Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Crithmum maritimum
2.2.1. Contents of Chlorophylls and Carotenoids of C. maritimum
2.2.2. Contents of Total Phenolic Compounds and Flavonoids of C. maritimum
2.2.3. Assessment of the Antioxidant Activity of C. maritimum
DPPH Radical Scavenging Activity
Ferric Reducing Antioxidant Power (FRAP)
2.3. Ultrasound Assisted Extraction of Bioactive Compounds to Sunflower Oil
2.4. Analyses of Sunflower Oil
2.4.1. Chemical Quality Parameters of Supplemented Sunflower Oils
2.4.2. Contents of Chlorophylls and Carotenoids in Sunflower Oils
2.4.3. Determination of Total Phenolic Compounds and Flavonoids in Sunflower Oils
2.4.4. Determination of Antioxidant Activity of Sunflower Oils
2.5. Sensory Evaluation of Supplemented Sunflower Oil
2.6. Oxidation Tests under Accelerated Conditions
2.7. Statistical Analyses
3. Results
3.1. Characterization of Crithmum maritimum
3.2. Quality Parameters of the Supplemented Sunflower Oil
3.2.1. Acidity
3.2.2. Oxidation Products
3.3. Green Pigments and Carotenoid Contents in Sunflower Oil
3.4. Determination of Total Phenolic Compounds, Flavonoids, and Antioxidant Activity
3.4.1. Total Phenolic Compounds and Flavonoids Contents
3.4.2. Antioxidant Activity of Supplemented Sunflower Oil Samples
3.5. Sensory Analysis
3.6. Accelerated Oxidation Tests
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farahmandfar, R.; Asnaashari, M.; Pourshayegan, M.; Maghsoudi, S.; Moniri, H. Evaluation of Antioxidant Properties of Lemon Verbena (Lippia citriodora) Essential Oil and Its Capacity in Sunflower Oil Stabilization during Storage Time. Food Sci. Nutr. 2018, 6, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Sing, Y.; Nawi, M.; Hashim, N.; Mohamedali, A.; Saleh, M.; Sulaiman, S.; Talib, K.; Ahmad, K. Determination of Synthetic Phenolic Antioxidants in Food Items Using Reversed-Phase HPLC. Food Chem. 2007, 105, 389–394. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as Source of Bioactive Phenolic Compounds and Their Potential Applications. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs Cultivated Halophytes: Nutritional and Functional Differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Pereira, C.G.; Barreira, L.; da Rosa Neng, N.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for New Sources of Innovative Products for the Food Industry within Halophyte Aromatic Plants: In Vitro Antioxidant Activity and Phenolic and Mineral Contents of Infusions and Decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the Prospects of Sea Fennel (Crithmum maritimum L.) as Emerging Vegetable Crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souid, A.; della Croce, C.M.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Hamed, K.B.; Magné, C.; Longo, V. Nutraceutical Potential of Leaf Hydro-Ethanolic Extract of the Edible Halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-Related Matrices. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Nde, D.; Foncha, A. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef] [Green Version]
- Sousa, G.; Trifunovska, M.; Antunes, M.; Miranda, I.; Moldão, M.; Alves, V.; Vidrih, R.; Lopes, P.A.; Aparicio, L.; Neves, M.; et al. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Pelvetia Canaliculata to Sunflower Oil. Foods 2021, 10, 1732. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids Measurement and UV-VIS Characterization. Curr. Protoc. Food Anal. Chem. 2001, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Rougereau, A.; Person, O.; Rougereau, G. Determination of Vitamins. In Analysis of Food Constituents; Multon, J.-L., Ed.; Wiley-VCH: New York, NY, USA, 1997; pp. 281–309. ISBN 0-471-18966-9. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant Activities and Phenolics Content of Eight Species of Seaweeds from North Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Laulloo, S.S.J.; Bhowon, M.G.; Hoolash, A. Influence Of Chemical Refining Processes On The Total Phenolics And Antioxidant Activity Of Sunflower Oil. Int. J. Nutr. 2015, 1, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Leardi, R. Experimental Design in Chemistry: A Tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Off. JL 1991, 248, 1–128. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01991R2568-20151016&from=EN (accessed on 1 November 2021).
