Bioactive, Mineral and Antioxidative Properties of Gluten-Free Chicory Supplemented Snack: Impact of Processing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Inulin Determination
2.2. Sesquiterpene Lactones (SLs) Content
2.3. Mineral Elements
2.4. Total Phenolic Content and Antioxidant Capacity
2.4.1. Free and Bound Polyphenols
2.4.2. Antioxidant Capacity
2.5. Artificial Neural Network
Local Sensitivity Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Inulin Content
3.2. Sesquiterpene Lactones (SLs)
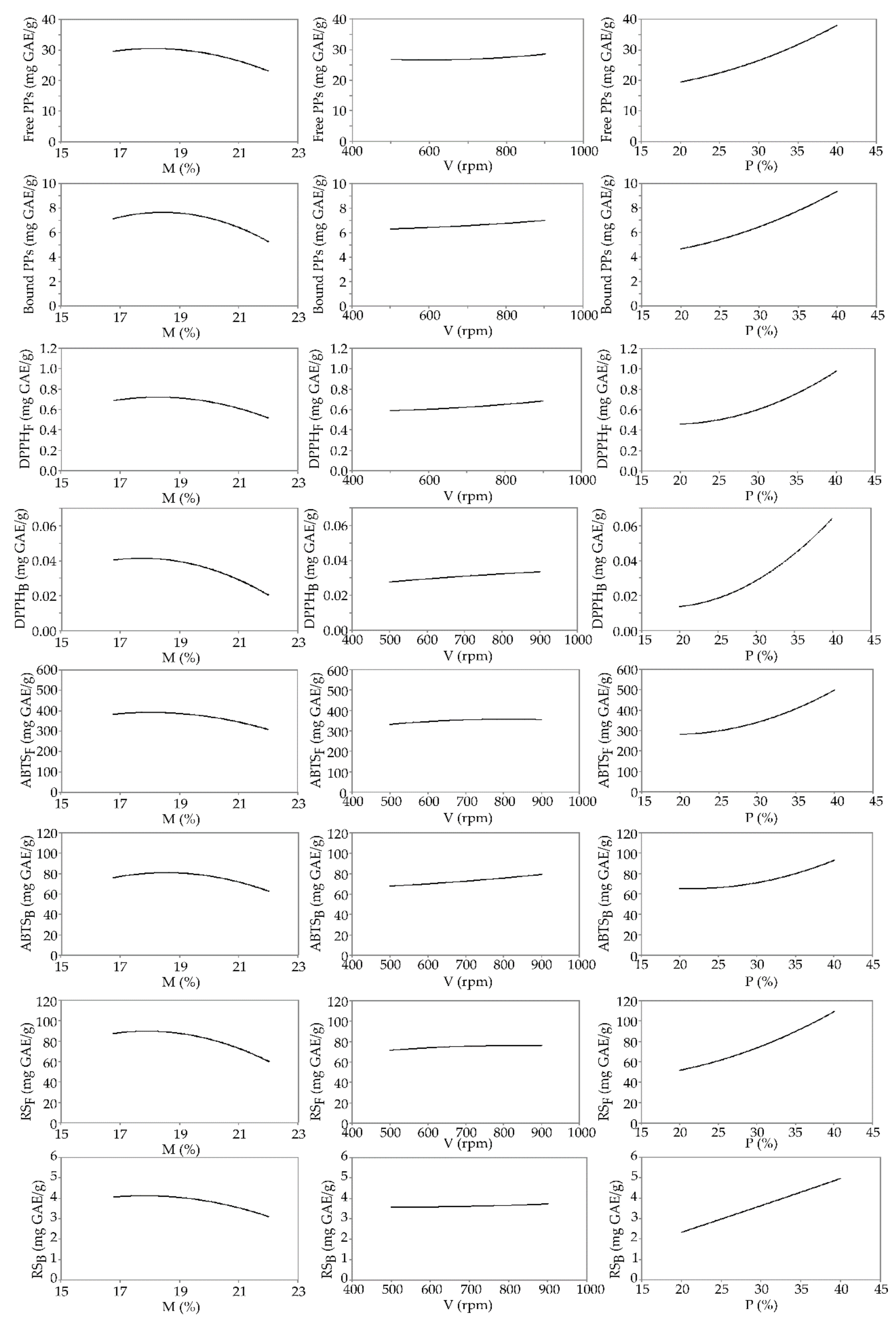
3.3. Total Phenolic Content and Antioxidant Capacity
3.4. Mineral Elements
3.5. Artificial Neural Network
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Výrostková, J.; Regecová, I.; Zigo, F.; Marcinčák, S.; Kožárová, I.; Kováčová, M.; Bertová, D. Detection of Gluten in Gluten-Free Foods of Plant Origin. Foods 2022, 11, 2011. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Taylor, J.; Campanella, O.H.; Hamaker, B.R. Functionality of the storage proteins in gluten-free cereals and pseudocereals in dough systems. J. Cereal Sci. 2016, 67, 22–34. [Google Scholar] [CrossRef]
- Torbica, A.; Hadnadev, M.; Dapčević, T. Rheological, textural and sensory properties of gluten-free bread formulations based on rice and buckwheat flour. Food Hydrocoll. 2010, 24, 626–632. [Google Scholar] [CrossRef]
- Arribas, C.; Cabellos, B.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive compounds, antioxidant activity, and sensory analysis of rice-based extruded snacks-like fortified with bean and carob fruit flours. Foods 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Pouille, C.L.; Jegou, D.; Dugardin, C.; Cudennec, B.; Ravallec, R.; Hance, P.; Rambaud, C.; Hilbert, J.L.; Lucau-Danila, A. Chicory root flour—A functional food with potential multiple health benefits evaluated in a mice model. J. Funct. Foods 2020, 74, 104174. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Kanayama, T.; Kitagawa, K.; Nishimura, H.; Nishihira, J. Effects of the extract from roasted chicory (Cichorium intybus L.) root containing inulin-type fructans on blood glucose, lipid metabolism, and fecal properties. J. Tradit. Complement. Med. 2015, 5, 161–167. [Google Scholar] [CrossRef]
- Ripoll, C.; Schmidt, M.B.; Ilic, N.; Poulev, A.; Dey, M.; Kurmukov, G.A.; Raskin, I. Anty-inflammatory effects of a sesquiterpene lactone extract from chicory (Cichorium intybus L) roots. Nat. Prod. Commun. 2007, 2, 717–722. [Google Scholar]
- Mihaylova, D.; Vrancheva, R.; Petkova, N.; Ognyanov, M.; Desseva, I.; Ivanov, I.; Popova, M.; Popova, A. Carotenoids, tocopherols, organic acids, charbohydrate and mineral content in different medicinal plant extracts. Z. Naturforsch.-C J. Biosci. 2018, 73, 439–448. [Google Scholar] [CrossRef]
- Harrington, K.C.; Thatcher, A.; Kemp, P.D. Mineral composition and nutritive value of some common pasture weeds. N. Z. Plant Prot. 2006, 59, 261–265. [Google Scholar] [CrossRef]
- Patil, S.S.; Kaur, C. Current trends in extrusion: Development of functional foods and novel ingredients. Food Sci. Technol. Res. 2018, 24, 23–34. [Google Scholar] [CrossRef]
