Impact of Flaxseed Gums on the Colloidal Changes and In Vitro Digestibility of Milk Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Milk Protein Food Models
2.3. In vitro Gastrointestinal Processing of the Milk Protein Food Models
2.4. Steady State Flow Rheological Measurements
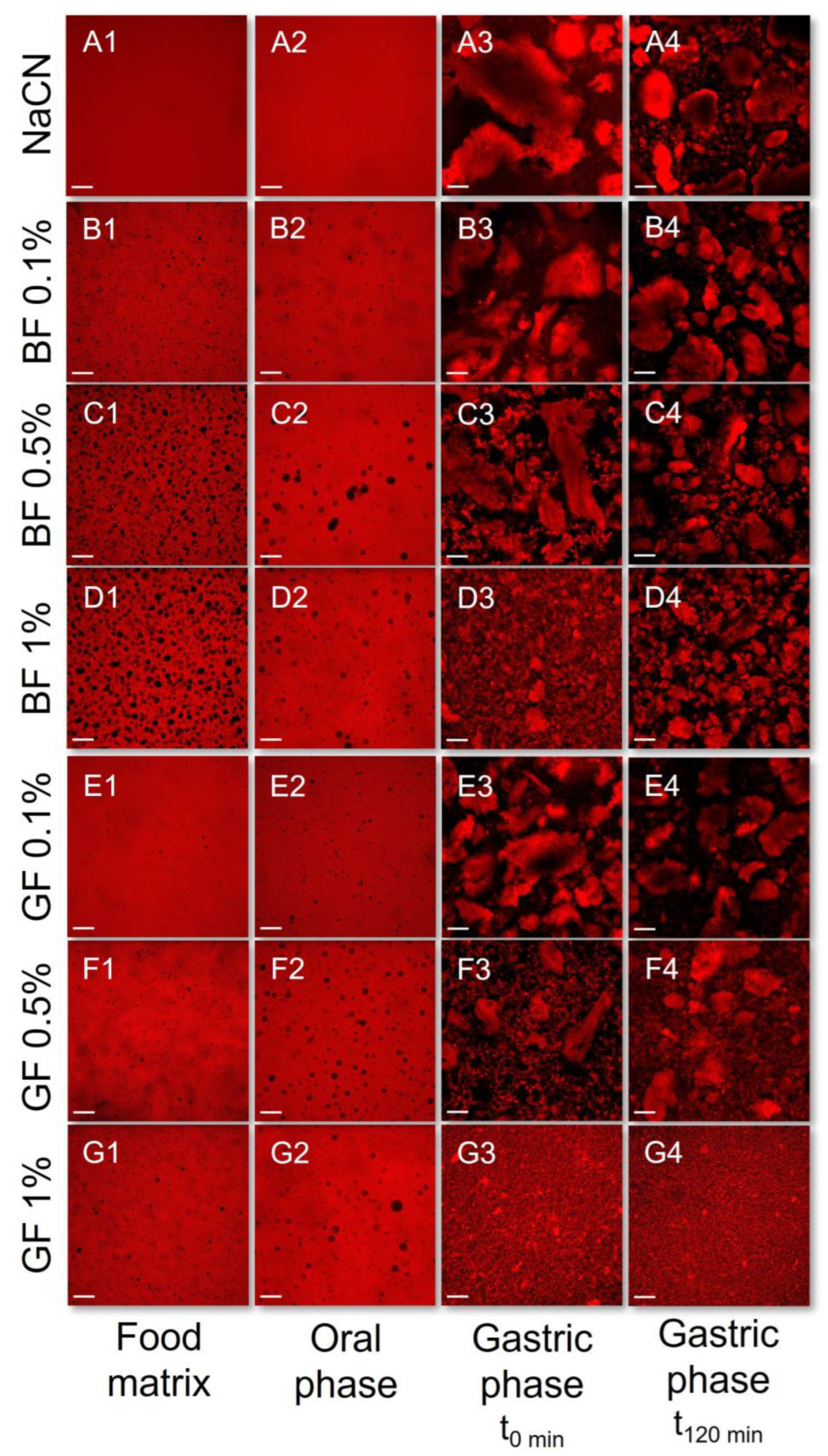
2.5. Confocal Laser Scanning Microscopy (CLSM)
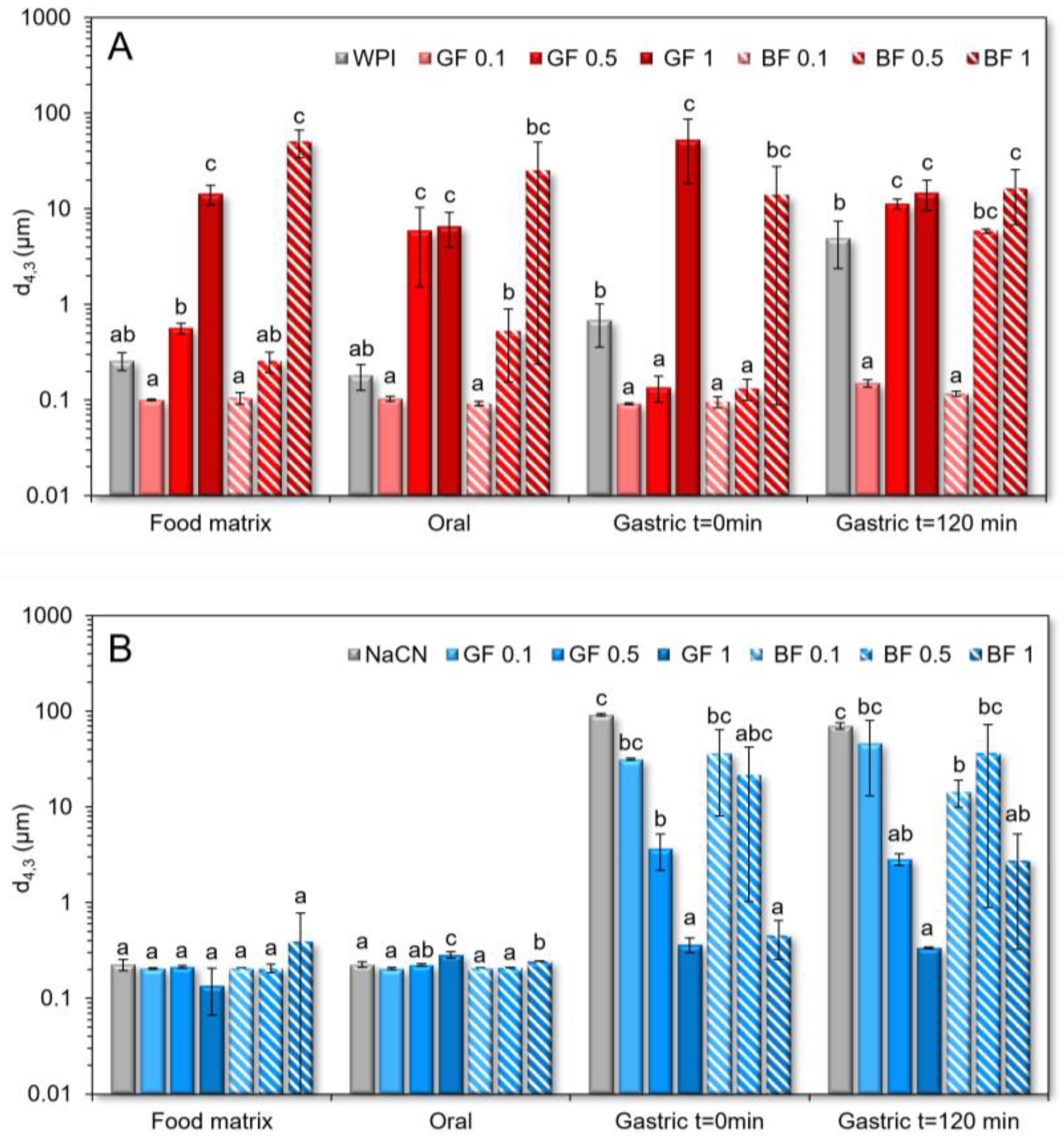
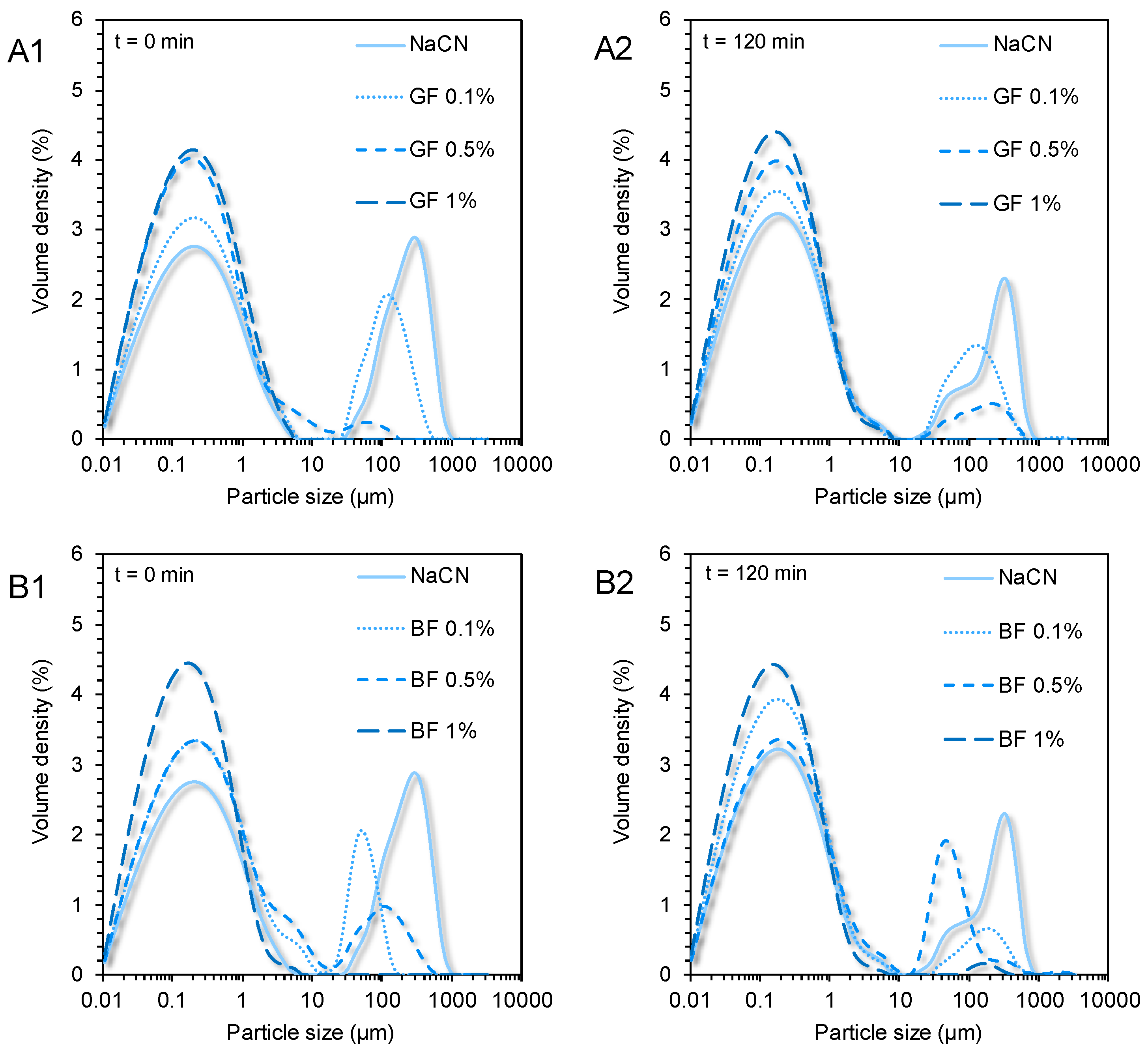
2.6. Particle Size Distribution Measurements
2.7. Protein Digestibility
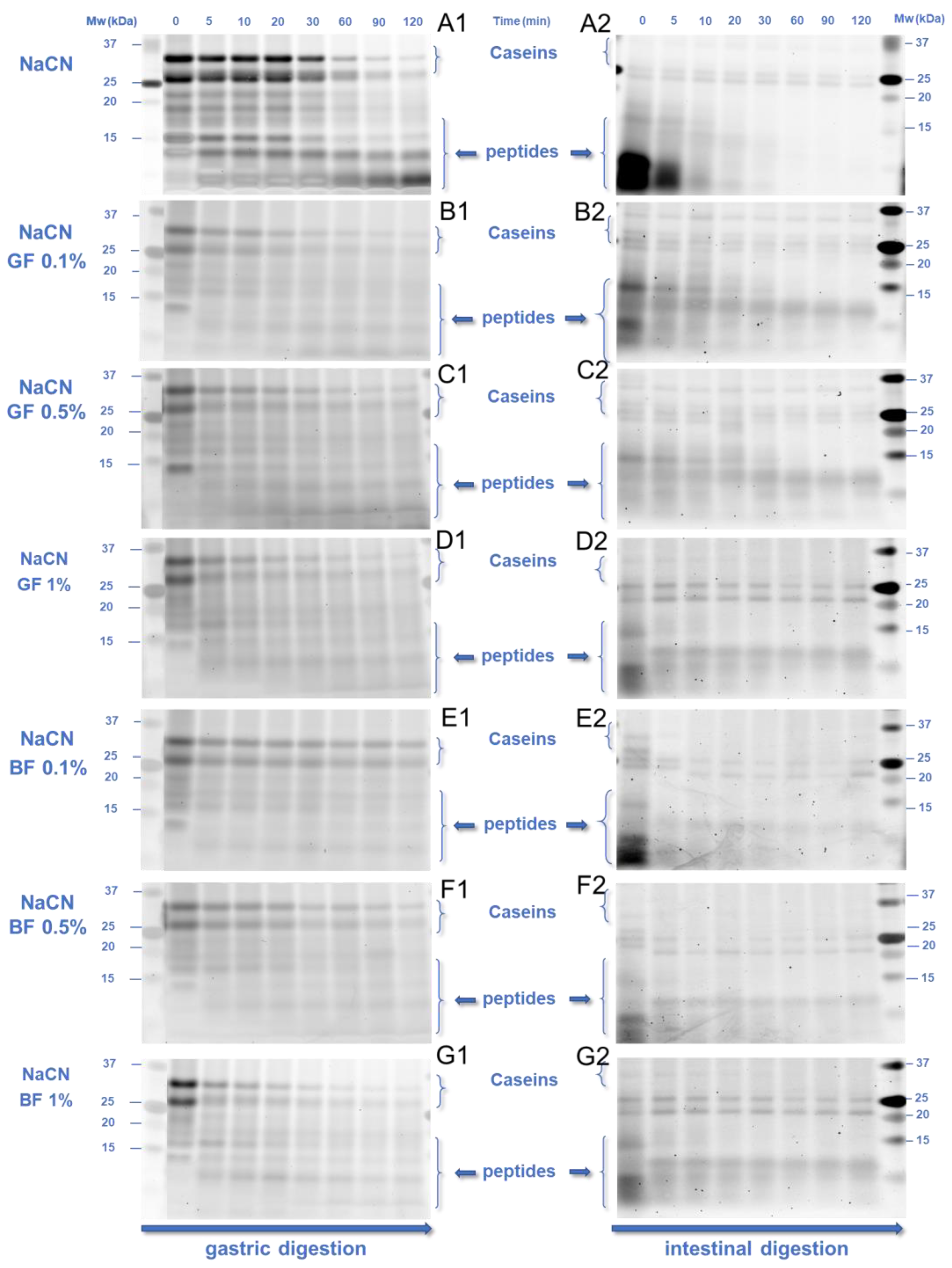
2.8. Protein Hydrolysis Quantification
2.9. Statistical Analyses
3. Results and Discussion
3.1. Rheological Behaviour and Colloidal Changes during the In Vitro Simulated Oral Processing
3.2. Rheological Behaviour and Colloidal Changes under In Vitro Gastro-Intestinal Conditions
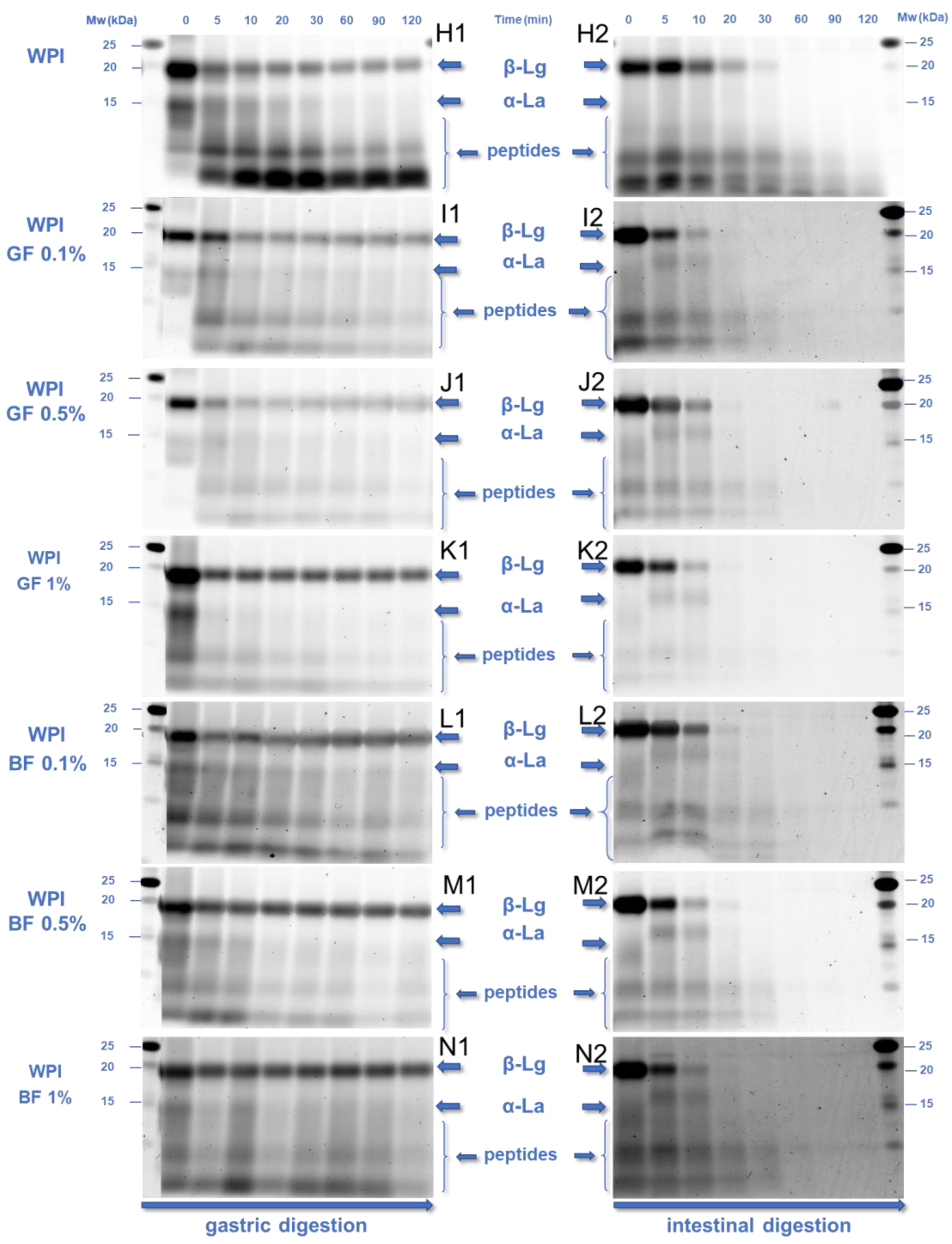
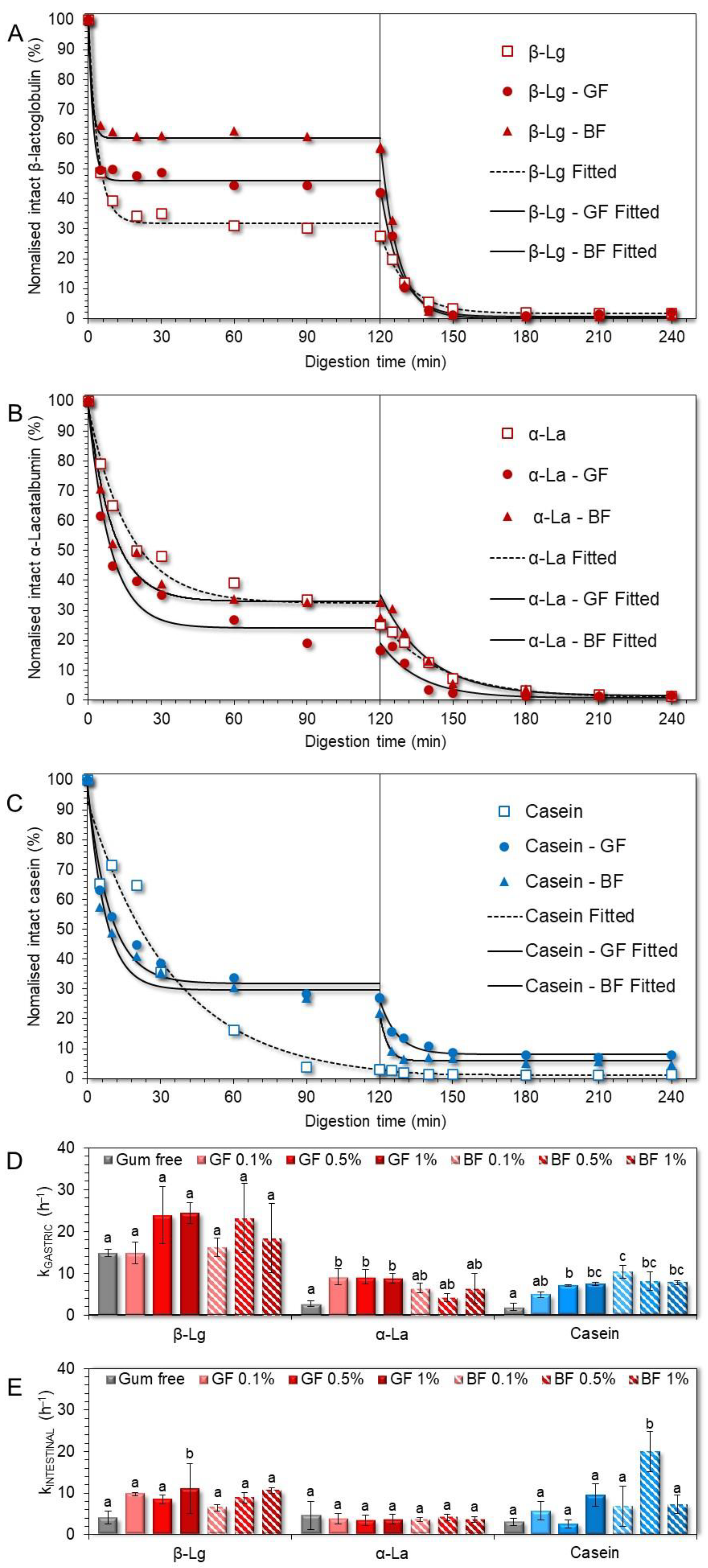
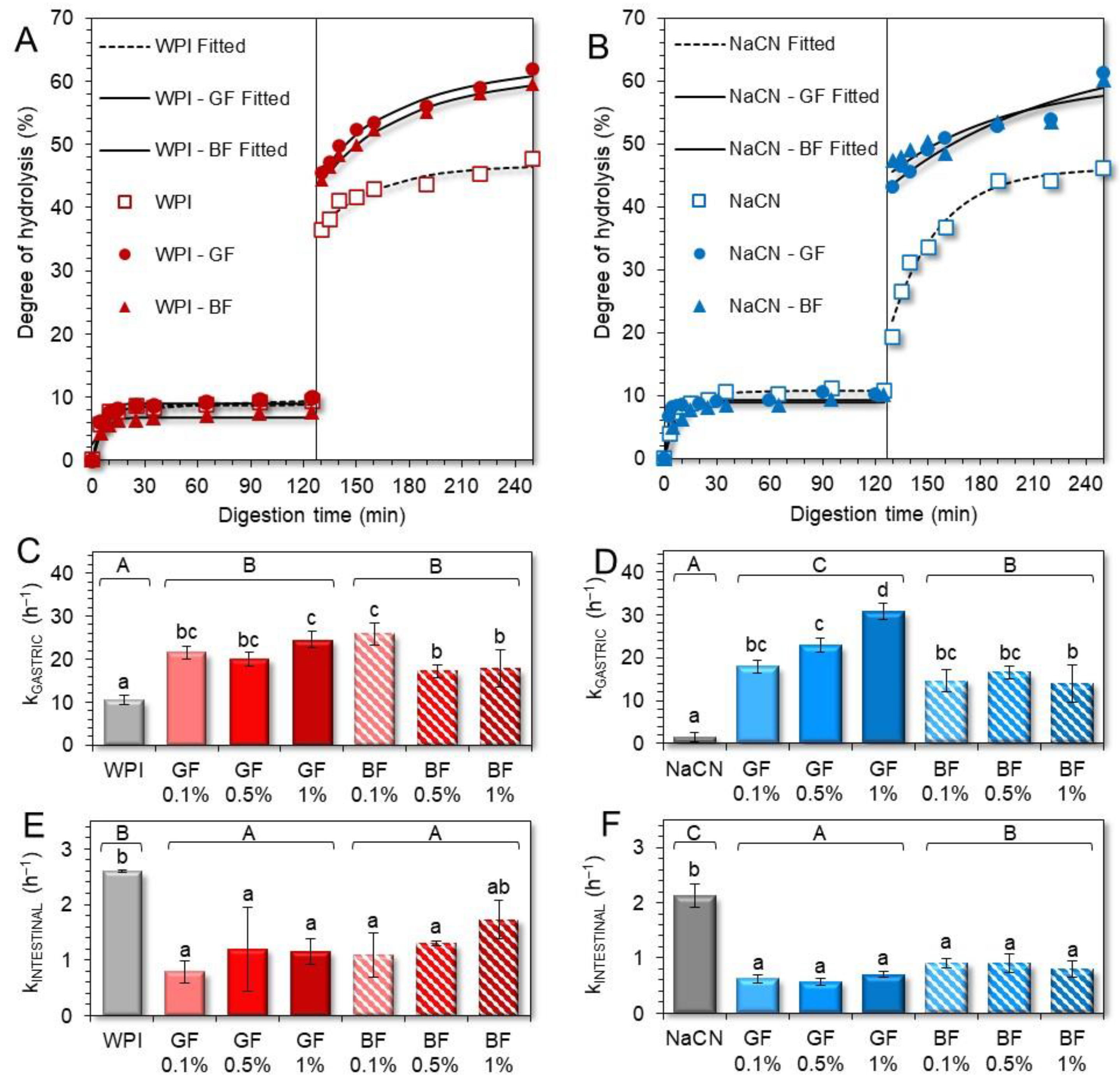
3.3. In Vitro Digestibility of the Milk Proteins
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baley, C.; Gomina, M.; Breard, J.; Bourmaud, A.; Davies, P. Variability of Mechanical Properties of Flax Fibres for Composite Reinforcement. A Review. Ind. Crops Prod. 2020, 145, 111984. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Tse, T.J.; Wang, Y.; Reaney, M.J.T. Flaxseed Gum a Versatile Natural Hydrocolloid for Food and Non-Food Applications. Trends Food Sci. Technol. 2018, 75, 146–157. [Google Scholar] [CrossRef]