- Pokorný, J.; Kalinova, L.; Dysseler, P. Determination of Chlorophyll Pigments in Crude Vegetable Oils: Results of a Collaborative Study and the Standardized Method (Technical Report). Pure Appl. Chem. 1995, 67, 1781–1787. [Google Scholar] [CrossRef]
- Hrncirik, K.; Fritsche, S. Comparability and Reliability of Different Techniques for the Determination of Phenolic Compounds in Virgin Olive Oil. Eur. J. Lipid Sci. Technol. 2004, 106, 540–549. [Google Scholar] [CrossRef]
- Haaland, P.D. Experimental Design in Biotechnology; Marcel Dekker, Inc.: New York, NY, USA, 1989; ISBN 0-8247-7881-2. [Google Scholar]
- Rodrigues, M.I.; Iemma, A.F. Experimental Design and Process Optimization; Casa do Espírito Amigo Fraternidade Fé e Amor: Campinas, Brazil, 2012; ISBN 978-85-60616-12-1. [Google Scholar]
- Mahoney, E.Y.; Milewska, M.; Mironczuk-Chodakowska, I.; Terlikowska, K.M. The Influence of Carotenoid and Chlorophyll Content on the Oxidative Processes in the Selected Vegetable Oils. Prog. Health Sci. 2018, 8, 144–151. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea Fennel (Crithmum Maritimum L.): From Underutilized Crop to New Dried Product for Food Use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burló, F.; Hernández, F.; Carbonell-Barrachina, Á.A.; Madani, K.; Larbat, R. Biological Activities and Secondary Compound Composition from Crithmum Maritimum Aerial Parts. Int. J. Food Prop. 2017, 20, 1843–1855. [Google Scholar] [CrossRef] [Green Version]