- Morales, P.; Berrios, J.D.J.; Varela, A.; Burbano, C.; Cuadrado, C.; Muzquiz, M.; Pedrosa, M.M. Novel fiber-rich lentil flours as snack-type functional foods: An extrusion cooking effect on bioactive compounds. Food Funct. 2015, 6, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Dilrukshi, H.N.N.; Torrico, D.D.; Brennan, M.A.; Brennan, C.S. Effects of extrusion processing on the bioactive constituents, in vitro digestibility, amino acid composition, and antioxidant potential of novel gluten-free extruded snacks fortified with cowpea and whey protein concentrate. Food Chem. 2022, 389, 133107. [Google Scholar] [CrossRef] [PubMed]
- Bokić, J.; Kojić, J.; Krulj, J.; Pezo, L.; Banjac, V.; Škrobot, D.; Tumbas Šaponjac, V.; Vidosavljević, S.; Stojkov, V.; Ilić, N.; et al. Development of a Novel Rice-Based Snack Enriched with Chicory Root: Physicochemical and Sensory Properties. Foods 2022, 11, 2393. [Google Scholar] [CrossRef]
- Perović, J.; Kojić, J.; Krulj, J.; Pezo, L.; Tumbas Šaponjac, V.; Ilić, N.; Bodroža-Solarov, M. Inulin determination by an improved HPLC-ELSD method. Food Anal. Methods 2022, 15, 1001–1010. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Ilic, N.; Poulev, A.; Raskin, I. Toxicological evaluation of a chicory root extract. Food Chem. Toxicol. 2007, 45, 1131–1139. [Google Scholar] [CrossRef]
- SRPS EN ISO 6869/2008; Animal feeding stuffs—Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- Singleton, L.V.; Rossi, A.J. Colorimetry of total phenolics with phospho¬molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Gaonkar, A.G.; Vasisht, N.; Khare, A.R.; Sobel, R. Microencapsulation in the Food Industry: A Practical Implementation Guide; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–282. [Google Scholar]
- Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- Mena, P.; Garcia-Viguera, C.; Navarro-Rico, J.; Moreno, A.D.; Bartual, J.; Saura, D.; Marti, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Kollo, T.; von Rosen, D. Advanced Multivariate Statistics with Matrices; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Trelea, I.C.; Raoult-Wack, A.L.; Trystram, G. Application of neural network modelling for the control of dewatering and impregnation soaking process (osmotic dehydration). Food Sci. Technol. Int. 1997, 3, 459–465. [Google Scholar] [CrossRef]
- Mohieddin, J.; Yinyin, W.; Ali, A.; Jing, T. Unsupervised Learning and Multipartite Network Models: A Promising Approach for Understanding Traditional Medicine. Front. Pharmacol. 2020, 11, 1319. [Google Scholar]
- Basheer, I.A.; Hajmeer, M. Artificial neural networks: Fundamentals, computing, design, and application. J. Microbiol. Methods 2000, 43, 3–31. [Google Scholar] [CrossRef]
- Yoon, Y.; Swales, G.; Margavio, T.M. A Comparison of Discriminant Analysis versus Artificial Neural Networks. J. Oper. Res. Soc. 2017, 44, 51–60. [Google Scholar] [CrossRef]
- Coussement, P.A.A. Inulin and oligofructose: Safe intakes and legal status. J. Nutr. 1999, 129 (Suppl. 7), 1412–1417. [Google Scholar] [CrossRef]
- Radovanovic, A.; Stojceska, V.; Plunkett, A.; Jankovic, S.; Milovanovic, D.; Cupara, S. The use of dry Jerusalem artichoke as a functional nutrient in developing extruded food with low glycaemic index. Food Chem. 2015, 177, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chaito, C.; Judprasong, K.; Puwastien, P. Inulin content of fortified food products in Thailand. Food Chem. 2016, 193, 102–105. [Google Scholar] [CrossRef]
