Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Sugar Components
2.3. Organic Acid Components
2.4. Amino Acid Components
2.5. Phenolic Acids and Flavonoids Components
2.6. Volatile Content by Electronic Nose Analysis
2.7. Activities of Enzymes Related to Sucrose Metabolism
2.8. Statistical Analysis
3. Results
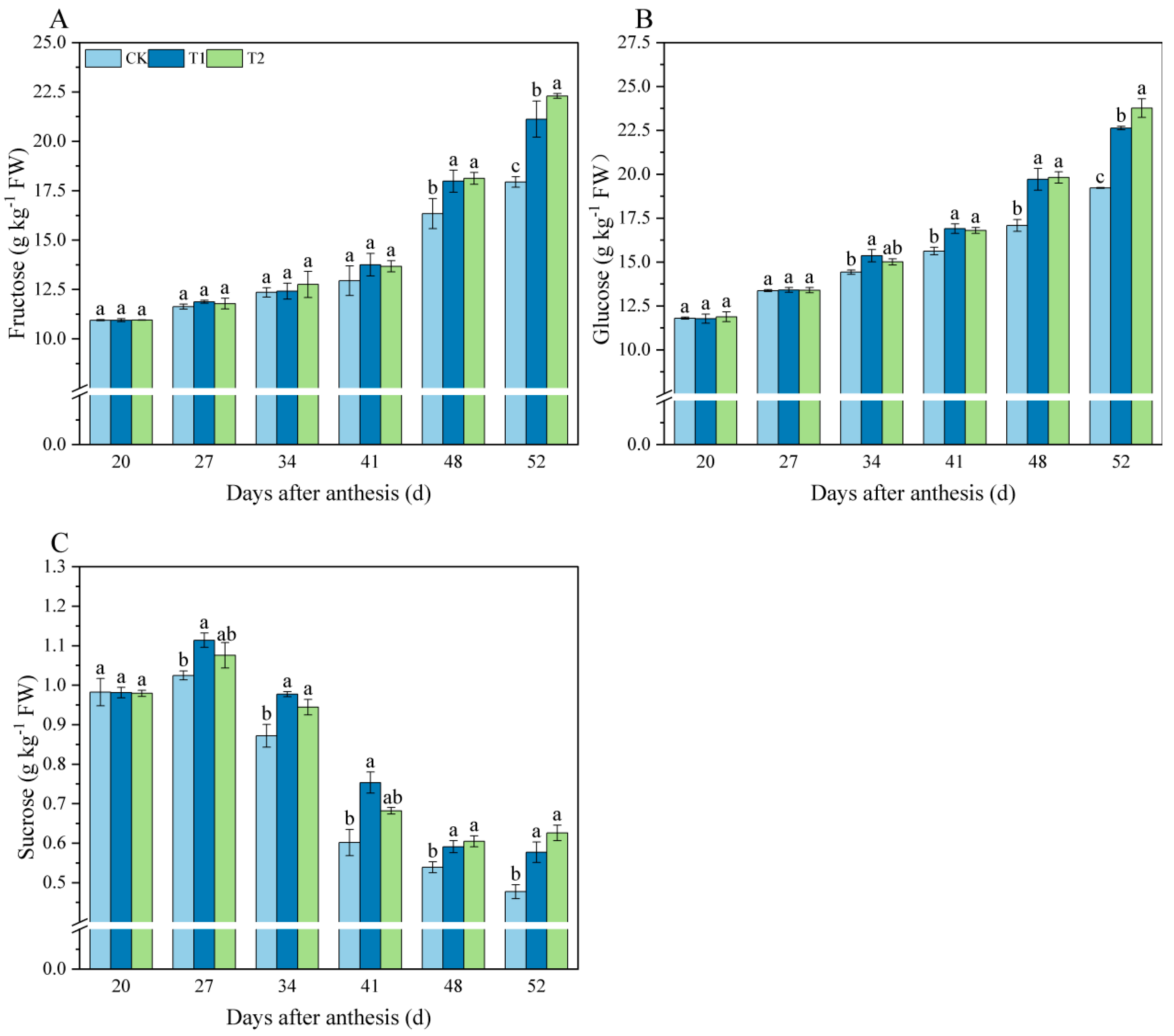
3.1. Effects of Exogenous Melatonin on Sugar Components in Tomato Fruit
3.2. Effects of Exogenous Melatonin on Organic Acid Components in Tomato Fruit
3.3. Effects of Exogenous Melatonin on Amino Acid Components in Tomato Fruit
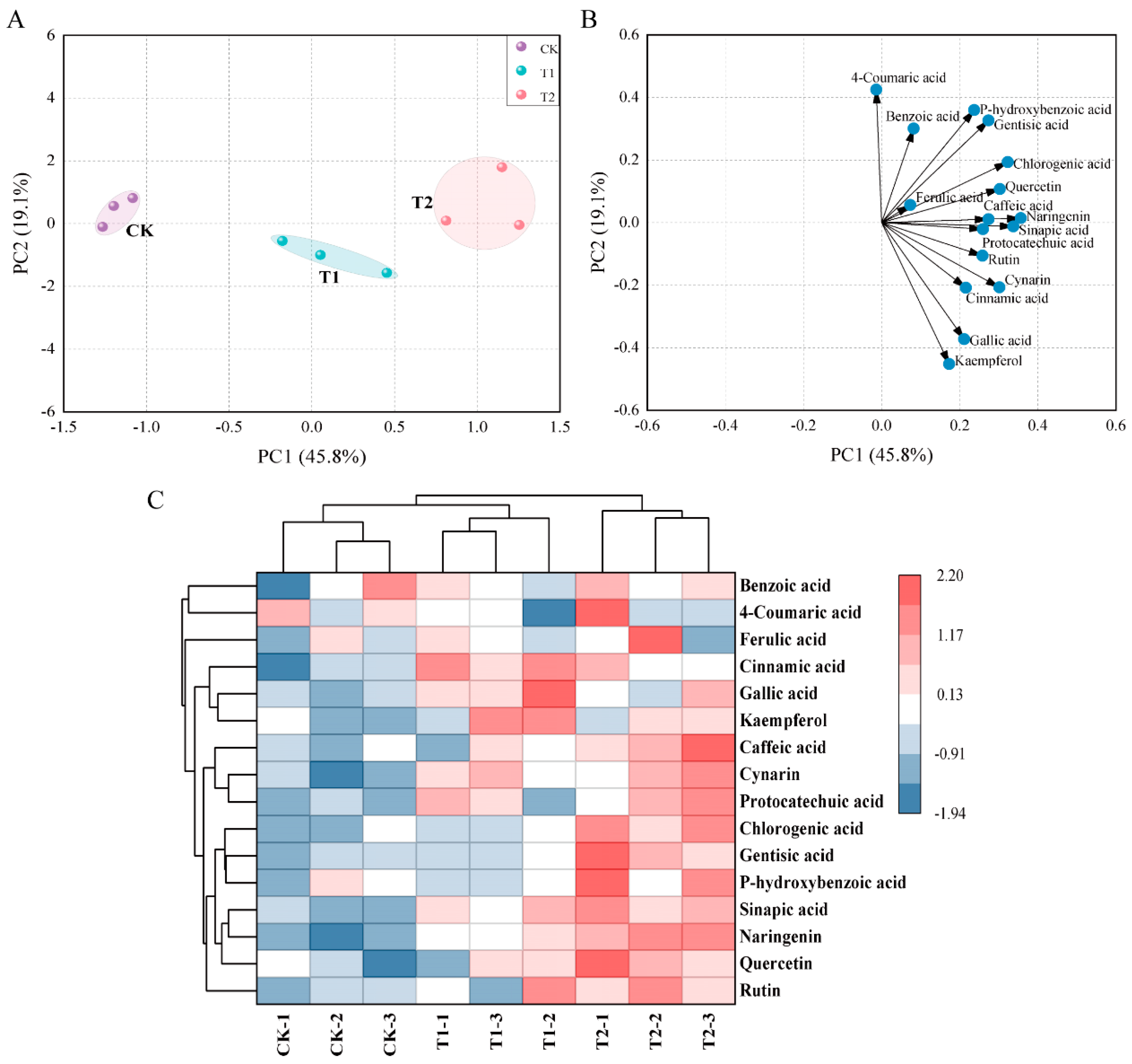
3.4. Effects of Exogenous Melatonin on Phenolic Acids and Flavonoids in Tomato Fruit
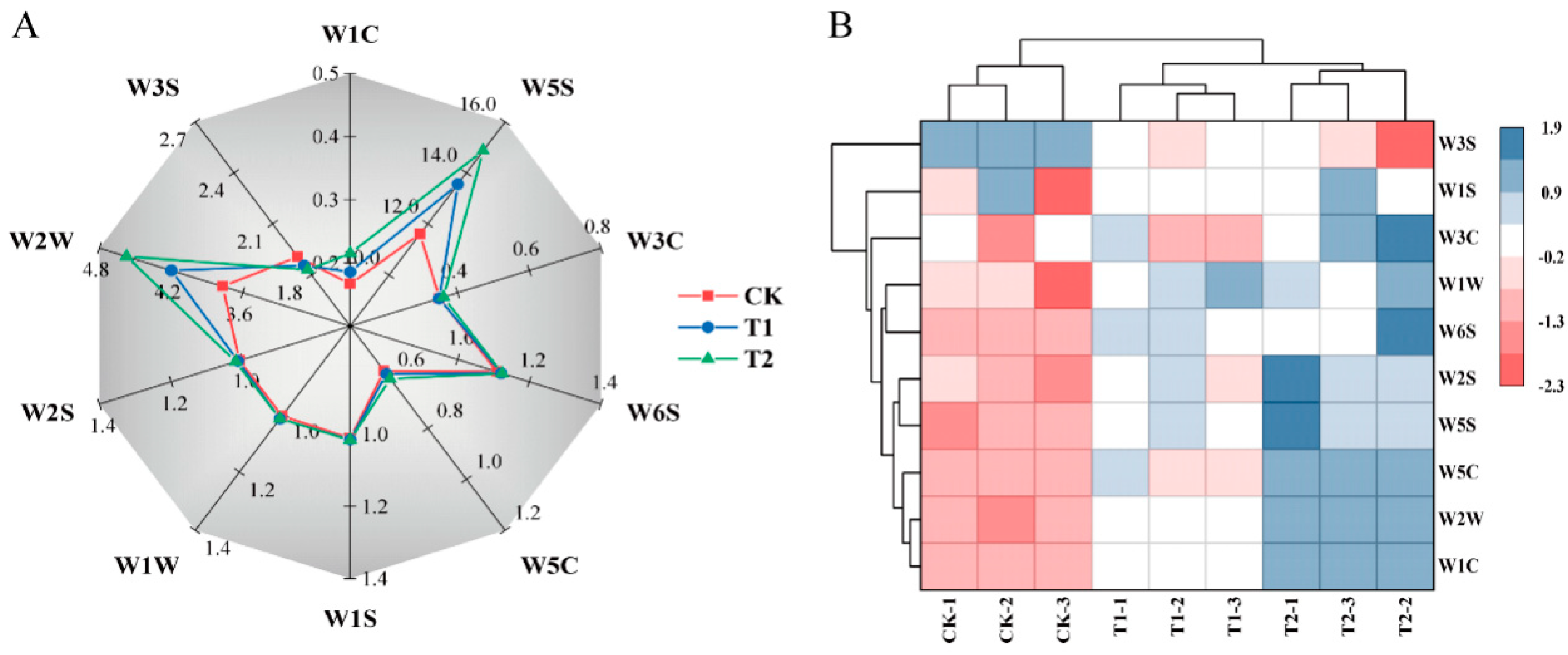
3.5. Effects of Exogenous Melatonin on Volatile Flavor Intensity in Tomato Fruit
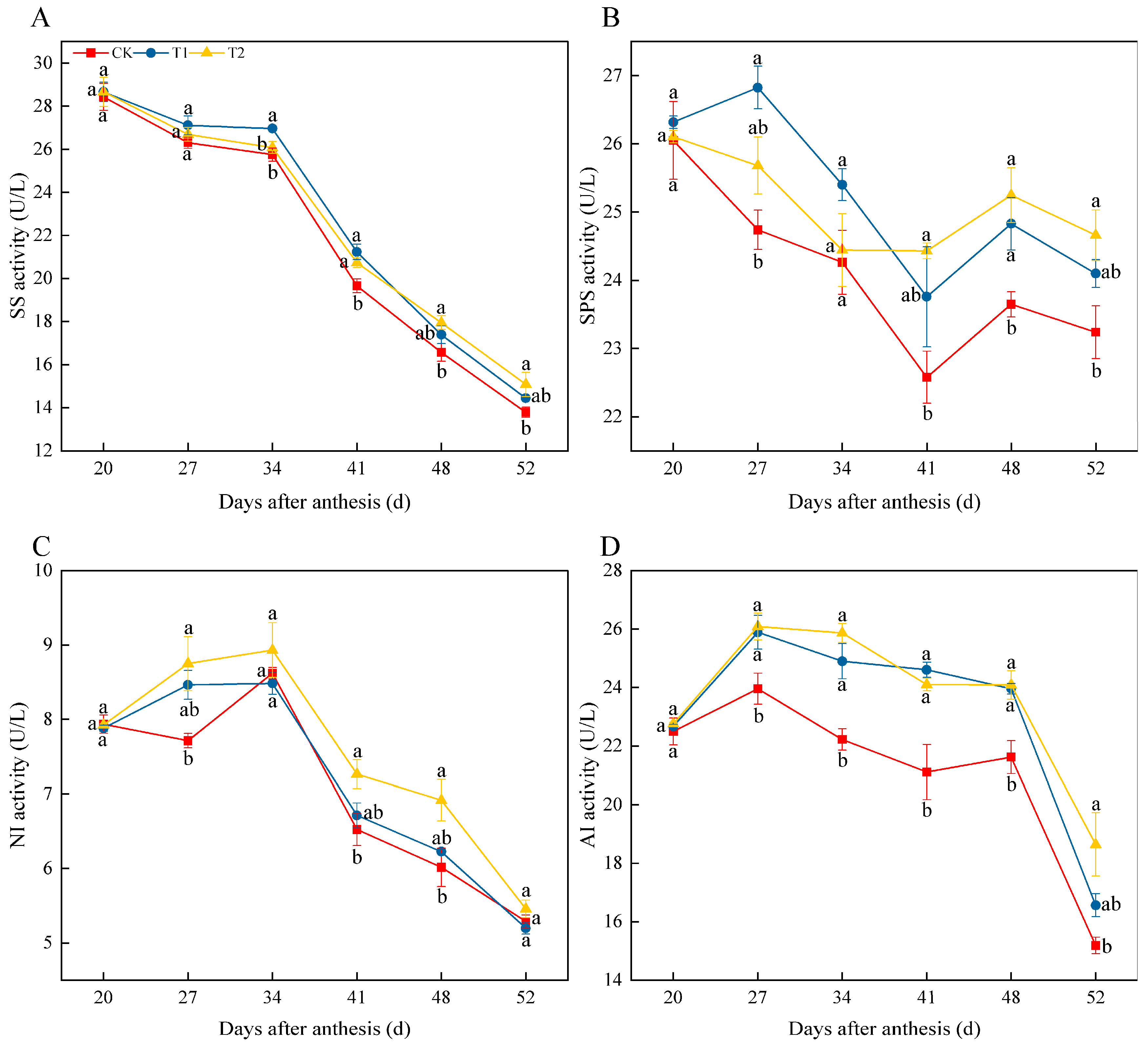
3.6. Effects of Exogenous Melatonin on Sucrose Metabolism-Related Enzyme Activities in Tomato Fruit
4. Discussion
4.1. Melatonin Application Increase Sugar Content in Tomato Fruit
4.2. Melatonin Application Decrease Organic Acid Content in Tomato Fruit
4.3. Melatonin Application Regulate Amino Acid Content in Tomato Fruit
4.4. Melatonin Application Regulate Secondary Metabolites Content in Tomato Fruit
4.5. Melatonin Application Increases the Volatile Substance Content in Tomato Fruit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, G.; Ma, T.; Deng, Z.; Gutierrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reiter, R.J.; et al. Plant-derived melatonin from food: A gift of nature. Food Funct. 2021, 12, 2829. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Sunera, A.; Saqib, S.; Uddin, S.; Zaman, W.; Ullah, F.; Ayaz, A.; Asghar, M.; Rehman, S.; Munis, M.; Chaudhary, H. Characterization and phytostimulatory activity of bacteria isolated from tomato (Lycopersicon esculentum Mill.) rhizosphere. Microb. Pathog. 2020, 140, 103966. [Google Scholar] [CrossRef]
- Naeem, M.; Shahzad, K.; Saqib, S.; Shahzad, A.; Younas, M.; Afridi, M.I. The Solanum melongena COP1LIKE manipulates fruit ripening and flowering time in tomato (Solanum lycopersicum). Plant Growth Regul. 2022, 96, 369–382. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; Abd Elbar, O.H.; Farag, R.; Hikal, M.; El-Kelish, A.; Abou El-Yazied, A.; Alkahtani, J.; Abd El-Gawad, H.G. Melatonin Counteracts Drought Induced Oxidative Damage and Stimulates Growth, Productivity and Fruit Quality Properties of Tomato Plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; Garcia, K.; Mendez, M.A.; Gonzalez, M.; Meisel, L.A.; Defilippi, B.G.; del Pozo, T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdam, M.S.; Luo, Z.; Razavi, F. Impact of Exogenous Melatonin Application on Chilling Injury in Tomato Fruits During Cold Storage. Food Bioprocess Technol. 2019, 12, 741–750. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Wang, K.-X.; Yan, M.-Y.; Kanwar, M.K.; Li, D.-Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin Alleviates High Temperature-Induced Pollen Abortion in Solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef]
