Soybean-Derived Tripeptide Leu–Ser–Trp (LSW) Protects Human Vascular Endothelial Cells from TNFα-Induced Oxidative Stress and Inflammation via Modulating TNFα Receptors and SIRT1
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culturing Protocol of Human Vascular Endothelial EA.hy926 Cells
2.3. Cytotoxicity Assay
2.4. Western Blotting
2.5. Measurement of TNFα in EA.hy926 Cells
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Superoxide Detection and Lipid Peroxidation Assay
2.8. Statistical Analysis
3. Results
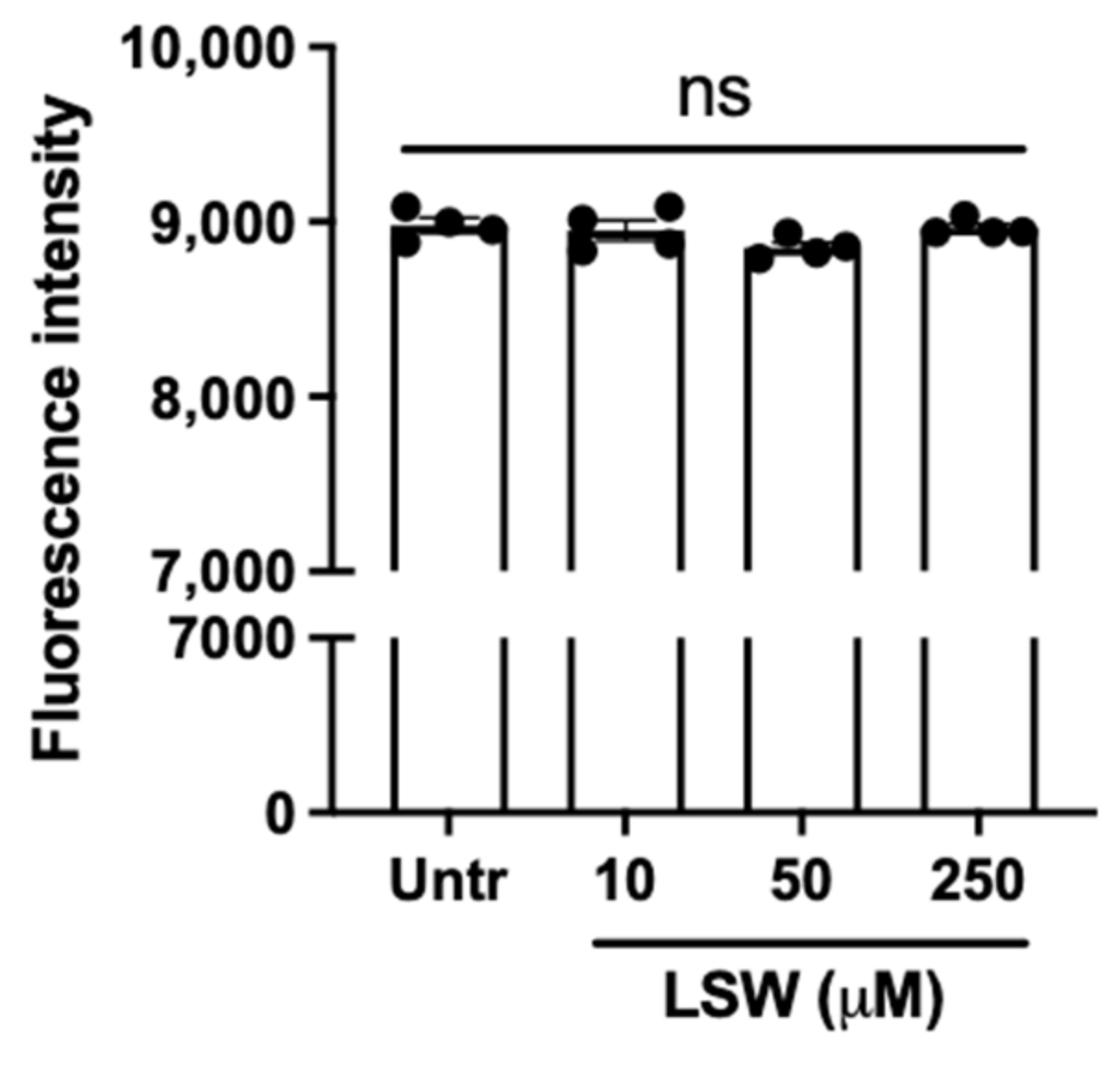
3.1. LSW Does Not Cause Cytotoxicity in TNFα-Stimulated EA.hy926 Cells
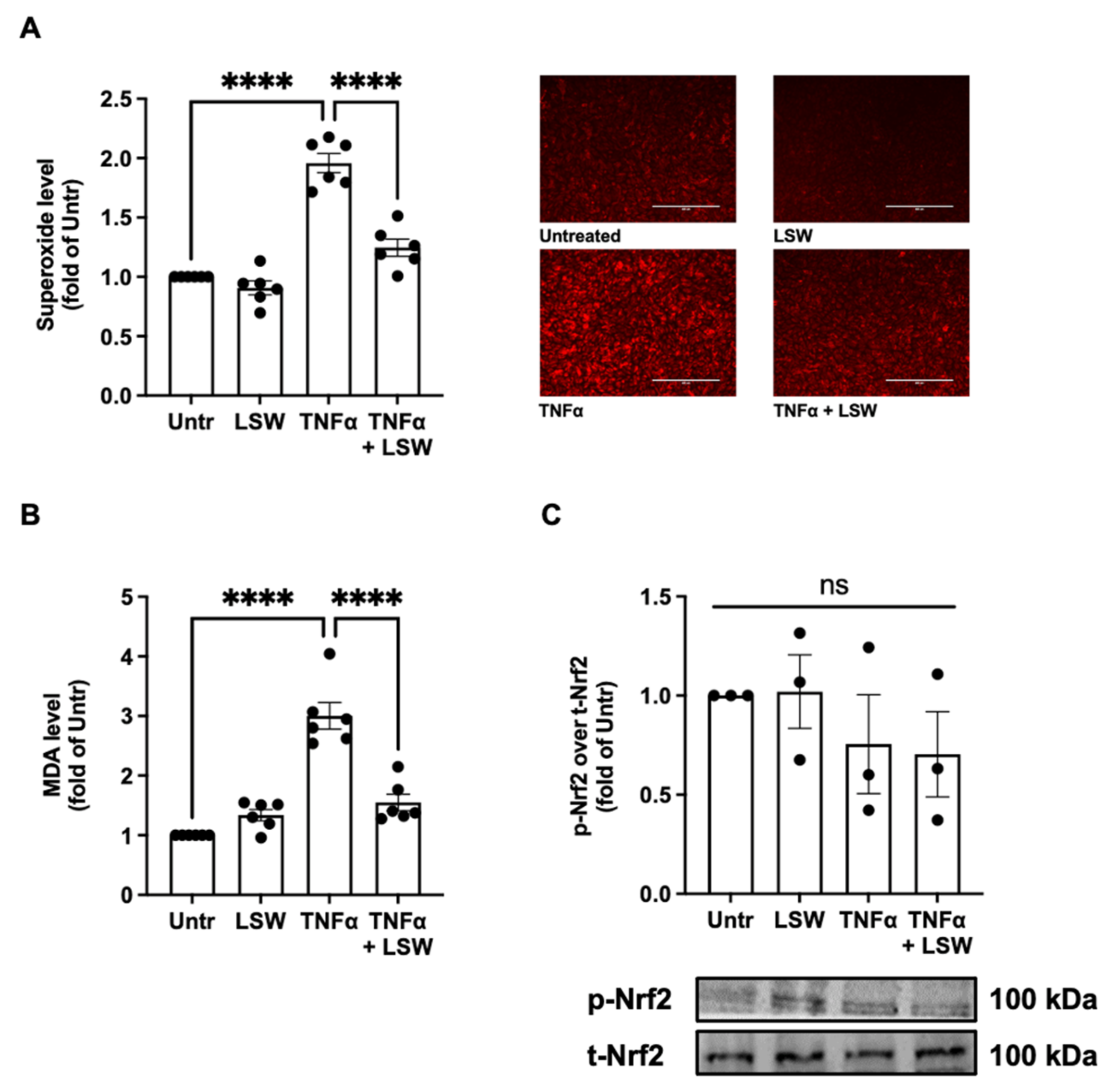
3.2. LSW Reduces Oxidative Stress in TNFα-Stimulated EA.hy926 Cells
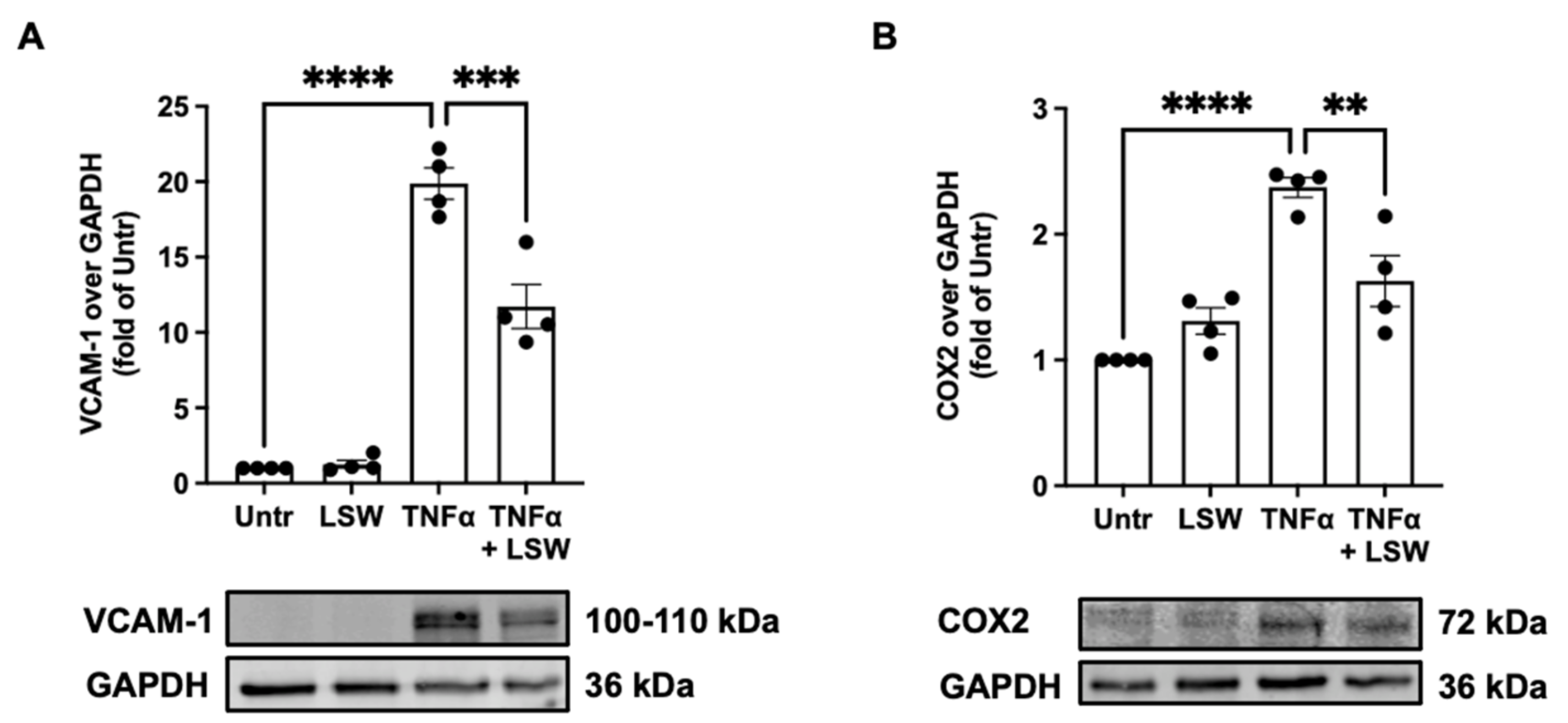
3.3. LSW Ameliorates Inflammation in TNFα-Stimulated EA.hy926 Cells
3.4. LSW Inhibits NF-κB and p38/JNK MAPK Signaling in TNFα-Stimulated EA.hy926 Cells
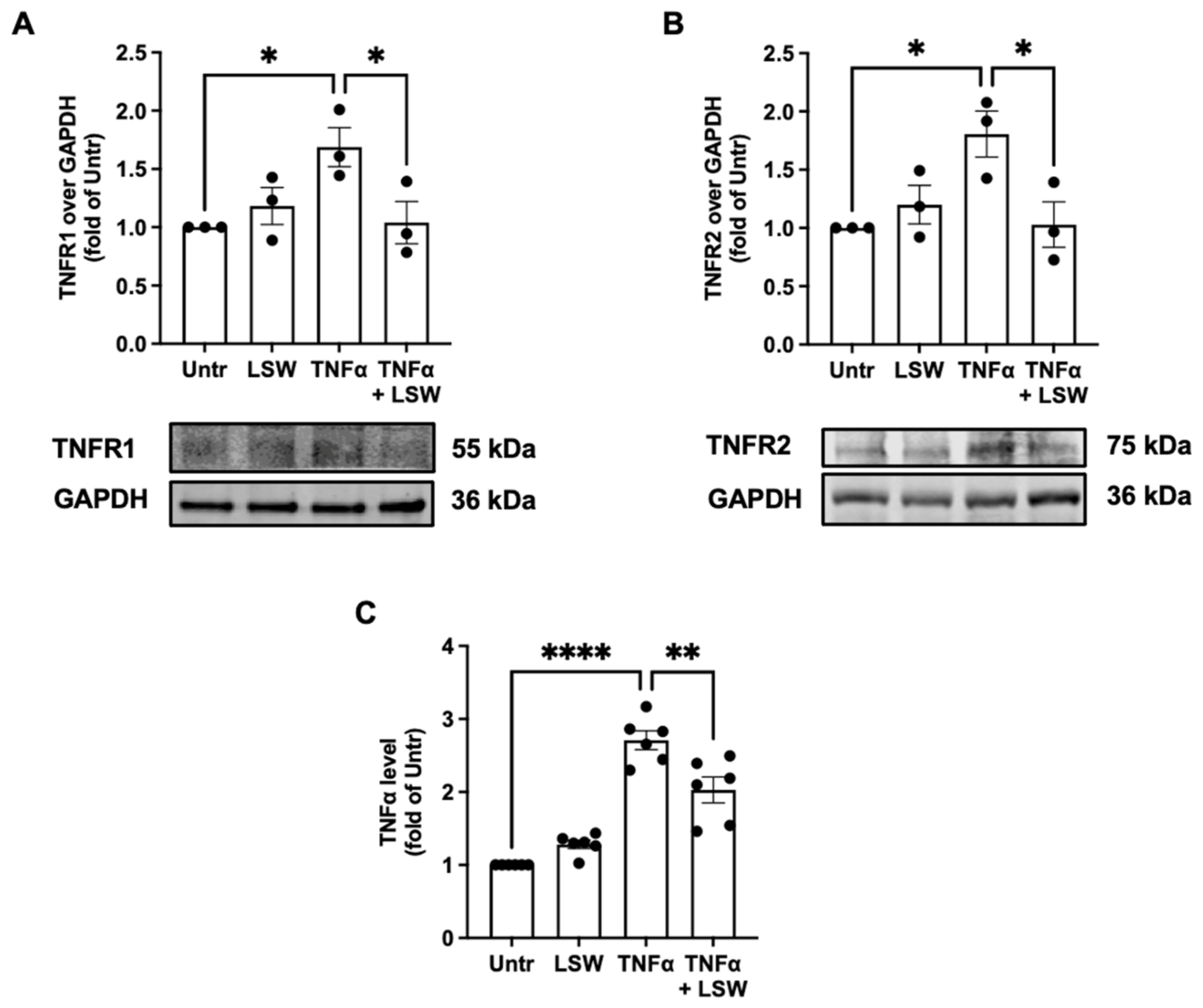
3.5. LSW Reduces TNFα/TNFR1/TNFR2 Expressions in TNFα-Stimulated EA.hy926 Cells
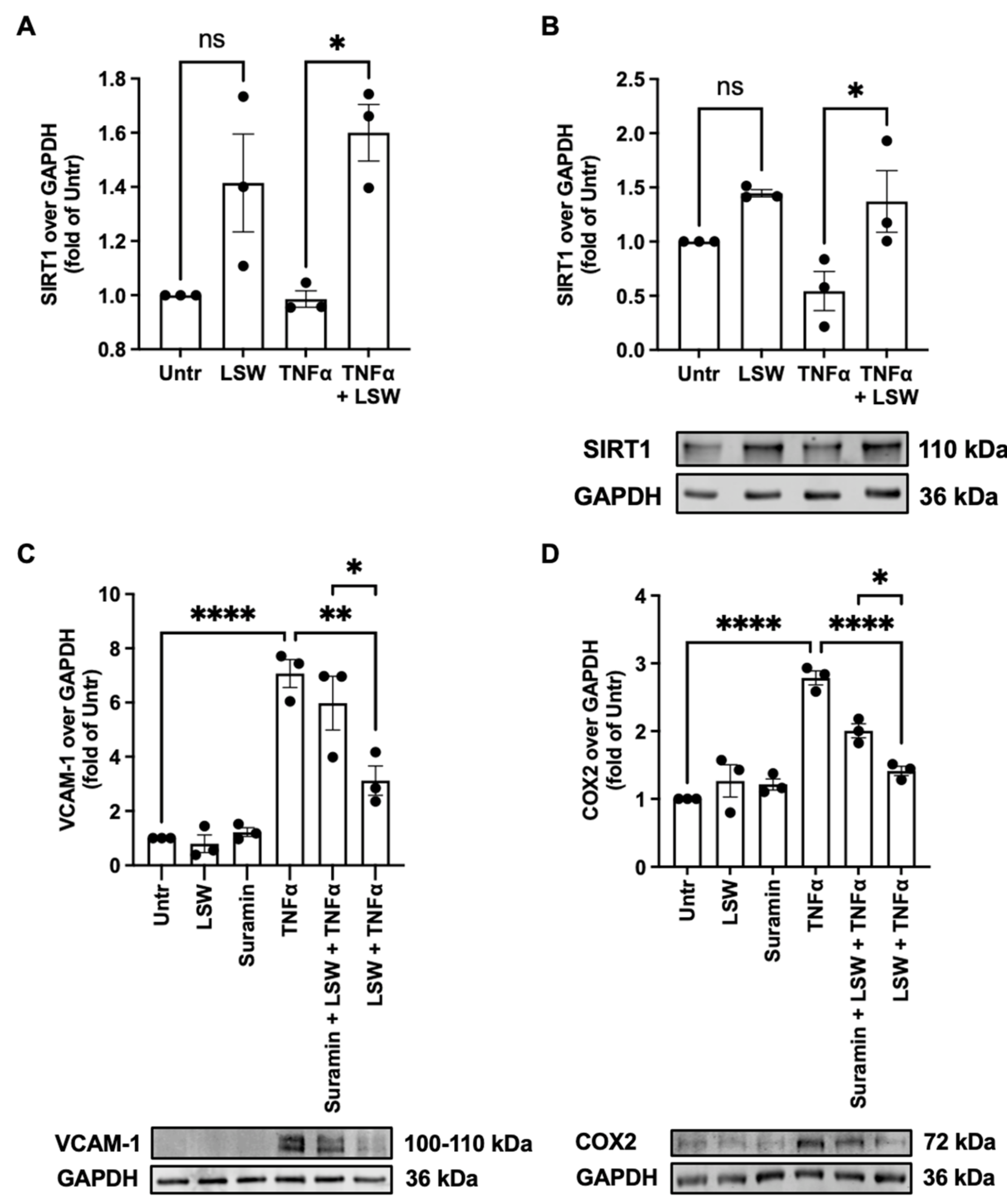
3.6. LSW Attenuates Inflammation Partially Dependent on SIRT1 in TNFα-Stimulated EA.hy926 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B. We have contact: Endothelial cell-smooth muscle cell interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.W.; Sarembock, I.J.; Linden, J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 2000, 67, 591–602. [Google Scholar] [CrossRef]