- Houta, O.; Akrout, A.; Neffati, M.; Amri, H. Phenolic Contents, Antioxidant and Antimicrobial Potentials of Crithmum Maritimum Cultivated in Tunisia Arid Zones. J. Biol. Act. Prod. Nat. 2011, 1, 138–143. [Google Scholar] [CrossRef]
- Fu, C.W.F.; Ho, C.W.; Yong, W.T.L.; Abas, F.; Tan, T.B.; Tan, C.P. Extraction of Phenolic Antioxidants from Four Selected Seaweeds Obtained from Sabah. Int. Food Res. J. 2016, 23, 2363–2369. [Google Scholar]
- Codex Alimentarius Commission. Standard for Named Vegetable Oils. CXS 210-1999. Codex Stan 2019, 210, 1–15. [Google Scholar]
- Corbu, A.R.; Rotaru, A.; Nour, V. Edible Vegetable Oils Enriched with Carotenoids Extracted from By-Products of Sea Buckthorn (Hippophae rhamnoides ssp. sinensis): The Investigation of Some Characteristic Properties, Oxidative Stability and the Effect on Thermal Behaviour. J. Ther. Anal. Calorim. 2020, 142, 735–747. [Google Scholar] [CrossRef]
- Karoune, S.; Kechebar, M.S.A.; Douffi, H.; Djellouli, A. Phenolic Compounds and Their Antioxidant Activities in Portulaca Oleracea L. Related to Solvent Extraction. Int. J. Biosci. (IJB) 2017, 11, 147–155. [Google Scholar] [CrossRef]