- Khuenpet, K.; Jittanit, W.; Sirisansaneeyakul, S.; Srichamnong, W. Inulin powder production from Jerusalem artichoke (Helianthus tuberosus L.) tuber powder and its application to commercial food products. J. Food Process. Preserv. 2017, 41, e13097. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Capriles, V.D.; Conti-Silva, A.C. Breakfast cereals with inulin obtained through thermoplastic extrusion: Chemical characteristics and physical and technological properties. LWT 2020, 137, 110390. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Extrusion of hulled barley affecting β-glucan and properties of extrudates. Food Bioproc. Technol. 2013, 6, 1374–1389. [Google Scholar] [CrossRef]
- Katsavou, I.D.; Tsokolar-Tsikopoulos, K.C.; Eleni, P.N.; Krokida, M.K. Sensorial, functional, optical and thermal properties of inulin enriched expanded products. Int. Food Res. J. 2019, 26, 671–687. [Google Scholar]
- Tsokolar-Tsikopoulos, K.C.; Katsavou, I.D.; Krokida, M.K. The effect of inulin addition on structural and textural properties of extruded products under several extrusion conditions. J. Food Sci. Technol. 2015, 52, 6170–6181. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Larroche, C. Biotechnological applications of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef]
- Weng, H.; He, L.; Zheng, J.; Li, Q.; Liu, X.; Wang, D. Low oral bioavailability and partial gut microbiotic and phase ii metabolism of brussels/witloof chicory sesquiterpene lactones in healthy humans. Nutrients 2020, 12, 3675. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.; Kelly, A.L.; Gough, T.; Jadhav, V.; Singh, K.K.; Paradkar, A. Application of hot melt extrusion for improving bioavailability of artemisinin a thermolabile drug. Drug Dev. Ind. Pharm. 2017, 44, 206–214. [Google Scholar] [CrossRef]
- Aberham, A.; Cicek, S.S.; Schneider, P.; Stuppner, H. Analysis of sesquiterpene lactones, lignans, and flavonoids in wormwood (Artemisia absinthium L.) using high-performance liquid chromatography (HPLC)-mass spectrometry, reversed phase HPLC, and HPLC-Solid phase extraction-nuclear magnetic resonance. J. Agric. Food Chem. 2010, 58, 10817–10823. [Google Scholar] [CrossRef]
- Willeman, H.; Hance, P.; Fertin, A.; Voedts, N.; Duhal, N.; Goossens, J.; Hilbert, J. A method for the simultaneous determination of chlorogenic acid and sesquiterpene lactone content in industrial chicory root foodstuffs. Sci. World J. 2014, 2014, 583180. [Google Scholar] [CrossRef] [PubMed]
- Jandrić, Z.; Cannavan, A. An investigative study on differentiation of citrus fruit/fruit juices by UPLC-QToF MS and chemometrics. Food Control 2017, 72, 173–180. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Berrios, J.D.J.; Tang, J.; Derito, C. Bioactivity of Antioxidants in Extruded Products Prepared from Purple Potato and Dry Pea Flours. J. Agric. Food Chem. 2011, 59, 8233–8243. [Google Scholar] [CrossRef]