- Asif, M.; Pervez, A.; Irshad, U.; Mehmood, Q.; Ahmad, R. Melatonin and plant growth-promoting rhizobacteria alleviate the cadmium and arsenic stresses and increase the growth of Spinacia oleracea L. Plant Soil Environ. 2020, 66, 234–241. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous Melatonin Improves Physiological Characteristics and Promotes Growth of Strawberry Seedlings Under Cadmium Stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Cao, L.; Kou, F.; Zhang, M.; Jin, X.; Ren, C.; Yu, G.; Zhang, Y.; Wang, M. Effect of Exogenous Melatonin on the Quality of Soybean and Natto Products under Drought Stress. J. Chem. 2021, 2021, 8847698. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin Function and Crosstalk with Other Phytohormones under Normal and Stressful Conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of Exogenous Application of Melatonin on Quality and Sugar Metabolism in “Zaosu” Pear Fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- Kanayama, Y. Sugar Metabolism and Fruit Development in the Tomato. Hortic. J. 2017, 86, 417–425. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims-A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Carrillo-Lopez, A.; Yahia, E. HPLC-DAD-ESI-MS Analysis of Phenolic Compounds During Ripening in Exocarp and Mesocarp of Tomato Fruit. J. Food Sci. 2013, 78, C1839–C1844. [Google Scholar] [CrossRef]
- Sorrequieta, A.; Ferraro, G.; Boggio, S.B.; Valle, E.M. Free amino acid production during tomato fruit ripening: A focus on l-glutamate. Amino Acids 2010, 38, 1523–1532. [Google Scholar] [CrossRef]
- Costa, F.; Baeta, M.d.L.; Saraiva, D.; Verissimo, M.T.; Ramos, F. Evolution of Mineral Contents in Tomato Fruits During the Ripening Process After Harvest. Food Anal. Methods 2011, 4, 410–415. [Google Scholar] [CrossRef]
- Paolo, D.; Bianchi, G.; Lo Scalzo, R.; Morelli, C.F.; Rabuffetti, M.; Speranza, G. The Chemistry behind Tomato Quality. Nat. Prod. Commun. 2018, 13, 1225–1232. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A. Current challenges and future potential of tomato breeding using omics approaches. Breed. Sci. 2013, 63, 31–41. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Mapelli-Brahm, P.; Hernanz, D.; Stinco, C.M.; Beltran-Sinchiguano, E.; Melendez-Martinez, A.J. Study of commercial quality parameters, sugars, phenolics, carotenoids and plastids in different tomato varieties. Food Chem. 2019, 277, 480–489. [Google Scholar] [CrossRef]
