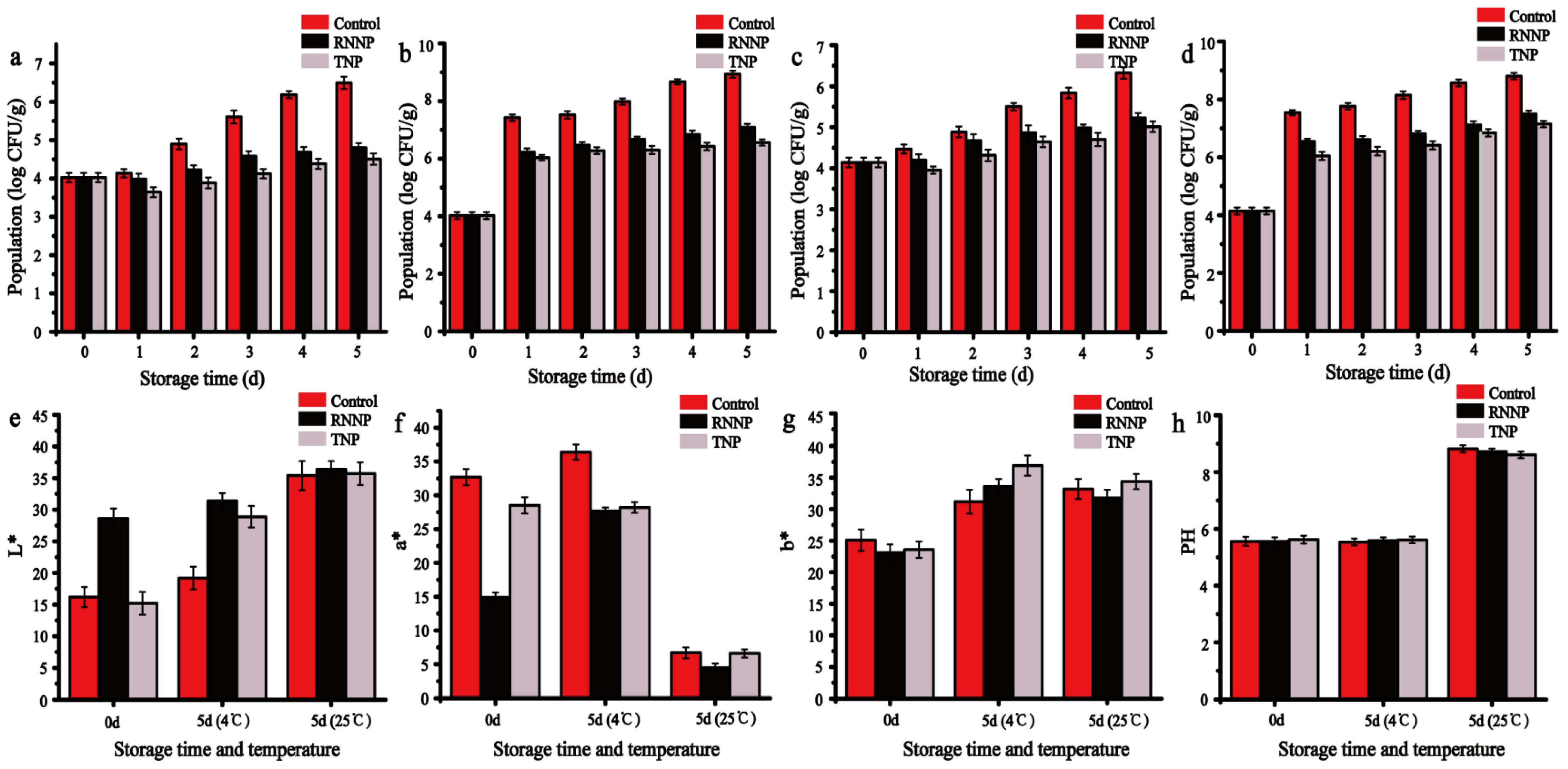
3.5. The Application of Nanoparticles against S. aureus and E. coli O157:H7 on Beef
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Umesha, S.; Manukumar, H.M. Advanced molecular diagnostic techniques for detection of food-borne pathogens: Current applications and future challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 84–104. [Google Scholar] [CrossRef] [PubMed]
- WHO. Food Safety 2014. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 23 July 2021).
- Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 4674235. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, V.; Sharma, V.; Sharma, R.; Kumar, S. Chitosan nanoemulsion: Gleam into the futuristic approach for preserving the quality of muscle foods. Int. J. Biol. Macromol. 2022, 199, 121–137. [Google Scholar] [CrossRef]
- Fucinos, C.; Fucinos, P.; Amado, I.R.; Miguez, M.; Fajardo, P.; Pastrana, L.M.; Rua, M.L. Chapter 28—Smart Nanohydrogels for Controlled Release of Food Preservatives. Antimicrob. Food Packag. 2016, 28, 349–362. [Google Scholar] [CrossRef]
- Zhou, C.; Abdel-Samie, M.A.; Li, C.; Cui, H.; Lin, L. Active packaging based on swim bladder gelatin/galangal root oil nanofibers: Preparation, properties and antibacterial application. Food Packag. Shelf Life 2020, 26, 100586. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Li, C.; Chen, C.; Cui, H. Fabrication of a dual-response intelligent antibacterial nanofiber and its application in beef preservation. LWT 2022, 154, 112606. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Plasma enhanced-nutmeg essential oil solid liposome treatment on the gelling and storage properties of pork meat batters. J. Food Eng. 2020, 266, 109696. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid T liposomes of clove oil. LWT-Food Sci. Technol. 2020, 117, 108656. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Zhou, P.; Feng, R.; Luo, Z.; Wang, L.; Gao, L. Synthesis, identification and bioavailability of Juglans regia L. polyphenols-Hohenbuehelia serotina polysaccharides nanoparticles. Food Chem. 2020, 329, 127158. [Google Scholar] [CrossRef]
- Li, G.; Chen, Q.; Su, C.; Wang, H.; He, S.; Liu, J.; Nag, A.; Yuan, Y. Soy protein-polysaccharide complex coacervate under physical treatment: Effects of pH, ionic sttrength and polysaccharide type. Innov. Food Sci. Emerg. Technol. 2021, 68, 102612. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Gao, X.; Li, G.; He, S. Sodium caseinate and soluble soybean polysaccharide complex as nanocarriers of curcumin. J. Food Meas. Charact. 2021, 15, 478–483. [Google Scholar] [CrossRef]
- Alex, L.C.; Carolina, M.J.; Danyxa, P.H.; Silvia, G. Cassava starch films containing rosemary nanoparticles produced by solvent displacement method. Food Hydrocoll. 2017, 71, 26–34. [Google Scholar] [CrossRef]
- Adriana, M.O.; Gatalina, M.B.; Miguel, A.E.; Miguel, A.J.; Silvia, M. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Catalina, S.R.; Karina, A.; Victoria, R.; Adrian, A.V.; Silvia, M. Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chem. 2009, 115, 456–461. [Google Scholar] [CrossRef]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT-Food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Bai, F.; Guo, D.; Wang, Y.; Zhang, S.; Li, J.; Zhi, K.; Shi, C.; Xia, X. The combined bactericidal effect of nisin and thymoquinone against Listeria monocytogenes in Tryptone Soy Broth and sterilized milk. Food Control. 2022, 135, 108771. [Google Scholar] [CrossRef]
- Mariel, C.; Héctor, B.E.; Omar, N.M.; Jose, P.; Ruth, P.; Edith, P. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT-Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Liu, C.; Zou, Y.; Huang, L.; Liang, Y.; Ren, J.; Liu, Y.; Lin, Q. Elaboration and characterization of curcumin-loaded soy soluble polysaccharide (SSPS)-based nanocarriers mediated by antimicrobial. Food Chem. 2021, 336, 127669. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wang, Q.; Zhao, X.; Yang, H.; Gong, F.; Guo, H. Preparation and antimicrobial activity of pectin-chitosan embedding nisin microcapsules. Eur. Polym. J. 2021, 157, 110676. [Google Scholar] [CrossRef]
- Prombutara, P.; Kulwatthanasal, Y.; Supaka, N.; Sramala, I.; Chareonpornwattana, S. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control. 2012, 24, 184–190. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Cleveland, j.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Cui, H.; Dai, Y.; Lin, L. Enhancing antibacterial efficacy of nisin in pork by poly-c-glutamic acid/poly-L-lysine nanoparticles encapsulation. J. Food Saf. 2018, 38, e12475. [Google Scholar] [CrossRef]