- Ng, Z.X.; Samsuri, S.N.; Yong, P.H. The Antioxidant Index and Chemometric Analysis of Tannin, Flavonoid, and Total Phenolic Extracted from Medicinal Plant Foods with the Solvents of Different Polarities. J. Food Process. Preserv. 2020, 44, e14680. [Google Scholar] [CrossRef]
- Bakhshi, S.; Abbaspour, H.; Saeidisar, S. Study of Phytochemical Changes, Enzymatic and Antioxidant Activity of Two Halophyte Plants: Salsola dendroides Pall and Limonium reniforme (Girard) Lincz in Different Seasons. J. Plant Environ. Physiol. 2018, 46, 79–92. [Google Scholar]
- Kulbat, K. The Role of Phenolic Compounds in Plant Resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary Extract Can Be Used as a Synthetic Antioxidant to Improve Vegetable Oil Oxidative Stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Siraj, N.; Shabbir, M.A.; Khan, M.R.; Rehman, K.U. Preventing Oxidation of Canola and Sunflower Oils by Addition of Pomegranate Seed Oil. Acta Aliment. 2019, 48, 18–27. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Accelerated Storage Conditions Effect on Ginger- and Turmeric-Enriched Soybean Oils with Comparing a Synthetic Antioxidant BHT. LWT 2020, 131, 109797. [Google Scholar] [CrossRef]
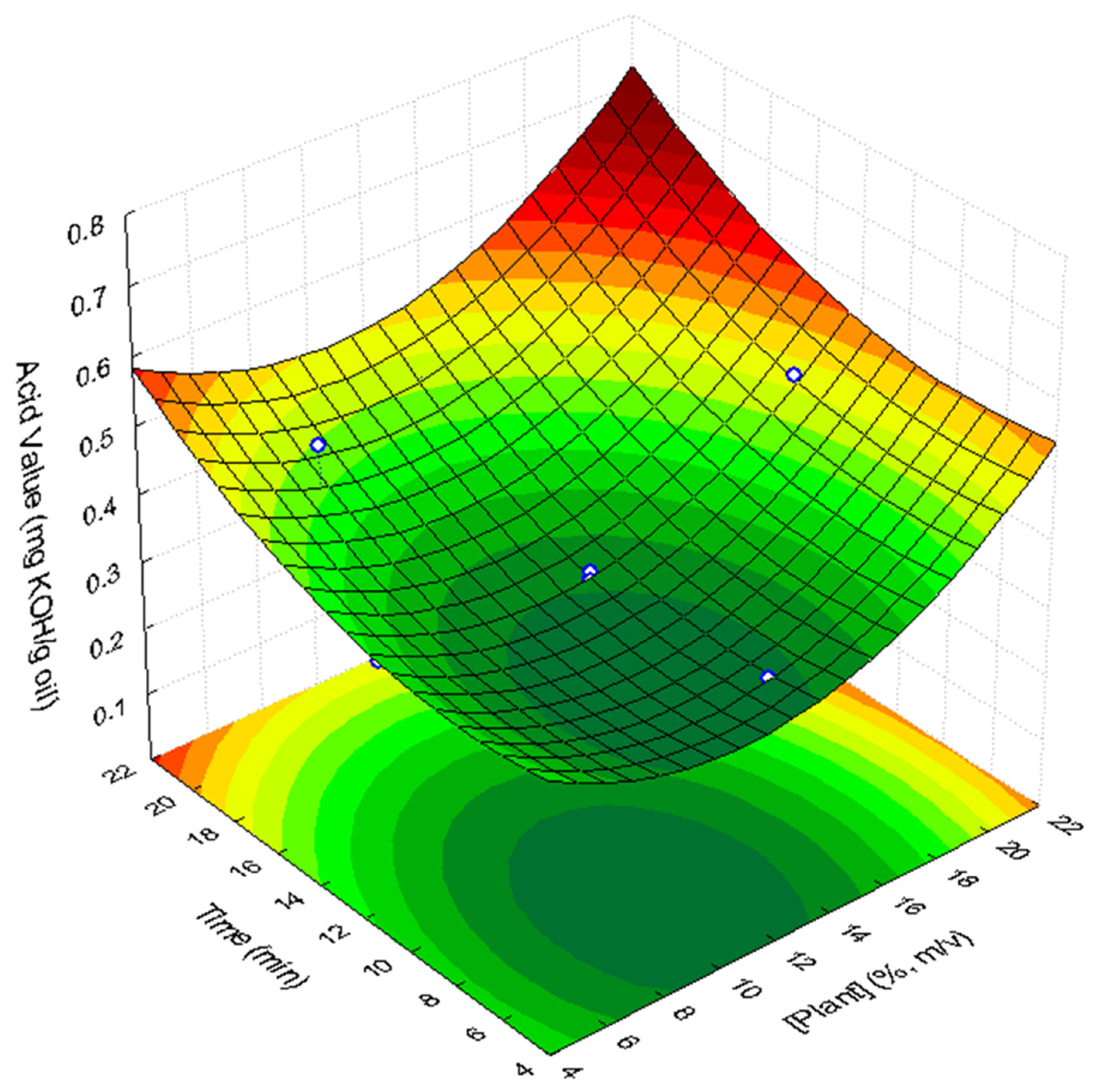


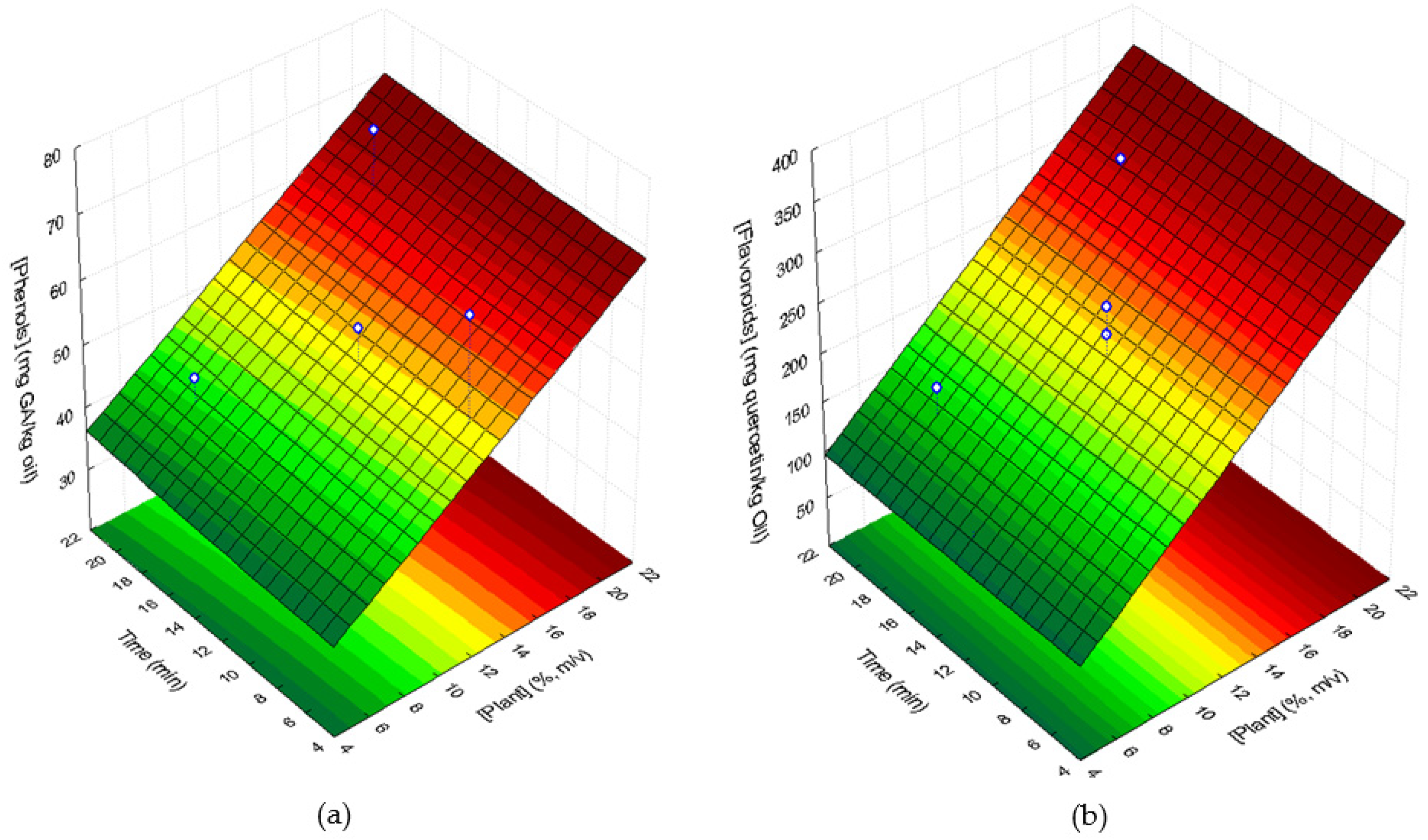