- Man, A.W.; Li, H.; Xia, N. Impact of lifestyles (diet and exercise) on vascular health: Oxidative stress and endothelial function. Oxidative Med. Cell. Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J. Food Peptides in Blood Pressure Regulation. In Food Proteins and Peptides: Emerging Biofunctions, Food and Biomaterial Applications; The Royal Society of Chemistry: London, UK, 2021; pp. 371–401. [Google Scholar]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of soybean products in terms of essential amino acids composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, J. LC-MS/MS coupled with QSAR modeling in characterising of angiotensin I-converting enzyme inhibitory peptides from soybean proteins. Food Chem. 2013, 141, 2682–2690. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, Q.; Bai, J.; Wu, W.; Hong, H.; Wu, J. Transport of soybean protein-derived antihypertensive peptide LSW across Caco-2 monolayers. J. Funct. Foods 2017, 39, 96–102. [Google Scholar] [CrossRef]
- Song, T.; Lv, M.; Zhou, M.; Huang, M.; Zheng, L.; Zhao, M. Soybean-derived antihypertensive peptide LSW (Leu–Ser–Trp) antagonizes the damage of angiotensin II to vascular endothelial cells through the trans-vesicular pathway. J. Agric. Food Chem. 2021, 69, 10536–10549. [Google Scholar] [CrossRef]