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Gomez, H.A.G.; Seabra Junior, S.; Maraschin, M.; Tecchio, M.A.; Borges, C.V. Functional and Nutraceutical Compounds of Tomatoes as Affected by Agronomic Practices, Postharvest Management, and Processing Methods: A Mini Review. Front. Nutr. 2022, 9, 868492. [Google Scholar] [CrossRef]
- Carrari, F.; Fernie, A.R. Metabolic regulation underlying tomato fruit development. J. Exp. Bot. 2006, 57, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Alseekh, S.; Fernie, A.R. On the regulation and function of secondary metabolism during fruit development and ripening. J. Exp. Bot. 2013, 65, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wu, Q.; Huang, S.; Zhu, B.; Chen, F.; Liu, B.; Cai, L.; Mao, L.; Luo, Z.; Li, L. Exogenous abscisic acid regulates primary metabolism in postharvest cherry tomato fruit during ripening. Sci. Hortic. 2022, 299, 111008. [Google Scholar] [CrossRef]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; et al. Response of Tomato Fruit Quality Depends on Period of LED Supplementary Light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Lv, J.; Li, J.; Gao, Y.; Patience, B.E.; Niu, T.; Yu, J.; Xie, J. Effect of Methyl Jasmonate Treatment on Primary and Secondary Metabolites and Antioxidant Capacity of the Substrate and Hydroponically Grown Chinese Chives. Front. Nutr. 2022, 9, 859035. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Meng, X.; Ma, X.; He, X.; Zhang, G.; Luo, S.; Lyu, J.; Yu, J. A Comprehensive Evaluation of Effects on Water-Level Deficits on Tomato Polyphenol Composition, Nutritional Quality and Antioxidant Capacity. Antioxidants 2022, 11, 1585. [Google Scholar] [CrossRef]
- Cai, J.-S.; Zhu, Y.-Y.; Ma, R.-H.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Effects of roasting level on physicochemical, sensory, and volatile profiles of soybeans using electronic nose and HS-SPME-GC-MS. Food Chem. 2021, 340, 127880. [Google Scholar] [CrossRef]
- Wei, L.; Wei, S.; Hu, D.; Feng, L.; Liu, Y.; Liu, H.; Liao, W. Comprehensive Flavor Analysis of Volatile Components During the Vase Period of Cut Lily (Lilium spp. “Manissa”) Flowers by HS-SPME/GC-MS Combined With E-Nose Technology. Front. Plant Sci. 2022, 13, 822956. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Hertog, M.L.A.T.M.; Van de Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.H.; Nicolai, B.M. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Lyu, J.; Jin, L.; Meng, X.; Jin, N.; Wang, S.; Hu, L.; Zhang, G.; Wu, Y.; Luo, S.; Yu, J. Exogenous Si Mitigates the Effects of Cinnamic-Acid-Induced Stress by Regulating Carbon Metabolism and Photosynthetic Pigments in Cucumber Seedlings. Agronomy 2022, 12, 1569. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Liang, D.; Hu, R.; Wang, Z.; et al. Melatonin application improves berry coloration, sucrose synthesis, and nutrient absorption in “Summer Black” grape. Food Chem. 2021, 356, 129713. [Google Scholar] [CrossRef]