- Imran, K.; Deog-Hwan, O. Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. Technol. 2016, 34, 376–384. [Google Scholar] [CrossRef]
- Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Aisaggaf, M.S.; Moussa, S.H.; Elguindy, N.M.; Tayel, A.A. Fungal chitosan and Lycium barbarum extract as anti-Listeria and quality preservatives in minced catfish. Int. J. Biol. Macromol. 2017, 104, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, F.; Luo, Q.; Wu, M.; Song, G.; Zhang, H.; Cao, J.; Wang, Y. Lycium barbarum polysaccharides grafted with doxorubicin: An efficient pH-responsive anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 121, 964–970. [Google Scholar] [CrossRef]
- Duan, G.L.; Yu, X. Isolation, purification, characterization, and antioxidant activity of low-molecular-weight polysaccharides from Sparassis latifolia. Int. J. Biol. Macromol. 2019, 137, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zha, X.; Li, Q.; Pan, L.; Luo, J. Hydrophobic interaction and hydrogen bonding driving the self-assembling of quinoa protein and flavonoids. Food Hydrocoll. 2021, 118, 106807. [Google Scholar] [CrossRef]
- Hamdeni, I.; Slim, S.; Sanaa, A.; Louhaichi, M.; Boulila, A.; Bettaieb, T. Rosemary essential oil enhances culture establishment and inhibits contamination and enzymatic browning: Applications for in vitro propagation of Aloe vera L. S. Afr. J. Bot. 2021. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with T pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [Green Version]
- Thakar, M.A.; Jha, S.S.; Phasinam, K.; Manne, R.; Qureshi, Y.; Babu, V. X ray diffraction (XRD) analysis and evaluation of antioxidant activity of copper oxide nanoparticles synthesized from leaf extract of Cissus vitiginea. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Novel electrospun gelatin-glycerin-ε-Poly-lysine nanofibers for controlling Listeria monocytogenes on beef. Food Packag. Shelf Life 2018, 18, 21–30. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Mohamed, A.; Lin, L. Cold plasma treated phlorotannin/Momordica charantia polysaccharide nanofiber for active food packaging. Carbohydr. Polym. 2020, 239, 116214. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Pan, D.D.; Cao, J.X.; Shao, X.F.; Chen, Y.J. Effect of black pepper essential oil on the quality of fresh pork during storage. Meat Sci. 2016, 117, 130–136. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food T packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant assisted nisin loaded chitosan-carageenan nanocapsule synthesis for controlling food pathogens. Food Control. 2014, 37, 158–164. [Google Scholar] [CrossRef]
- Chang, R.; Lu, H.; Li, M.; Zhang, S.; Xiong, L.; Sun, Q. Preparation of extra-small nisin nanoparticles for enhanced antibacterial activity after autolave treatment. Food Chem. 2018, 245, 756–760. [Google Scholar] [CrossRef]
- Narahari, A.; Swamy, M.J. Tryptophan exposure and accessibility in the chitooligosaccharide-specific phloem exudate lectin from pumpkin (Cucurbita maxima). A fluorescence study. J. Photochem. Photobiol. B Biol. 2009, 97, 40–47. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Rostami, E. Spectroscopic investigation of the interaction of BSA with cationic surfactant. Chem. Eng. Technol. 2010, 31, 1265–1271. [Google Scholar] [CrossRef]
- Li, J.; Pan, D.; Yi, J.; Hao, L.; Kang, Q.; Liu, X.; Lu, L.; Lu, J. Protective effect of β-cyclodextrin on stability of nisin and corresponding interactions involved. Carbohydr. Polym. 2019, 223, 115115. [Google Scholar] [CrossRef]
- Radhakrishna, M.; Grimaldi, J.; Belfort, G.; Kumar, S.K. Stability of Proteins Inside a Hydrophobic Cavity. Am. Chem. Soc. 2003, 29, 8922–8928. [Google Scholar] [CrossRef]
- Dhouibi, I.; Masmoudi, F.; Bouaziz, M.; Masmoudi, M. A study of the anti-corrosive effects of essential oils of rosemary and myrtle for copper corrosion in chloride media. Arab. J. Chem. 2021, 14, 102961. [Google Scholar] [CrossRef]
- Stamarkou, M.; Oikonomopoulou, V.; Missirli, T.; Thanassoulia, I.; Krokida, M. Encapsulation of Rosemary Essential Oil into Biodegradable Polymers for Application in Crop Management. J. Polym. Environ. 2020, 28, 2161–2177. [Google Scholar] [CrossRef]