- Hellebois, T.; Fortuin, J.; Xu, X.; Shaplov, A.S.; Gaiani, C.; Soukoulis, C. Structure Conformation, Physicochemical and Rheological Properties of Flaxseed Gums Extracted under Alkaline and Acidic Conditions. Int. J. Biol. Macromol. 2021, 192, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant Seed Mucilage as Emerging Biopolymer in Food Industry Applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Walstra, P.; Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-429-11614-8. [Google Scholar]
- Dupont, D.; Tomé, D. Chapter 20—Milk Proteins: Digestion and Absorption in the Gastrointestinal Tract. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 701–714. ISBN 978-0-12-815251-5. [Google Scholar]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Chapter 2—Milk Proteins: An Overview. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–98. ISBN 978-0-12-815251-5. [Google Scholar]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Ménard, O.; Le Gouar, Y.; Buffière, C.; Famelart, M.-H.; Laroche, B.; Le Feunteun, S.; Dupont, D.; Rémond, D. The Heat Treatment and the Gelation Are Strong Determinants of the Kinetics of Milk Proteins Digestion and of the Peripheral Availability of Amino Acids. Food Chem. 2013, 136, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Böttger, F.; Dupont, D.; Marcinkowska, D.; Bajka, B.; Mackie, A.; Macierzanka, A. Which Casein in Sodium Caseinate Is Most Resistant to in Vitro Digestion? Effect of Emulsification and Enzymatic Structuring. Food Hydrocoll. 2019, 88, 114–118. [Google Scholar] [CrossRef]
- van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How Processing May Affect Milk Protein Digestion and Overall Physiological Outcomes: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. 1—Introduction to Food Hydrocolloids. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 1–22. ISBN 978-1-84569-414-2. [Google Scholar]
- Turgeon, S.L.; Beaulieu, M.; Schmitt, C.; Sanchez, C. Protein–Polysaccharide Interactions: Phase-Ordering Kinetics, Thermodynamic and Structural Aspects. Curr. Opin. Colloid Interface Sci. 2003, 8, 401–414. [Google Scholar] [CrossRef]
- Ye, A. Gastric Colloidal Behaviour of Milk Protein as a Tool for Manipulating Nutrient Digestion in Dairy Products and Protein Emulsions. Food Hydrocoll. 2021, 115, 106599. [Google Scholar] [CrossRef]
- Borreani, J.; Llorca, E.; Larrea, V.; Hernando, I. Adding Neutral or Anionic Hydrocolloids to Dairy Proteins under in Vitro Gastric Digestion Conditions. Food Hydrocoll. 2016, 57, 169–177. [Google Scholar] [CrossRef]