- Colak, N.G.; Eken, N.T.; Ulger, M.; Frary, A.; Doganlar, S. Exploring wild alleles from Solanum pimpinellifolium with the potential to improve tomato flavor compounds. Plant Sci. 2020, 298, 110567. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kasemsap, P.; Vercambre, G.; Gay, F.; Phattaralerphong, J.; Gautier, H. Impact of red and blue nets on physiological and morphological traits, fruit yield and quality of tomato (Solanum lycopersicum Mill.). Sci. Hortic. 2020, 264, 109185. [Google Scholar] [CrossRef]
- Pal, H.; Kundu, A.; Sahu, R.; Sethi, A.; Hazra, P.; Chatterjee, S. Unraveling the metabolic behavior in tomato high pigment mutants (hp-1, hp-2dg, og(c)) and non ripening mutant (rin) during fruit ripening. Sci. Hortic. 2019, 246, 652–663. [Google Scholar] [CrossRef]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Just, D.; Benard, N.; Destrac-Irvine, A.; Baldet, P.; Hernould, M.; Causse, M.; Raymond, P.; Rothan, C. A fruit-specific phospho enolpyruvate carboxylase is related to rapid growth of tomato fruit. Planta 2002, 214, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Salvioli, A.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Biol. 2012, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Pratta, G.; Zorzoli, R.; Boggio, S.B.; Picardi, L.A.; Valle, E.M. Glutamine and glutamate levels and related metabolizing enzymes in tomato fruits with different shelf-life. Sci. Hortic. 2004, 100, 341–347. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Li, J.; Dawuda, M.M.; Ali, B.; Wu, Y.; Yu, J.; Tang, Z.; Lyu, J.; Xiao, X.; et al. Exogenous Application of 5-Aminolevulinic Acid Promotes Coloration and Improves the Quality of Tomato Fruit by Regulating Carotenoid Metabolism. Front. Plant Sci. 2021, 12, 683868. [Google Scholar] [CrossRef]
- Bellisle, F. Glutamate and the UMAMI taste: Sensory, metabolic, nutritional and behavioural considerations. A review of the literature published in the last 10 years. Neurosci. Biobehav. Rev. 1999, 23, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Phillips, R.S.; Li, J. Editorial: Aromatic Amino Acid Metabolism. Front. Mol. Biosci. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, L.; Liao, Y.; Zhou, Y.; Xu, X.; Dong, F.; Yang, Z. An alternative pathway for the formation of aromatic aroma compounds derived from L-phenylalanine via phenylpyruvic acid in tea (Camellia sinensis (L.) O. Kuntze) leaves. Food Chem. 2019, 270, 17–24. [Google Scholar] [CrossRef]