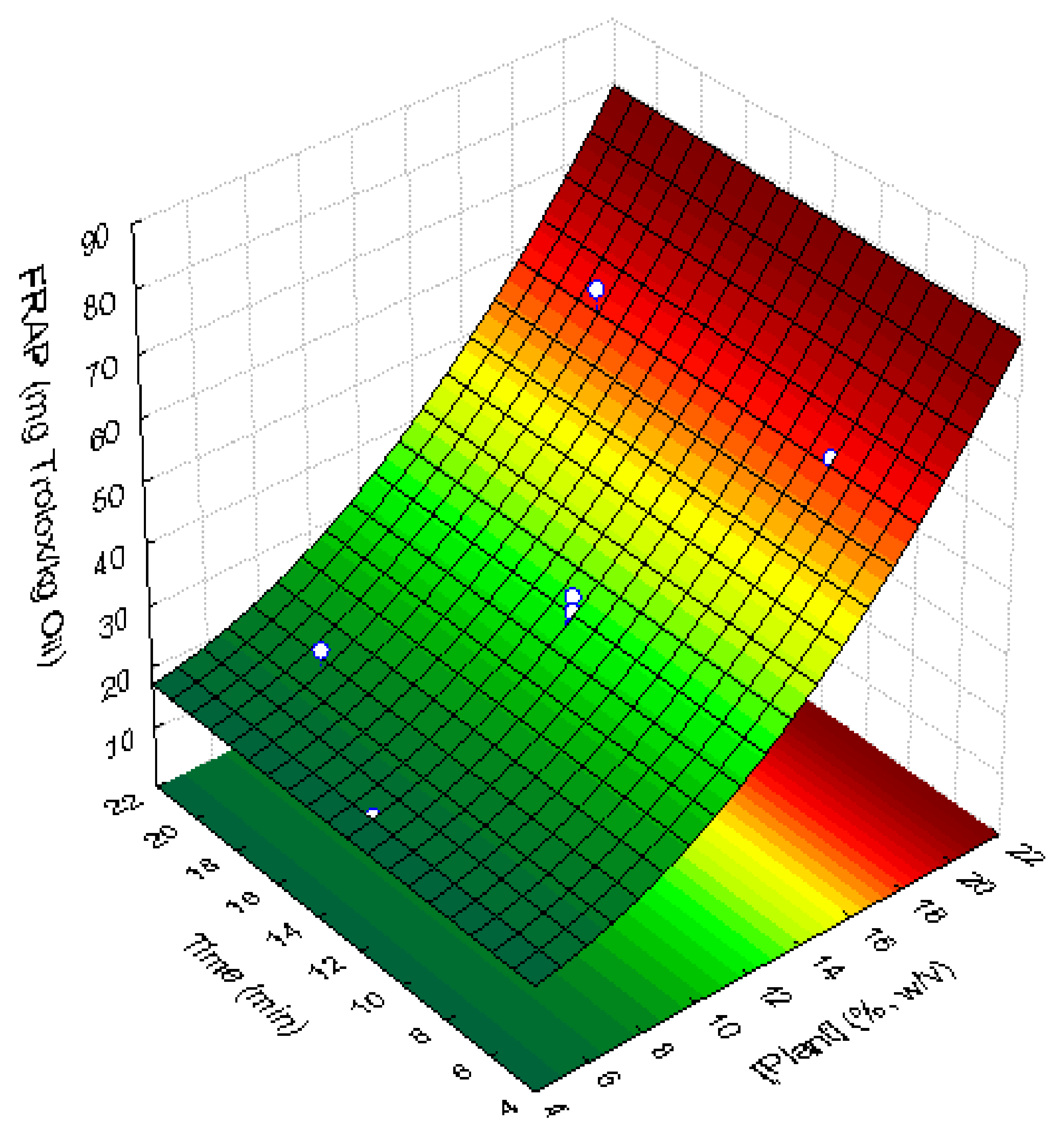


| Factor (Variable) | Coded Levels | ||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| (Plant) (% m/v) | 5.0 | 7.2 | 12.5 | 17.8 | 20.0 |
| UAE Time (min) | 5.0 | 7.2 | 12.5 | 17.8 | 20.0 |
| Compound | Amount (mg/kg Halophyte) |
|---|---|
| Chlorophylls | 1092 ± 8 |
| Carotenoids (β-carotene) | 470 ± 40 |
| Phenolics (gallic acid) | 8110 ± 210 |
| Flavonoids (quercetin) | 56,202 ± 4019 |
| DPPH, % RSA | 87.88 ± 1.66 |
| DPPH (Trolox) | 7320 ± 140 |
| FRAP (Trolox) | 20,997 ± 222 |
| Experimental Conditions | Green Pigments | Carotenoids | ||||
|---|---|---|---|---|---|---|
| Assay | (Plant) (% m/v) Decoded Values | UAE Time (min) Decoded Values | (mg Pheophytin a/kg Oil) | Extraction Yield (%) | (mg β-Carotene/kg Oil) | Extraction Yield (%) |
| 1 | 7.2 | 7.2 | 14.63 ± 0.06 | 17.1 | 19.03 ± 0.62 | 48.7 |
| 2 | 7.2 | 17.8 | 17.83 ± 0.21 | 20.8 | 21.79 ± 0.16 | 56.3 |
| 3 | 17.8 | 7.2 | 44.24 ± 0.13 | 20.9 | 53.47 ± 0.85 | 57.9 |
| 4 | 17.8 | 17.8 | 42.48 ± 0.08 | 20.1 | 42.56 ± 5.15 | 45.8 |
| 5 | 5.0 | 12.5 | 9.14 ± 0.39 | 15.4 | 12.63 ± 0.64 | 44.9 |
| 6 | 20.0 | 12.5 | 29.92 ± 0.13 | 12.6 | 32.61 ± 1.46 | 30.9 |
| 7 | 12.5 | 5.0 | 27.60 ± 0.20 | 18.6 | 33.56 ± 0.63 | 51.0 |
| 8 | 12.5 | 20.0 | 29.16 ± 0.19 | 19.6 | 34.66 ± 0.10 | 52.7 |