- David, S.; Wojciechowska, A.; Portmann, R.; Shpigelman, A.; Lesmes, U. The Impact of Food-Grade Carrageenans and Consumer Age on the in Vitro Proteolysis of Whey Proteins. Food Res. Int. 2020, 130, 108964. [Google Scholar] [CrossRef] [PubMed]
- Koutina, G.; Ray, C.A.; Lametsch, R.; Ipsen, R. The Effect of Protein-to-Alginate Ratio on in Vitro Gastric Digestion of Nanoparticulated Whey Protein. Int. Dairy J. 2018, 77, 10–18. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. Influence of Nanocellulose on in Vitro Digestion of Whey Protein Isolate. Carbohydr. Polym. 2019, 210, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lin, Z.; Wu, Y.; Gao, Z.; Hu, B.; Xu, L.; Fang, Y.; Nishinari, K. Modulating the in Vitro Gastric Digestion of Heat-Induced Beta-Lactoglobulin Aggregates: Incorporation with Polysaccharide. Food Chem. 2021, 354, 129506. [Google Scholar] [CrossRef]
- Markussen, J.Ø.; Madsen, F.; Young, J.F.; Corredig, M. A Semi Dynamic in Vitro Digestion Study of Milk Protein Concentrate Dispersions Structured with Different Polysaccharides. Curr. Res. Food Sci. 2021, 4, 250–261. [Google Scholar] [CrossRef]
- Hellebois, T.; Gaiani, C.; Soukoulis, C. Impact of Alfalfa (Medicago Sativa L.) Galactomannan on the Microstructural and Physicochemical Changes of Milk Proteins under Static in-Vitro Digestion Conditions. Food Chem. X 2022, 14, 100330. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Hellebois, T.; Gaiani, C.; Paris, C.; Planchon, S.; Renaut, J.; Soukoulis, C. Data on the In-Vitro Digestibility of Acid Gels Prepared from Brewers’ Spent Grain Protein Isolates. Data Brief 2021, 37, 107160. [Google Scholar] [CrossRef]