- Rivero Meza, S.L.; Tobaruela, E.d.C.; Pascoal, G.B.; Massaretto, I.L.; Purgatto, E. Post-Harvest Treatment with Methyl Jasmonate Impacts Lipid Metabolism in Tomato Pericarp (Solanum lycopersicum L. cv. Grape) at Different Ripening Stages. Foods 2021, 10, 877. [Google Scholar] [CrossRef]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the Health-Promoting Effects of Tomato Fruit for Biofortified Food. Mediat. Inflamm. 2014, 2014, 139873. [Google Scholar] [CrossRef]
- Ballester, A.-R.; Tikunov, Y.; Molthoff, J.; Grandillo, S.; Viquez-Zamora, M.; de Vos, R.; de Maagd, R.A.; van Heusden, S.; Bovy, A.G. Identification of Loci Affecting Accumulation of Secondary Metabolites in Tomato Fruit of a Solanum lycopersicum x Solanum chmielewskii Introgression Line Population. Front. Plant Sci. 2016, 7, 1428. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Metabolomics-Inspired Insight into Developmental, Environmental and Genetic Aspects of Tomato Fruit Chemical Composition and Quality. Plant Cell Physiol. 2015, 56, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, J.; He, X.; Yang, Z.; Fang, W.; Tao, J. Role of exogenous melatonin involved in phenolic acid metabolism of germinated hulless barley under NaCl stress. Plant Physiol. Biochem. 2022, 170, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Z.; Ban, Z.; Jiang, N.; Yang, M.; Li, L. Role of exogenous melatonin involved in phenolic metabolism of Zizyphus jujuba fruit. Food Chem. 2021, 341, 128268. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Deng, B.; Xia, C.; Tian, S.; Shi, H. Melatonin reduces pesticide residue, delays senescence, and improves antioxidant nutrient accumulation in postharvest jujube fruit. Postharvest Biol. Technol. 2021, 173, 111419. [Google Scholar] [CrossRef]
- Shang, F.; Liu, R.; Wu, W.; Han, Y.; Fang, X.; Chen, H.; Gao, H. Effects of melatonin on the components, quality and antioxidant activities of blueberry fruits. Lwt Food Sci. Technol. 2021, 147, 111582. [Google Scholar] [CrossRef]
- Li, W.; Lu, X.; Li, J. The effect of organic nutrient solution on flavor in ripe cherry tomato fruit-Transcriptome and metabolomic analyses. Environ. Exp. Bot. 2022, 194, 104721. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent Advance in Aromatic Volatile Research in Tomato Fruit: The Metabolisms and Regulations. Food Bioprocess Technol. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Meng, J.-F.; Xu, T.-F.; Song, C.-Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.-W.; Xi, Z.-M. Melatonin treatment of pre-veraison grape berries to increase size and synchronicity of berries and modify wine aroma components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, X.; Ai, X.; Luo, Z.; Mao, L.; Ying, T.; Li, L. Effect of exogenous auxin on aroma volatiles of cherry tomato (Solanum lycopersicum L.) fruit during postharvest ripening. Postharvest Biol. Technol. 2018, 146, 108–116. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]