| 9 | 12.5 | 12.5 | 25.16 ± 0.14 | 16.9 | 30.26 ± 0.29 | 45.8 |
| 10 | 12.5 | 12.5 | 29.04 ± 0.15 | 19.6 | 32.88 ± 0.36 | 49.9 |
| 11 | 12.5 | 12.5 | 28.56 ± 0.14 | 19.2 | 30.52 ± 0.59 | 46.2 |
| 12 | 12.5 | 12.5 | 24.88 ± 0.16 | 16.7 | 24.52 ± 0.03 | 36.7 |
| 13 | 0 | 0 | 0.03 ± 0.01 | - | 1.25 ± 0.66 | - |
| 14 | 0 | 20 | 0.02 ± 0.01 | - | 1.25 ± 0.32 | - |
| Phenolic Content | Flavonoids | DPPH | FRAP | ||||
|---|---|---|---|---|---|---|---|
| Assay | (mg Gallic Acid/kg Oil) | Extraction Yield (%) | (mg Quercetin/kg Oil) | Extraction Yield (%) | % RSA | (mg Trolox/kg Oil) | (mg Trolox/kg Oil) |
| 1 | 39.05 ± 7.60 | 5.5 | 119.39 ± 0.09 | 1.9 | 25.52 ± 6.34 | 12.81 ± 3.18 | 17.50 ± 1.89 |
| 2 | 47.19 | 6.8 | 181.11 ± 16.52 | 3.4 | 27.45 ± 0.21 | 13.72 ± 0.10 | 26.34 ± 0.39 |
| 3 | 60.24 ± 6.78 | 3.6 | 306.76 ± 39.91 | 2.5 | 27.90 ± 1.31 | 21.71 ± 1.02 | 61.67 ± 22.90 |
| 4 | 72.13 ± 0.67 | 4.3 | 319.68 ± 99.56 | 2.6 | 35.16 ± 4.97 | 26.79 ± 3.78 | 63.62 ± 10.91 |
| 5 | 32.66 ± 9.58 | 6.5 | 90.41 ± 2.69 | 1.9 | 20.53 ± 2.44 | 10.29 ± 1.22 | 18.29 ± 0.39 |
| 6 | 56.38 ± 6.46 | 3.0 | 301.11 ±33.09 | 2.2 | 30.58 ± 4.88 | 26.87 ± 4.28 | 59.99 ± 8.78 |
| 7 | 70.42 ± 7.88 | 6.0 | 202.25 ± 20.89 | 2.2 | 42.66 ± 4.73 | 29.26 ± 3.24 | 36.75 ± 3.61 |