- Fan, H.; Bhullar, K.S.; Wu, J. Spent hen muscle protein-derived RAS regulating peptides show antioxidant activity in vascular cells. Antioxidants 2021, 10, 290. [Google Scholar] [CrossRef]
- Fan, H.; Liao, W.; Davidge, S.T.; Wu, J. Chicken muscle-derived ACE2 upregulating peptide VVHPKESF inhibits angiotensin II-stimulated inflammation in vascular smooth muscle cells via the ACE2/Ang (1–7)/MasR axis. J. Agric. Food Chem. 2022, 70, 6397–6406. [Google Scholar] [CrossRef]
- Fan, H.; Bhullar, K.S.; Wang, Z.; Wu, J. Chicken muscle protein-derived peptide VVHPKESF reduces TNFα-induced inflammation and oxidative stress by suppressing TNFR1 signaling in human vascular endothelial cells. Mol. Nutr. Food Res. 2022, 66, 2200184. [Google Scholar] [CrossRef]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in endothelial dysfunction and cardiovascular aging. Front. Physiol. 2021, 12, 1589. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R.; Sanchez, O.A.; Goff, D.C.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Galle, J.; Quaschning, T.; Seibold, S.; Wanner, C. Endothelial dysfunction and inflammation: What is the link? Kidney Int. 2003, 63, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, B.S.; Seals, D.R.; Zigler, M.L.; Sindler, A.L. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 2012, 11, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yu, W.; Liao, W.; Wu, J. Spent hen protein hydrolysate with good gastrointestinal stability and permeability in caco-2 cells shows antihypertensive activity in SHR. Foods 2020, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liao, W.; Spaans, F.; Pasha, M.; Davidge, S.T.; Wu, J. Chicken muscle hydrolysate reduces blood pressure in spontaneously hypertensive rats, upregulates ACE2, and ameliorates vascular inflammation, fibrosis, and oxidative stress. J. Food. Sci. 2022, 87, 1292–1305. [Google Scholar] [CrossRef]
- Baranska, P.; Jerczynska, H.; Pawlowska, Z.; Koziolkiewicz, W.; Cierniewski, C.S. Expression of integrins and adhesive properties of human endothelial cell line EA.hy 926. Cancer Genom. Proteom. 2005, 2, 265–269. [Google Scholar]
- Fan, H.; Liao, W.; Wu, J. Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J. Food. Biochem. 2019, 43, e12572. [Google Scholar] [CrossRef]
- Thornhill, M.; Li, J.; Haskard, D. Leucocyte endothelial cell adhesion: A study comparing human umbilical vein endothelial cells and the endothelial cell line EA-hy-926. Scand. J. Immunol. 1993, 38, 279–286. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Touyz, R.; Schiffrin, E. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem. Cell Biol. 2004, 122, 339–352. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory effects of a pea-derived peptide Leu-Arg-Trp (LRW) on dysfunction of rat aortic vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Maralani, M.N.; Movahedian, A.; Javanmard, S.H. Antioxidant and cytoprotective effects of L-Serine on human endothelial cells. Res. Pharm. Sci. 2012, 7, 209. [Google Scholar]
- Chen, Y.; Zhang, H.; Mats, L.; Liu, R.; Deng, Z.; Mine, Y.; Tsao, R. Anti-inflammatory effect and cellular uptake mechanism of peptides from common bean (Phaseolus vulga L.) milk and yogurts in Caco-2 mono-and Caco-2/EA. hy926 co-culture models. J. Agric. Food Chem. 2019, 67, 8370–8381. [Google Scholar]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophs. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Zhou, Z.; Connell, M.C.; MacEwan, D.J. TNFR1-induced NF-κB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell. Signal. 2007, 19, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Lima, S.L.; Alves, N.E.; Assis, A.; Moreira, M.E.; Toledo, R.C.; Rosa, C.O.; Teixeira, O.R.; Bassinello, P.Z.; De Mejía, E.G. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.D.; Pangloli, P.; Krishnan, H.B.; Dia, V.P. BG-4, a novel bioactive peptide from Momordica charantia, inhibits lipopolysaccharide-induced inflammation in THP-1 human macrophages. Phytomedicine 2018, 42, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Dumeus, S.; Shibu, M.A.; Lin, W.