- dos Santos D’Almeida, C.T.; Mameri, H.; dos Santos Menezes, N.; de Carvalho, C.W.P.; Queiroz, V.A.V.; Cameron, L.C.; Morel, M.H.; Takeiti, C.Y.; Ferreira, M.S.L. Effect of extrusion and turmeric addition on phenolic compounds and kafirin properties in tannin and tannin-free sorghum. Food Res. Int. 2021, 149, 110663. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and / or alternative livestock feed supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Lu, X.; Narciso, J.O.; Li, W.; Qin, Y.; Brennan, M.A.; Brennan, C.S. Physical, predictive glycaemic response and antioxidative properties of black ear mashroom (Auricularia auricula) extrudates. Plant Foods Hum. Nutr. 2017, 72, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Igual, M.; Chiş, M.S.; Socaci, S.A.; Vodnar, D.C.; Ranga, F.; Martínez-Monzó, J.; García-Segovia, P. Effect of Medicago sativa Addition on Physicochemical, Nutritional and Functional Characteristics of Corn Extrudates. Foods 2021, 10, 928. [Google Scholar] [CrossRef]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Yaǧci, S.; Göǧüş, F. Response surface methodology for evaluation of physical and functional properties of extruded snack foods developed from food-by-products. J. Food Eng. 2008, 86, 122–132. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Application of extrusion technology in plant food processing byproducts: An overview. Compr. Rev. Food Sci. Food Saf. 2020, 19, 218–246. [Google Scholar] [CrossRef]
- Chalermchaiwat, P.; Jangchud, K.; Jangchud, A.; Charunuch, C.; Prinyawiwatkul, W. Antioxidant activity, free gamma-aminobutyric acid content, selected physical properties and consumer acceptance of germinated brown rice extrudates as affected by extrusion process. LWT-Food Sci. Technol. 2015, 64, 490–496. [Google Scholar] [CrossRef]
- Natabirwa, H.; Nakimbugwe, D.; Lungáho, M.; Muyonga, J.H. Optimization of Roba1 extrusion conditions and bean extrudate properties using response surface methodology and multi-response desirability function. LWT 2018, 96, 411–418. [Google Scholar] [CrossRef]
- Boue, S.M.; Daigle, K.; Beaulieu, J.C.; Heiman, M. Rice flour and bran enriched with blueberry polyphenols increases storage stability and decreases arsenic content in bran. Foods 2019, 8, 276. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zarroug, Y.; Abdelkarim, A.; Dorra, S.T.; Hamdaoui, G.; Felah, M.E.L.; Hassouna, M. Biochemical characterization of Tunisian Cichorium Intybus L. roots and optimization of ultrasonic inulin extraction. Mediter. J. Chem. 2016, 6, 674–685. [Google Scholar] [CrossRef]
- Lenntech, B.V. Recommended Daily Intake of Vitamins and Minerals; Lenntech: Rotterdamseweg, The Netherlands, 2013. [Google Scholar]
- Danbaba, N.; Nkama, I.; Badau, M.H. Application of response surface methodology (RSM) and central composite design (CCD) to optimize minerals composition of rice-cowpea composite blends during extrusion cooking. Int. J. Food Sci. Nut. Eng. 2015, 5, 40–52. [Google Scholar] [CrossRef]
- Camire, M.E.; Camire, A.; Krumhar, K. Chemical and nutritional changes in foods during extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Bergman, C.J.; Gualberto, D.G.; Weber, C.W. Mineral binding capacity of dephytinized insoluble fiber from extruded wheat, oat and rice brans. Plant Foods Hum. Nutr. 1997, 51, 295–310. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Irungu, F.G.; Mutungi, C.M.; Faraj, A.K.; Affognon, H.; Tanga, C.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Minerals content of extruded fish feeds containing cricket (Acheta domesticus) and black soldier fly larvae (Hermetia illucens) fractions. Int. Aquat. Res. 2018, 10, 101–113. [Google Scholar] [CrossRef]