| Wavelength | 240 nm | 280 nm | 322 nm |
|---|---|---|---|
| Polyphenolic compounds | Rutin | Gallic acid | Gentisic acid |
| Protocatechuic acid | 4-coumaric acid | Caffeic acid | |
| Quercetin | Cinnamic acid | Cynarin | |
| Chlorogenic acid | Benzoic acid | Sinapic acid | |
| P-hydroxybenzoic acid | Ferulic acid Naringenin | Kaempferol |
| Array Serial Number | Sensor Name | Substance Types | Sensor Performance Description |
|---|---|---|---|
| 1 | W1C | Aromatic | Aromatic components, benzenes |
| 2 | W5S | Broadrange | High sensitivity, sensitive to nitrogen oxides |
| 3 | W3C | Aromatic | Sensitive aromatic components, ammonia |
| 4 | W6S | Hydrogen | Mainly selective to hydride |
| 5 | W5C | Arom-aliph | Aromatic components of short-chain alkanes |
| 6 | W1S | Broad-methane | Sensitive to methyl groups |
| 7 | W1W | Sulfur-organic | Sensitive to sulfides |
| 8 | W2S | Broad-alcohol | Sensitive to aldehydes, alcohols, ketones |
| 9 | W2W | Sulph-chlor | Aromatic components, sensitive organic sulfides |
| 10 | W3S | Methane-aliph | Sensitive to long-chain alkanes |
| Days after Anthesis (d) | Treatments | Essential Amino Acids (mg·g−1 DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lysine | Threonine | Phenylalanine | Tryptophan | Leucine | Isoleucine | Valine | Methionine | ||
| 20 | CK | 1.083 ± 0.031 a | 2.393 ± 0.064 a | 2.391 ± 0.053 a | 0.396 ± 0.002 a | 0.697 ± 0.004 a | 0.715 ± 0.016 a | 1.385 ± 0.007 a | 0.128 ± 0.002 a |
| T1 | 1.071 ± 0.007 a | 2.461 ± 0.003 a | 2.448 ± 0.004 a | 0.391 ± 0.002 a | 0.644 ± 0.001 b | 0.697 ± 0.004 a | 1.386 ± 0.001 a | 0.129 ± 0.001 a | |
| T2 | 1.067 ± 0.002 a | 2.463 ± 0.000 a | 2.450 ± 0.000 a | 0.388 ± 0.005 a | 0.692 ± 0.004 a | 0.693 ± 0.007 a | 1.386 ± 0.000 a | 0.129 ± 0.000 a | |
| 27 | CK | 1.029 ± 0.002 a | 2.315 ± 0.012 c | 2.318 ± 0.017 b | 0.432 ± 0.019 a | 0.796 ± 0.006 a | 0.814 ± 0.008 a | 1.312 ± 0.013 a | 0.133 ± 0.001 a |
| T1 | 1.008 ± 0.048 a | 2.469 ± 0.000 a | 2.451 ± 0.003 a | 0.441 ± 0.004 a | 0.797 ± 0.001 a | 0.809 ± 0.001 a | 1.250 ± 0.006 b | 0.114 ± 0.002c | |
| T2 | 1.090 ± 0.005 a | 2.387 ± 0.023 b | 2.368 ± 0.034 b | 0.442 ± 0.001 a | 0.798 ± 0.000 a | 0.809 ± 0.000 a | 1.303 ± 0.016 a | 0.122 ± 0.001 b | |
| 34 | CK | 1.053 ± 0.017 a | 3.082 ± 0.004 a | 3.099 ± 0.012 a | 0.477 ± 0.001 a | 0.813 ± 0.004 a | 0.825 ± 0.007 a | 1.320 ± 0.004 b | 0.146 ± 0.001 a |
| T1 | 1.092 ± 0.011 a | 3.036 ± 0.009 b | 3.037 ± 0.002 b | 0.485 ± 0.013 a | 0.840 ± 0.005 a | 0.847 ± 0.004 a | 1.361 ± 0.003 a | 0.135 ± 0.001 b | |