- Bai, L.; Liu, F.; Xu, X.; Huan, S.; Gu, J.; McClements, D.J. Impact of Polysaccharide Molecular Characteristics on Viscosity Enhancement and Depletion Flocculation. J. Food Eng. 2017, 207, 35–45. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P.; Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-Protein Interactions and Their Relevance in Food Colloids; IntechOpen: London, UK, 2012; ISBN 978-953-51-0819-1. [Google Scholar]
- Musampa, R.M.; Alves, M.M.; Maia, J.M. Phase Separation, Rheology and Microstructure of Pea Protein–Kappa-Carrageenan Mixtures. Food Hydrocoll. 2007, 21, 92–99. [Google Scholar] [CrossRef]
- Hellebois, T.; Gaiani, C.; Cambier, S.; Noo, A.; Soukoulis, C. Exploration of the Co-Structuring and Stabilising Role of Flaxseed Gum in Whey Protein Isolate Based Cryo-Hydrogels. Carbohydr. Polym. 2022, 289, 119424. [Google Scholar] [CrossRef] [PubMed]
- Doublier, J.-L.; Garnier, C.; Renard, D.; Sanchez, C. Protein–Polysaccharide Interactions. Curr. Opin. Colloid Interface Sci. 2000, 5, 202–214. [Google Scholar] [CrossRef]