- Krivorotova, T.; Cirkovas, A.; Maciulyte, S.; Staneviciene, R.; Budriene, S.; Serviene, E.; Sereikaite, J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll. 2016, 54, 49–56. [Google Scholar] [CrossRef]
- Tan, H.F.; Gan, C.Y. Polysaccharide with antioxidant, amylase inhibitory and ACE inhibitory activities from Momordica charantia. Int. J. Biol. Macromol. 2016, 85, 487–496. [Google Scholar] [CrossRef]
- Gong, F.; Qian, J.; Chen, Y.; Yao, S.; Tong, J.; Guo, H. Preparation and properties of gum arabic cross-link binding nisin microparticles. Carbohydr. Polym. 2018, 197, 608–613. [Google Scholar] [CrossRef]
- Cui, H.; Lu, J.; Li, C.; Rashed, M.; Lin, L. Antibacterial and physical effects of cationic starch nanofibers containing carvacrol@casein nanoparticles against Bacillus cereus in soy products. Inter. J. Food Microbiol. 2022, 364, 109530. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Zou, H.; Zhang, X.; Sheng, R.; Zhang, Y.; Zhang, B.; Li, C.; Qi, Y. An enhanced antibacterial nanoflowers AgPW@PDA@Nisin constructer from polyoxometalate and nisin. J. Inorg. Biochem. 2020, 212, 111212. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Shen, Z.; Dong, S. Prediction of total volatile basic nitrogen (TVB-N) content of chilled beef for freshness evaluation by using viscoelasticity based on airflow and laser technique. Food Chem. 2019, 287, 126–132. [Google Scholar] [CrossRef]
| Nanoparticles | Nisin:LBP (V/V) | Particle Size (nm) | PDI | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| Nanoparticles-I | 1:0 | 167.8 ± 4.8 | 0.242 ± 0.008 | 53.7 ± 0.6 |
| Nanoparticles-II | 3:1 | 386.5 ± 2.3 a | 0.302 ± 0.022 a | 84.4 ± 0.3 c |
| Nanoparticles-III | 1:1 | 211.5 ± 8.1 d | 0.241 ± 0.002 c | 86.6 ± 0.2 a |
| Nanoparticles-VI | 1:3 | 266.7 ± 7.7 b | 0.269 ± 0.005 b | 86.4 ± 0.1 a |
| Nanoparticles-V | 1:5 | 256.3 ± 8.4 c | 0.227 ± 0.015 c | 85.6 ± 0.3 b |
| Treatment | Texture Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Hardness (g) | Adhesiveness (g.sec) | Resilience (%) | Cohesion | Springiness (%) | Gumminess | Chewiness | ||
| 0 d | Control | 25.916 ± 0.482 a | −47.244 ± 1.572c | 47.372 ± 2.758 b | 0.801 ± 0.003 b | 51.237 ± 2.573 a | 8.235 ± 0.628 a | 3.514 ± 0.089 b |
| RNNP | 25.985 ± 0.893 a | −19.499 ± 0.724 a | 51.061 ± 5.641 a | 0.853 ± 0.004 a | 53.423 ± 3.091 a | 8.703 ± 0.773 a | 3.825 ± 0.068 a | |
| TNP | 26.328 ± 0.737 a | −26.424 ± 0.857 b | 40.243 ± 2.986 c | 0.763 ± 0.002 c | 47.279 ± 2.942 a | 8.255 ± 0.395 a | 3.301 ± 0.046 c | |
| 5 d (4 °C) | Control | 18.855 ± 0.961 b | −41.829 ± 1.147 c | 38.297 ± 2.675 a | 0.597 ± 0.002 c | 39.582 ± 1.862 a | 6.425 ± 0.413 a | 2.125 ± 0.031 c |
| RNNP | 20.275 ± 0.725 b | −13.351 ± 1.426 a | 45.847 ± 4.842 a | 0.752 ± 0.006 a | 43.51 ± 3.427 a | 7.214 ± 0.386 a | 2.532 ± 0.073 a | |
| TNP | 23.016 ± 0.515 a | −25.934 ± 0.984 b | 38.961 ± 2.967 a | 0.665 ± 0.005 b | 40.21 ± 2.074 a | 7.013 ± 0.514 a | 2.289 ± 0.062 b | |
| 5 d (25 °C) | Control | 11.616 ± 0.472 c | −34.455 ± 0.963 c | 30.566 ± 1.653b | 0.468 ± 0.002 c | 32.221 ± 1.143 a | 5.438 ± 0.327 a | 1.752 ± 0.057 c |
| RNNP | 14.734 ± 0.357 b | −10.345 ± 1.273 a | 40.968 ± 3.547 a | 0.638 ± 0.004 a | 37.061 ± 1.984 a | 6.217 ± 0.551 a | 2.316 ± 0.071 a | |
| TNP | 19.564 ± 0.416 a | −21.362 ± 1.527 b | 36.805 ± 1.604 a | 0.615 ± 0.008 b | 35.415 ± 2.573 a | 5.941 ± 0.426 a | 2.046 ± 0.054 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Luo, C.; Li, C.; Chen, X.; Cui, H. A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation. Foods 2022, 11, 438. https://doi.org/10.3390/foods11030438
Lin L, Luo C, Li C, Chen X, Cui H. A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation. Foods. 2022; 11(3):438. https://doi.org/10.3390/foods11030438
Chicago/Turabian StyleLin, Lin, Chencheng Luo, Changzhu Li, Xiaochen Chen, and Haiying Cui. 2022. "A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation" Foods 11, no. 3: 438. https://doi.org/10.3390/foods11030438
APA StyleLin, L., Luo, C., Li, C., Chen, X., & Cui, H. (2022). A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation. Foods, 11(3), 438. https://doi.org/10.3390/foods11030438