| T2 | 1.021 ± 0.034 a | 3.020 ± 0.009 b | 3.016 ± 0.002 b | 0.497 ± 0.013 a | 0.839 ± 0.014 a | 0.818 ± 0.034 a | 1.238 ± 0.015 c | 0.125 ± 0.001 c | |
| 41 | CK | 1.088 ± 0.020 b | 3.319 ± 0.168 b | 3.272 ± 0.175 b | 0.498 ± 0.009 b | 0.724 ± 0.043 b | 0.734 ± 0.033 c | 0.841 ± 0.055 b | 0.095 ± 0.007 c |
| T1 | 1.041 ± 0.012 b | 3.526 ± 0.003 ab | 3.463 ± 0.004 b | 0.509 ± 0.004 a b | 0.836 ± 0.006 a | 0.847 ± 0.006 b | 1.117 ± 0.007 a | 0.133 ± 0.001 a | |
| T2 | 1.146 ± 0.009 a | 3.854 ± 0.063 a | 3.858 ± 0.063 a | 0.537 ± 0.011 a | 0.922 ± 0.018 a | 0.921 ± 0.004 a | 1.058 ± 0.027 a | 0.113 ± 0.002 b | |
| 48 | CK | 1.029 ± 0.011 b | 3.004 ± 0.052 b | 2.995 ± 0.040 b | 0.625 ± 0.014 c | 0.485 ± 0.006 a | 0.481 ± 0.013 a | 0.387 ± 0.005 a | 0.130 ± 0.003 b |
| T1 | 1.067 ± 0.042 ab | 3.042 ± 0.001 b | 3.101 ± 0.009 b | 0.679 ± 0.000 b | 0.469 ± 0.018 a | 0.414 ± 0.000 b | 0.283 ± 0.001 c | 0.146 ± 0.001 a | |
| T2 | 1.121 ± 0.005 a | 3.683 ± 0.037 a | 3.689 ± 0.050 a | 0.752 ± 0.020 a | 0.482 ± 0.012 a | 0.493 ± 0.010 a | 0.333 ± 0.006 b | 0.148 ± 0.003 a | |
| 52 | CK | 1.068 ± 0.009 b | 2.997 ± 0.008 b | 2.990 ± 0.033 b | 0.738 ± 0.006 b | 0.251 ± 0.003 c | 0.339 ± 0.002 b | 0.237 ± 0.001 b | 0.206 ± 0.001 a |
| T1 | 1.177 ± 0.000 a | 2.931 ± 0.064 b | 2.933 ± 0.079 b | 0.758 ± 0.026 b | 0.333 ± 0.006 b | 0.329 ± 0.013 b | 0.222 ± 0.011 b | 0.220 ± 0.011 a | |
| T2 | 1.197 ± 0.032 a | 3.433 ± 0.031 a | 3.441 ± 0.029 a | 0.897 ± 0.041 a | 0.436 ± 0.005 a | 0.436 ± 0.008 a | 0.318 ± 0.008 a | 0.218 ± 0.007 a | |
| Days after Anthesis (d) | Treatments | Non-Essential Amino Acids (mg·g−1 DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cysteine | Arginine | Glutamate | Tyrosine | Aspartic Acid | Serine | Glycine | Alanine | ||
| 20 | CK | 1.604 ± 0.130 a | 0.363 ± 0.110 a | 3.983 ± 0.037 a | 0.315 ± 0.009 a | 0.910 ± 0.009 a | 1.085 ± 0.009 a | 0.015 ± 0.002 a | 1.687 ± 0.013 a |
| T1 | 1.697 ± 0.104 a | 0.343 ± 0.021 a | 3.995 ± 0.104 a | 0.303 ± 0.007 a | 0.913 ± 0.002 a | 1.081 ± 0.002 a | 0.014 ± 0.000 a | 1.688 ± 0.002 a | |
| T2 | 1.599 ± 0.019 a | 0.335 ± 0.005 a | 3.998 ± 0.019 a | 0.314 ± 0.004 a | 0.914 ± 0.000 a | 1.080 ± 0.000 a | 0.014 ± 0.000 a | 1.689 ± 0.000 a | |
| 27 | CK | 1.852 ± 0.144 a | 0.370 ± 0.016 b | 3.982 ± 0.030 b | 0.344 ± 0.006 a | 1.012 ± 0.006 a | 0.833 ± 0.011 a | 0.011 ± 0.001 b | 1.451 ± 0.010 a |
| T1 | 1.883 ± 0.024 a | 0.460 ± 0.010 a | 4.066 ± 0.004 b | 0.347 ± 0.001 a | 1.083 ± 0.012 a | 0.796 ± 0.003 b | 0.015 ± 0.001 a | 1.244 ± 0.001 b | |
| T2 | 1.885 ± 0.004 a | 0.406 ± 0.036 ab | 4.188 ± 0.035 a | 0.347 ± 0.000 a | 1.064 ± 0.085 a | 0.788 ± 0.003 b | 0.012 ± 0.000 b | 1.216 ± 0.006 c | |