- Vakula, A.; Pavlić, B.; Pezo, L.; Tepić Horecki, A.; Daničić, T.; Raičević, L.; Ljubojević, M.; Šumić, Z. Vacuum drying of sweet cherry: Artificial neural networks approach in process optimization. J. Food Process. Preserv. 2020, 44, e14863. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Manikantan, M.R.; Sunoj, S.; Sreejith, S.; Beegum, S. Modeling of coconut milk residue incorporated rice-corn extrudates properties using multiple linear regression and artificial neural network. J. Food Process Eng. 2019, 42, e12981. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, A.; Rao, P.S. Optimization, modeling, and characterization study for the physicochemical properties of raw banana and defatted soy composite extrudates. Food Chem. 2021, 339, 127865. [Google Scholar] [CrossRef]
- Kothakota, A.; Pandiselvam, R.; Siliveru, K.; Pandey, J.P.; Sagarika, N.; Srinivas, C.H.S.; Kumar, A.; Singh, A.; Prakash, S.D. Modeling and optimization of process parameters for nutritional enhancement in enzymatic milled rice by multiple linear regression (MLR) and artificial neural network (ANN). Foods 2021, 10, 2975. [Google Scholar] [CrossRef]



| CCD Design | ||||||||
|---|---|---|---|---|---|---|---|---|
| SLs | ||||||||
| S.No. | M, % | V, rpm | P, % | Inulin, % | Lactucin, µg/g | Lactucopicrin, µg/g | Dihydrolactucin, µg/g | Dihydrolactucopicrin, µg/g |
| 1 | 21.2 | 820 | 35.9 | 5.52 ± 0.26 c | 7.35 ± 0.89 e | 4.15 ± 0.49 gh | 4.15 ± 0.49 gh | 9.79 ± 0.97 h |
| 2 | 19.4 | 700 | 30.0 | 7.26 ± 0.16 e | 4.29 ± 0.56 cd | 2.74 ± 0.34 ef | 2.64 ± 0.34 de | 5.09 ± 0.57 de |
| 3 | 17.6 | 820 | 35.9 | 7.15 ± 0.06 e | 9.13 ± 0.93 f | 4.61 ± 0.41 h | 4.61 ± 0.41 h | 9.47 ± 0.86 h |
| 4 | 19.4 | 700 | 30.0 | 7.55 ± 0.41 e | 4.35 ± 0.47 cd | 2.72 ± 0.29 ef | 2.62 ± 0.29 de | 4.97 ± 0.43 de |
| 5 | 21.2 | 820 | 24.1 | 4.28 ± 0.42 b | 1.92 ± 0.21 b | 1.13 ± 0.13 ab | 1.13 ± 0.13 ab | 2.45 ± 0.25 b |
| 6 | 22.5 | 700 | 30.0 | 9.17 ± 0.18 g | 4.97 ± 0.52 d | 2.89 ± 0.12 ef | 2.89 ± 0.12 ef | 5.68 ± 0.46 ef |
| 7 | 19.4 | 900 | 30.0 | 8.39 ± 0.14 f | 4.77 ± 0.61 d | 2.53 ± 0.23 de | 2.53 ± 0.23 de | 4.76 ± 0.51 cde |
| 8 | 17.6 | 820 | 24.1 | 5.64 ± 1.05 c | 3.97 ± 0.38 cd | 1.64 ± 0.16 bc | 1.64 ± 0.16 bc | 3.68 ± 0.33 bcd |
| 9 | 19.4 | 700 | 30.0 | 7.77 ± 0.55 e | 4.53 ± 0.50 cd | 2.50 ± 0.36 de | 2.50 ± 0.36 de | 5.05 ± 0.48 de |
| 10 | 21.2 | 580 | 24.1 | 4.18 ± 0.49 b | 3.12 ± 0.43 bc | 1.94 ± 0.20 cd | 1.94 ± 0.20 cd | 4.16 ± 0.53 cde |
| 11 | 16.3 | 700 | 30.0 | 5.36 ± 0.10 c | 5.05 ± 0.57 d | 2.22 ± 0.41 cde | 2.22 ± 0.41 cde | 4.54 ± 0.40 cde |
| 12 | 19.4 | 700 | 40.0 | 9.49 ± 0.69 g | 7.28 ± 0.75 e | 4.18 ± 0.52 h | 4.18 ± 0.52 h | 8.61 ± 0.76 gh |
| 13 | 19.4 | 500 | 30.0 | 7.61 ± 0.64 e | 0.51 ± 0.12 a | 0.56 ± 0.10 a | 0.56 ± 0.10 a | 0.49 ± 0.09 a |
| 14 | 19.4 | 700 | 30.0 | 7.66 ± 0.66 e | 4.42 ± 0.34 cd | 2.62 ± 0.44 de | 2.62 ± 0.44 de | 5.14 ± 0.55 de |
| 15 | 17.6 | 580 | 24.1 | 6.58 ± 0.14 d | 2.16 ± 0.23 b | 1.52 ± 0.17 bc | 1.52 ± 0.17 bc | 3.28 ± 0.39 bc |