- Farrer, D.; Lips, A. On the Self-Assembly of Sodium Caseinate. Int. Dairy J. 1999, 9, 281–286. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Shen, J.; Wang, Y.; Reaney, M.J.T. Whey Protein Isolate and Flaxseed (Linum Usitatissimum L.) Gum Electrostatic Coacervates: Turbidity and Rheology. Food Hydrocoll. 2017, 64, 18–27. [Google Scholar] [CrossRef]
- Soukoulis, C.; Cambier, S.; Serchi, T.; Tsevdou, M.; Gaiani, C.; Ferrer, P.; Taoukis, P.S.; Hoffmann, L. Rheological and Structural Characterisation of Whey Protein Acid Gels Co-Structured with Chia (Salvia Hispanica L.) or Flax Seed (Linum Usitatissimum L.) Mucilage. Food Hydrocoll. 2019, 89, 542–553. [Google Scholar] [CrossRef]
- Xavier, A.A.; Mariutti, L.R. Static and Semi-Dynamic in Vitro Digestion Methods: State of the Art and Recent Achievements towards Standardization. Curr. Opin. Food Sci. 2021, 41, 260–273. [Google Scholar] [CrossRef]
- Le Feunteun, S.; Verkempinck, S.; Floury, J.; Janssen, A.; Kondjoyan, A.; Marze, S.; Mirade, P.-S.; Pluschke, A.; Sicard, J.; van Aken, G.; et al. Mathe matical Modelling of Food Hydrolysis during in Vitro Digestion: From Single Nutrient to Complex Foods in Static and Dynamic Conditions. Trends Food Sci. Technol. 2021, 116, 870–883. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Guevara-Zambrano, J.M.; Infantes-Garcia, M.R.; Naranjo, M.C.; Soliva-Fortuny, R.; Elez-Martínez, P.; Grauwet, T. Gastric and Small Intestinal Lipid Digestion Kinetics as Affected by the Gradual Addition of Lipases and Bile Salts. Food Biosci. 2022, 46, 101595. [Google Scholar] [CrossRef]