| 34 | CK | 1.796 ± 0.052 b | 0.360 ± 0.121 a | 4.071 ± 0.005 c | 0.453 ± 0.005 b | 2.674 ± 0.018 a | 0.933 ± 0.008 a | 0.094 ± 0.001 a | 1.371 ± 0.003 b |
| T1 | 1.869 ± 0.012 b | 0.301 ± 0.122 a | 4.395 ± 0.016 a | 0.472 ± 0.004 a | 2.599 ± 0.004 b | 0.914 ± 0.004 a | 0.073 ± 0.001 b | 1.558 ± 0.000 a | |
| T2 | 2.161 ± 0.038 a | 0.217 ± 0.024 a | 4.251 ± 0.031 b | 0.403 ± 0.005 c | 2.579 ± 0.019 b | 0.805 ± 0.006 b | 0.077 ± 0.008 b | 1.285 ± 0.016 c | |
| 41 | CK | 1.336 ± 0.110 b | 0.593 ± 0.018 a | 4.325 ± 0.272 b | 0.452 ± 0.001 c | 5.092 ± 0.239 b | 1.408 ± 0.068 b | 0.122 ± 0.008 a | 0.807 ± 0.054 b |
| T1 | 1.975 ± 0.099 a | 0.404 ± 0.121 a | 5.673 ± 0.005 a | 0.477 ± 0.005 b | 5.430 ± 0.017 b | 1.336 ± 0.021 b | 0.118 ± 0.001 a | 0.923 ± 0.000 a | |
| T2 | 1.708 ± 0.158 a b | 0.353 ± 0.110 a | 4.397 ± 0.097 b | 0.522 ± 0.004 a | 6.165 ± 0.133 a | 1.827 ± 0.016 a | 0.135 ± 0.001 a | 0.957± 0.020 a | |
| 48 | CK | 1.639 ± 0.021 a | 0.384 ± 0.018 a | 14.042 ± 0.129 c | 0.373 ± 0.009 b | 6.151 ± 0.077 b | 1.722 ± 0.051 a | 0.097 ± 0.001 a | 0.447 ± 0.006 a |
| T1 | 1.805 ± 0.477 a | 0.324 ± 0.065 a | 19.331 ± 0.013 a | 0.348 ± 0.004 b | 6.746 ± 0.037 a | 1.419 ± 0.024 b | 0.097 ± 0.003 a | 0.384 ± 0.006 b | |
| T2 | 1.834 ± 0.033 a | 0.361 ± 0.065 a | 17.452 ± 0.274 b | 0.464 ± 0.015 a | 6.752 ± 0.232 a | 1.482 ± 0.090 b | 0.110 ± 0.010 a | 0.361 ± 0.007 c | |
| 52 | CK | 1.793 ± 0.095 a | 0.411 ± 0.023 a | 25.736 ± 0.574 b | 0.260 ± 0.007 b | 8.590 ± 0.135 b | 1.303 ± 0.009 b | 0.091 ± 0.000 b | 0.454 ± 0.013 c |
| T1 | 1.739 ± 0.039 a | 0.421 ± 0.048 a | 27.085 ± 0.249 ab | 0.197 ± 0.001 c | 9.121 ± 0.266 ab | 1.426 ± 0.028 a | 0.099 ± 0.005 ab | 0.713 ± 0.019 a | |
| T2 | 1.914 ± 0.007 a | 0.357 ± 0.020 a | 28.161 ± 0.919 a | 0.337 ± 0.005 a | 9.238 ± 0.087 a | 1.333 ± 0.019 b | 0.105 ± 0.003 a | 0.540 ± 0.004 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, J.; Wang, J.; Tang, Z.; Yu, J.; Wu, Y.; Liu, Z.; Wang, J.; Wang, G.; Tian, Q. Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites. Foods 2022, 11, 4097. https://doi.org/10.3390/foods11244097
Dou J, Wang J, Tang Z, Yu J, Wu Y, Liu Z, Wang J, Wang G, Tian Q. Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites. Foods. 2022; 11(24):4097. https://doi.org/10.3390/foods11244097
Chicago/Turabian StyleDou, Jianhua, Jie Wang, Zhongqi Tang, Jihua Yu, Yue Wu, Zeci Liu, Junwen Wang, Guangzheng Wang, and Qiang Tian. 2022. "Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites" Foods 11, no. 24: 4097. https://doi.org/10.3390/foods11244097
APA StyleDou, J., Wang, J., Tang, Z., Yu, J., Wu, Y., Liu, Z., Wang, J., Wang, G., & Tian, Q. (2022). Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites. Foods, 11(24), 4097. https://doi.org/10.3390/foods11244097