| 16 | 21.2 | 580 | 35.9 | 4.23 ± 0.03 b | 8.72 ± 0.88 f | 5.52 ± 0.64 i | 5.52 ± 0.64 i | 12.39 ± 0.99 i |
| 17 | 17.6 | 580 | 35.9 | 10.10 ± 0.27 h | 4.64 ± 0.63 d | 3.38 ± 0.39 fg | 3.38 ± 0.39 fg | 5.38 ± 0.67 e |
| 18 | 19.4 | 700 | 20.0 | 3.29 ± 0.02 a | 6.40 ± 0.72 e | 2.86 ± 0.38 ef | 2.86 ± 0.38 ef | 7.21 ± 0.78 fg |
| 19 | 19.4 | 700 | 30.0 | 7.42 ± 0.64 e | 4.46 ± 0.54 cd | 2.63 ± 0.40 de | 2.63 ± 0.40 de | 5.22 ± 0.72 de |
| 20 | 19.4 | 700 | 30.0 | 7.59 ± 1.31 e | 4.45 ± 0.47 cd | 2.60 ± 0.48 de | 2.60 ± 0.48 de | 4.97 ± 0.68 de |
| CS | 18.0 | 800 | 00.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| CV | 2.87 | 1.93 | 3.31 | 1.98 | 1.93 | |||
| CCD Design | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S.No. | M, % | V, rpm | P, % | Free PPs, mg GAE/g d.w. | Bound PPs, mg GAE/g d.w. | DDPHF, mmol TE/g d.w. | DDPHB, mmol TE/g d.w. | ABTSF, mmol TE/g d.w. | ABTSB, mmol TE/g d.w. | RCF, mmol TE/g d.w. | RCB, mmol TE/g d.w. |
| 1 | 21.2 | 820 | 35.9 | 33.26 ± 0.64 h | 8.13 ± 0.06 fg | 0.79 ± 0.00 efg | 0.05 ± 0.00 f | 400.17 ± 28.52 efg | 80.41 ± 3.26 bc | 91.99 ± 7.06 efg | 4.54 ± 0.00 h |
| 2 | 19.4 | 700 | 30.0 | 27.12 ± 0.13 efg | 6.96 ± 0.11 def | 0.61 ± 0.07 cde | 0.03 ± 0.00 d | 356.28 ± 12.64 cdef | 71.28 ± 3.69 bc | 79.54 ± 4.16 cdefg | 3.97 ± 0.00 f |
| 3 | 17.6 | 820 | 35.9 | 36.87 ± 0.98 i | 9.06 ± 0.32 g | 1.03 ± 0.00 h | 0.06 ± 0.00 g | 473.69 ± 12.97 gh | 96.47 ± 15.02 c | 108.97 ± 2.71 g | 5.14 ± 0.01 j |
| 4 | 19.4 | 700 | 30.0 | 27.01 ± 0.02 efg | 6.57 ± 0.24 def | 0.62 ± 0.07 cde | 0.03 ± 0.00 d | 347.23 ± 11.54 cdef | 70.79 ± 1.29 bc | 79.1 ± 3.15 cdefg | 3.76 ± 0.00 f |
| 5 | 21.2 | 820 | 24.1 | 23.11 ± 0.54 cd | 5.51 ± 0.12 bcd | 0.54 ± 0.09 bcd | 0.02 ± 0.00 c | 313.69 ± 2.69 bcde | 65.97 ± 13.06 bc | 60.12 ± 7.56 bcdef | 3.11 ± 0.00 d |
| 6 | 22.5 | 700 | 30.0 | 20.47 ± 0.06 c | 4.25 ± 0.57 bc | 0.48 ± 0.01 bc | 0.01 ± 0.00 b | 289.36 ± 24.56 bcd | 61.17 ± 4.15 bc | 51.89 ± 2.61 bc | 2.83 ± 0.00 c |
| 7 | 19.4 | 900 | 30.0 | 24.55 ± 0.69 de | 6.05 ± 0.09 de | 0.55 ± 0.01 bcd | 0.02 ± 0.00 c | 316.16 ± 5.25 bcde | 67.69 ± 5.50 bc | 62.06 ± 2.97 bcde | 3.09 ± 0.00 d |
| 8 | 17.6 | 820 | 24.1 | 26.98 ± 0.39 efg | 6.90 ± 0.25 def | 0.60 ± 0.00 de | 0.03 ± 0.00 d | 340.17 ± 23.69 bcde | 89.33 ± 16.58 bc | 77.14 ± 9.88 bcdefg | 3.47 ± 0.01 e |
| 9 | 19.4 | 700 | 30.0 | 26.68 ± 0.21 efg | 6.85 ± 0.04 def | 0.63 ± 0.00 cde | 0.03 ± 0.00 d | 353.69 ± 16.87 cdef | 69.79 ± 2.58 bc | 77.47 ± 1.65 cdefg | 3.86 ± 0.02 f |
| 10 | 21.2 | 580 | 24.1 | 20.81 ± 0.03 c | 4.41 ± 0.39 bc | 0.48 ± 0.07 bc | 0.01 ± 0.00 b | 279.36 ± 21.49 bc | 62.09 ± 7.28 bc | 53.71 ± 8.63 bcd | 2.91 ± 0.00 c |
| 11 | 16.3 | 700 | 30.0 | 28.54 ± 0.03 g | 7.01 ± 0.03 def | 0.66 ± 0.02 cde | 0.04 ± 0.00 e | 374.63 ± 13.64 def | 75.87 ± 2.14 bc | 84.65 ± 9.23 defg | 3.94 ± 0.00 f |
| 12 | 19.4 | 700 | 40.0 | 36.14 ± 0.87 i | 8.94 ± 0.01 g | 0.91 ± 0.01 gh | 0.06 ± 0.00 g | 498.21 ± 14.36 h | 91.63 ± 2.41 bc | 103.89 ± 1.47 g | 4.69 ± 0.02 i |
| 13 | 19.4 | 500 | 30.0 | 25.77 ± 0.36 def | 6.13 ± 0.68 de | 0.56 ± 0.00 bcd | 0.03 ± 0.00 d | 324.61 ± 2.57 bcde | 67.48 ± 2.11 bc | 71.62 ± 9.46 bcdef | 3.51 ± 0.00 e |