- Huppertz, T.; Chia, L.W. Milk Protein Coagulation under Gastric Conditions: A Review. Int. Dairy J. 2021, 113, 104882. [Google Scholar] [CrossRef]
- Halabi, A.; Croguennec, T.; Bouhallab, S.; Dupont, D.; Deglaire, A. Modification of Protein Structures by Altering the Whey Protein Profile and Heat Treatment Affects in Vitro Static Digestion of Model Infant Milk Formulas. Food Funct. 2020, 11, 6933–6945. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Böttger, F.; Lansonneur, L.; Groizard, R.; Jean, A.-S.; Rigby, N.M.; Cross, K.; Wellner, N.; Mackie, A.R. The Effect of Gel Structure on the Kinetics of Simulated Gastrointestinal Digestion of Bovine β-Lactoglobulin. Food Chem. 2012, 134, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Brownlee, I.A.; Pearson, J.P. Alginate as a Protease Inhibitor in Vitro and in a Model Gut System; Selective Inhibition of Pepsin but Not Trypsin. Carbohydr. Polym. 2015, 131, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Pälchen, K.; Bredie, W.L.P.; Duijsens, D.; Isaac Alfie Castillo, A.; Hendrickx, M.; Van Loey, A.; Raben, A.; Grauwet, T. Effect of Processing and Microstructural Properties of Chickpea-Flours on in Vitro Digestion and Appetite Sensations. Food Res. Int. 2022, 157, 111245. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. In Vitro Gastrointestinal Digestion of Liquid and Semi-Liquid Dairy Matrixes. LWT—Food Sci. Technol. 2014, 57, 99–105. [Google Scholar] [CrossRef]