-T.; Wang, M.-F.; Lai, C.-H.; Shen, C.-Y.; Lin, Y.-M.; Viswanadha, V.P.; Kuo, W.-W.; Huang, C.-Y. Bioactive peptide improves diet-induced hepatic fat deposition and hepatocyte proinflammatory response in SAMP8 ageing mice. Cell. Physiol. Biochem. 2018, 48, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wu, K.; Wu, J. Pea-derived tripeptide LRW fails to reduce blood pressure in spontaneously hypertensive rats due to its low gastrointestinal stability and transepithelial permeability. Food Biosci. 2022, 49, 101964. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-α: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Wang, S.; Shang, Y.C.; Maiese, K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012, 8, 89–100. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- De Gregorio, E.; Colell, A.; Morales, A.; Marí, M. Relevance of SIRT1-NF-κB axis as therapeutic target to ameliorate inflammation in liver disease. Int. J. Mol. Sci. 2020, 21, 3858. [Google Scholar] [CrossRef]
- Lai, S.-W.; Liu, Y.-S.; Lu, D.-Y.; Tsai, C.-F. Melatonin modulates the microenvironment of glioblastoma multiforme by targeting sirtuin 1. Nutrients 2019, 11, 1343. [Google Scholar] [CrossRef]
- Qiang, L.; Sample, A.; Liu, H.; Wu, X.; He, Y.-Y. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci. Rep. 2017, 7, 14110. [Google Scholar] [CrossRef]