| 14 | 19.4 | 700 | 30.0 | 27.36 ± 1.06 efg | 7.16 ± 0.01 def | 0.61 ± 0.00 cde | 0.03 ± 0.00 d | 351.98 ± 2.65 cdef | 72.24 ± 5.79 bc | 78.73 ± 2.65 cdefg | 3.92 ± 0.00 f |
| 15 | 17.6 | 580 | 24.1 | 23.55 ± 0.28 d | 5.81 ± 0.08 cde | 0.53 ± 0.03 bcd | 0.02 ± 0.00 c | 301.21 ± 14.65 bcd | 65.26 ± 1.36 bc | 62.35 ± 7.45 bcde | 3.15 ± 0.00 d |
| 16 | 21.2 | 580 | 35.9 | 32.05 ± 0.43 h | 8.01 ± 0.09 fg | 0.71 ± 0.03 def | 0.05 ± 0.00 f | 395.27 ± 14.57 efg | 78.25 ± 11.69 bc | 91.43 ± 6.93 efg | 4.15 ± 0.03 g |
| 17 | 17.6 | 580 | 35.9 | 34.56 ± 0.91 hi | 8.32 ± 0.11 fg | 0.84 ± 0.01 fgh | 0.04 ± 0.00 e | 436.11 ± 23.14 fgh | 81.24 ± 6.54 bc | 96.36 ± 5.87 fg | 4.69 ± 0.00 i |
| 18 | 19.4 | 700 | 20.0 | 16.83 ± 0.07 b | 4.12 ± 0.08 b | 0.39 ± 0.00 b | 0.01 ± 0.00 b | 254.96 ± 6.43 b | 57.37 ± 0.12 b | 45.62 ± 2.32 b | 1.87 ± 0.03 b |
| 19 | 19.4 | 700 | 30.0 | 26.96 ± 0.13 efg | 6.87 ± 0.21 def | 0.61 ± 0.06 cde | 0.03 ± 0.00 d | 352.21 ± 17.61 cdef | 70.06 ± 5.69 bc | 78.14 ± 0.39 cdefg | 3.69 ± 0.00 f |
| 20 | 19.4 | 700 | 30.0 | 27.08 ± 0.09 efg | 6.99 ± 0.56 def | 0.62 ± 0.00 cde | 0.03 ± 0.00 d | 353.28 ± 29.64 cdef | 70.31 ± 6.91 bc | 80.11 ± 5.28 cdefg | 3.76 ± 0.04 f |
| CS | 18.0 | 800 | 00.0 | 8.39 ± 0.11 a | 2.45 ± 0.35 a | 0.11 ± 0.01 a | 0.00 ± 0.00 a | 85.73 ± 0.97 a | 35.36 ± 1.05 a | 14.85 ± 0.41 a | 0.76 ± 0.01 a |
| CV | 0.82 | 2.84 | 1.68 | 0.00 | 0.85 | 1.28 | 1.21 | 2.81 | |||
| CCD Design | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S.No. | M, % | V, rpm | P, % | Ca | K | Mg | Na | Fe | Mn | Zn | Cu |
| 1 | 21.2 | 820 | 35.9 | 639.29 ± 1.32 j | 2242.72 ± 4.32 f | 501.48 ± 0.98 de | 2076.30 ± 3.03 j | 54.13 ± 0.26 i | 7.36 ± 0.06 de | 15.75 ± 0.22 i | 3.75 ± 0.09 f |
| 2 | 19.4 | 700 | 30.0 | 534.07 ± 1.04 g | 2292.48 ± 3.13 h | 512.78 ± 0.83 fg | 1562.49 ± 2.76 e | 33.63 ± 0.13 e | 6.91 ± 0.11 bcde | 14.77 ± 0.37 ghi | 2.83 ± 0.03 bcde |
| 3 | 17.6 | 820 | 35.9 | 427.90 ± 1.12 d | 2159.61 ± 2.59 d | 514.25 ± 1.01 fg | 1358.71 ± 2.02 d | 28.41 ± 0.11 c | 6.40 ± 0.09 bc | 15.59 ± 0.45 i | 2.27 ± 0.11 bc |
| 4 | 19.4 | 700 | 30.0 | 535.51 ± 2.01 g | 2290.68 ± 3.24 h | 539.55 ± 0.76 fg | 1561.92 ± 2.14 e | 35.25 ± 0.27 e | 6.93 ± 0.09 bcde | 14.76 ± 0.51 ghi | 2.95 ± 0.09 bcde |
| 5 | 21.2 | 820 | 24.1 | 483.27 ± 1.00 f | 2214.95 ± 2.11 e | 534.14 ± 1.12 ij | 1398.47 ± 1.98 d | 31.04 ± 0.31 d | 6.90 ± 0.07 bcde | 11.21 ± 0.20 bc | 2.26 ± 0.21 bc |
| 6 | 22.5 | 700 | 30.0 | 556.71 ± 0.98 h | 2544.48 ± 2.27 j | 545.94 ± 1.22 k | 1700.58 ± 2.04 fg | 34.47 ± 0.44 e | 6.53 ± 0.10 bcd | 15.60 ± 0.11 i | 2.78 ± 0.17 bcde |
| 7 | 19.4 | 900 | 30.0 | 533.90 ± 1.03 g | 2565.08 ± 3.01 k | 533.04 ± 0.99 jk | 1702.68 ± 2.15 fg | 35.18 ± 0.34 e | 6.84 ± 0.12 bcde | 15.48 ± 0.27 hi | 3.15 ± 0.23 def |
| 8 | 17.6 | 820 | 24.1 | 417.43 ± 1.15 c | 2070.44 ± 1.09 c | 487.20 ± 0.73 c | 1242.16 ± 1.72 c | 26.05 ± 0.25 bc | 6.07 ± 0.07 b | 14.50 ± 0.35 gh | 2.29 ± 0.31 b |
| 9 | 19.4 | 700 | 30.0 | 531.03 ± 1.02 g | 2293.79 ± 1.56 h | 508.63 ± 1.01 fg | 1560.18 ± 1.84 e | 36.34 ± 0.61 e | 6.91 ± 0.09 bcde | 15.01 ± 0.19 ghi | 2.88 ± 0.14 bcde |
| 10 | 21.2 | 580 | 24.1 | 482.28 ± 0.89 f | 2240.59 ± 1.48 f | 510.00 ± 0.94 ef | 1729.49 ± 2.01 g | 26.25 ± 0.23 bc | 6.43 ± 0.10 bc | 10.72 ± 0.29 b | 3.07 ± 0.19 def |