- Chai, C.; Lee, J.; Huang, Q. The Effect of Ionic Strength on the Rheology of PH-Induced Bovine Serum Albumin/κ-Carrageenan Coacervates. LWT—Food Sci. Technol. 2014, 59, 356–360. [Google Scholar] [CrossRef]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and Precipitation in Polysaccharide–Protein Systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellebois, T.; Fortuin, J.; Gaiani, C.; Soukoulis, C. Impact of Flaxseed Gums on the Colloidal Changes and In Vitro Digestibility of Milk Proteins. Foods 2022, 11, 4096. https://doi.org/10.3390/foods11244096
Hellebois T, Fortuin J, Gaiani C, Soukoulis C. Impact of Flaxseed Gums on the Colloidal Changes and In Vitro Digestibility of Milk Proteins. Foods. 2022; 11(24):4096. https://doi.org/10.3390/foods11244096
Chicago/Turabian StyleHellebois, Thierry, Jennyfer Fortuin, Claire Gaiani, and Christos Soukoulis. 2022. "Impact of Flaxseed Gums on the Colloidal Changes and In Vitro Digestibility of Milk Proteins" Foods 11, no. 24: 4096. https://doi.org/10.3390/foods11244096
APA StyleHellebois, T., Fortuin, J., Gaiani, C., & Soukoulis, C. (2022). Impact of Flaxseed Gums on the Colloidal Changes and In Vitro Digestibility of Milk Proteins. Foods, 11(24), 4096. https://doi.org/10.3390/foods11244096