| 8 | 51.33 ± 3.31 | 4.3 | 205.54 ± 3.34 | 2.2 | 32.16 ± 5.34 | 23.05 ± 3.82 | 29.63 ± 7.67 |
| 9 | 56.49 ± 14.55 | 4.8 | - | - | 37.92 ± 6.24 | 29.25 ± 4.80 | 32.52 ± 2.11 |
| 10 | 44.24 ± 4.50 | 3.6 | 244.74 ± 43.15 | 2.8 | 26.14 ± 6.90 | 19.25 ± 5.07 | 37.52 ± 3.55 |
| 11 | 35.00 ± 1.16 | 2.8 | 182.71 ±17.56 | 2.0 | - | - | 27.52 ± 1.62 |
| 12 | 45.19 ± 1.74 | 3.7 | 272.54 ± 9.03 | 3.1 | 16.40 ± 2.02 | 12.13 ± 1.49 | 34.93 ± 0.70 |
| 13 | 4.12 ± 0.95 | - | 33.74 ± 3.01 | - | 0.64 ± 0.09 | 0.35 ± 0.04 | 2.91 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, G.; Alves, M.I.; Neves, M.; Tecelão, C.; Ferreira-Dias, S. Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods 2022, 11, 439. https://doi.org/10.3390/foods11030439
Sousa G, Alves MI, Neves M, Tecelão C, Ferreira-Dias S. Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods. 2022; 11(3):439. https://doi.org/10.3390/foods11030439
Chicago/Turabian StyleSousa, Gabriela, Mariana I. Alves, Marta Neves, Carla Tecelão, and Suzana Ferreira-Dias. 2022. "Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L." Foods 11, no. 3: 439. https://doi.org/10.3390/foods11030439
APA StyleSousa, G., Alves, M. I., Neves, M., Tecelão, C., & Ferreira-Dias, S. (2022). Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods, 11(3), 439. https://doi.org/10.3390/foods11030439