| 11 | 16.3 | 700 | 30.0 | 563.83 ± 1.17 i | 2526.92 ± 3.22 i | 534.87 ± 1.04 ij | 1693.07 ± 1.87 fg | 36.02 ± 0.56 e | 6.44 ± 0.11 bc | 11.32 ± 0.35 bc | 2.63 ± 0.09 bcd |
| 12 | 19.4 | 700 | 40.0 | 713.51 ± 2.09 k | 3018.60 ± 4.02 n | 526.52 ± 0.73 hi | 2074.80 ± 2.90 j | 51.11 ± 0.72 h | 7.32 ± 0.07 e | 13.30 ± 0.27 ef | 3.75 ± 0.07 f |
| 13 | 19.4 | 500 | 30.0 | 555.55 ± 1.23 h | 2525.85 ± 3.12 i | 564.65 ± 1.12 l | 1668.48 ± 1.78 f | 36.62 ± 0.45 e | 6.59 ± 0.12 bcde | 12.48 ± 0.41 de | 2.96 ± 0.08 cdef |
| 14 | 19.4 | 700 | 30.0 | 532.15 ± 1.11 g | 2285.53 ± 2.12 h | 552.90 ± 1.01 fg | 1560.16 ± 1.09 e | 36.77 ± 0.37 e | 6.90 ± 0.15 bcde | 14.91 ± 0.61 ghi | 2.86 ± 0.11 bcde |
| 15 | 17.6 | 580 | 24.1 | 472.51 ± 0.99 e | 2260.06 ± 1.87 g | 522.93 ± 0.95 gh | 1381.76 ± 1.23 d | 24.21 ± 0.22 b | 6.87 ± 0.07 bcde | 12.16 ± 0.37 cd | 2.43 ± 0.12 bcd |
| 16 | 21.2 | 580 | 35.9 | 644.01 ± 2.01 j | 2777.22 ± 2.35 l | 493.60 ± 0.84 cd | 1899.85 ± 2.34 h | 46.03 ± 0.48 g | 7.09 ± 0.09 cde | 14.12 ± 0.59 fg | 3.76 ± 0.21 f |
| 17 | 17.6 | 580 | 35.9 | 643.34 ± 1.92 j | 2824.91 ± 2.47 m | 507.18 ± 1.01 ef | 2027.11 ± 2.76 i | 43.99 ± 0.58 g | 7.29 ± 0.11 de | 14.20 ± 0.63 fg | 3.49 ± 0.31 ef |
| 18 | 19.4 | 700 | 20.0 | 385.94 ± 1.35 b | 1835.95 ± 1.03 b | 427.24 ± 0.78 b | 1145.93 ± 1.03 b | 41.78 ± 0.33 f | 6.81 ± 0.08 bcde | 12.60 ± 0.43 de | 3.08 ± 0.19 def |
| 19 | 19.4 | 700 | 30.0 | 532.69 ± 1.22 g | 2295.36 ± 2.25 h | 506.46 ± 1.17 fg | 1560.14 ± 1.54 e | 35.45 ± 0.28 e | 6.92 ± 0.07 bcde | 14.68 ± 0.52 ghi | 2.82 ± 0.11 bcde |
| 20 | 19.4 | 700 | 30.0 | 533.35 ± 1.24 g | 2293.32 ± 2.11 h | 528.88 ± 0.72 fg | 1562.28 ± 1.86 e | 35.14 ± 0.31 e | 6.93 ± 0.08 bcde | 14.47 ± 0.47 ghi | 2.98 ± 0.10 bcde |
| CS | 18.0 | 800 | 00.0 | 43.82 ± 0.26 a | 934.98 ± 0.89 a | 380.18 ± 0.45 a | 56.10 ± 0.37 a | 3.42 ± 0.41 a | 4.79 ± 0.07 a | 9.91 ± 0.06 a | 1.08 ± 0.05 a |
| CV | 0.29 | 0.15 | 3.58 | 0.07 | 3.09 | 0.18 | 1.27 | 2.25 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokić, J.; Kojić, J.; Krulj, J.; Pezo, L.; Banjac, V.; Tumbas Šaponjac, V.; Travičić, V.; Moreno, D.A.; Bodroža-Solarov, M. Bioactive, Mineral and Antioxidative Properties of Gluten-Free Chicory Supplemented Snack: Impact of Processing Conditions. Foods 2022, 11, 3692. https://doi.org/10.3390/foods11223692
Bokić J, Kojić J, Krulj J, Pezo L, Banjac V, Tumbas Šaponjac V, Travičić V, Moreno DA, Bodroža-Solarov M. Bioactive, Mineral and Antioxidative Properties of Gluten-Free Chicory Supplemented Snack: Impact of Processing Conditions. Foods. 2022; 11(22):3692. https://doi.org/10.3390/foods11223692
Chicago/Turabian StyleBokić, Jelena, Jovana Kojić, Jelena Krulj, Lato Pezo, Vojislav Banjac, Vesna Tumbas Šaponjac, Vanja Travičić, Diego A. Moreno, and Marija Bodroža-Solarov. 2022. "Bioactive, Mineral and Antioxidative Properties of Gluten-Free Chicory Supplemented Snack: Impact of Processing Conditions" Foods 11, no. 22: 3692. https://doi.org/10.3390/foods11223692
APA StyleBokić, J., Kojić, J., Krulj, J., Pezo, L., Banjac, V., Tumbas Šaponjac, V., Travičić, V., Moreno, D. A., & Bodroža-Solarov, M. (2022). Bioactive, Mineral and Antioxidative Properties of Gluten-Free Chicory Supplemented Snack: Impact of Processing Conditions. Foods, 11(22), 3692. https://doi.org/10.3390/foods11223692