- Ho, J.-H.; Baskaran, R.; Wang, M.-F.; Mohammedsaleh, Z.M.; Yang, H.-S.; Balasubramanian, B.; Lin, W.-T. Dipeptide IF and exercise training attenuate hypertension in SHR rats by inhibiting fibrosis and hypertrophy and activating AMPKα1, SIRT1, and PGC1α. Int. J. Mol. Sci. 2022, 23, 8167. [Google Scholar] [CrossRef]
- Tsai, B.C.-K.; Kuo, W.-W.; Day, C.H.; Hsieh, D.J.-Y.; Kuo, C.-H.; Daddam, J.; Chen, R.-J.; Padma, V.V.; Wang, G.; Huang, C.-Y. The soybean bioactive peptide VHVV alleviates hypertension-induced renal damage in hypertensive rats via the SIRT1-PGC1α/Nrf2 pathway. J. Funct. Foods 2020, 75, 104255. [Google Scholar] [CrossRef]
- Ho, J.-H.; Baskaran, R.; Wang, M.-F.; Yang, H.-S.; Lo, Y.-H.; Mohammedsaleh, Z.M.; Lin, W.-T. Bioactive peptides and exercise modulate the AMPK/SIRT1/PGC-1α/FOXO3 pathway as a therapeutic approach for hypertensive rats. Pharmaceuticals 2022, 15, 819. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Ashkar, F.; Wu, J. Peptides GWN and GW protect kidney cells against Dasatinib induced mitochondrial injury in a SIRT1 dependent manner. Food Chem. Mol. Sci. 2022, 4, 100069. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Son, M.; Kerek, E.; Cromwell, C.R.; Wingert, B.M.; Wu, K.; Jovel, J.; Camacho, C.J.; Hubbard, B.P.; Wu, J. Tripeptide IRW upregulates NAMPT protein levels in cells and obese C57BL/6J mice. J. Agric. Food Chem. 2021, 69, 1555–1566. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Bhullar, K.S.; Wang, Z.; Wu, J. Soybean-Derived Tripeptide Leu–Ser–Trp (LSW) Protects Human Vascular Endothelial Cells from TNFα-Induced Oxidative Stress and Inflammation via Modulating TNFα Receptors and SIRT1. Foods 2022, 11, 3372. https://doi.org/10.3390/foods11213372
Fan H, Bhullar KS, Wang Z, Wu J. Soybean-Derived Tripeptide Leu–Ser–Trp (LSW) Protects Human Vascular Endothelial Cells from TNFα-Induced Oxidative Stress and Inflammation via Modulating TNFα Receptors and SIRT1. Foods. 2022; 11(21):3372. https://doi.org/10.3390/foods11213372
Chicago/Turabian StyleFan, Hongbing, Khushwant S. Bhullar, Zihan Wang, and Jianping Wu. 2022. "Soybean-Derived Tripeptide Leu–Ser–Trp (LSW) Protects Human Vascular Endothelial Cells from TNFα-Induced Oxidative Stress and Inflammation via Modulating TNFα Receptors and SIRT1" Foods 11, no. 21: 3372. https://doi.org/10.3390/foods11213372
APA StyleFan, H., Bhullar, K. S., Wang, Z., & Wu, J. (2022). Soybean-Derived Tripeptide Leu–Ser–Trp (LSW) Protects Human Vascular Endothelial Cells from TNFα-Induced Oxidative Stress and Inflammation via Modulating TNFα Receptors and SIRT1. Foods, 11(21), 3372. https://doi.org/10.3390/foods11213372





