Cultivar-Dependent Effects of Non-Saccharomyces Yeast Starter on the Oenological Properties of Wines Produced from Two Autochthonous Grape Cultivars in Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Winemaking
2.3. Chemical Analyses
2.4. Analysis of Volatile Compounds
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
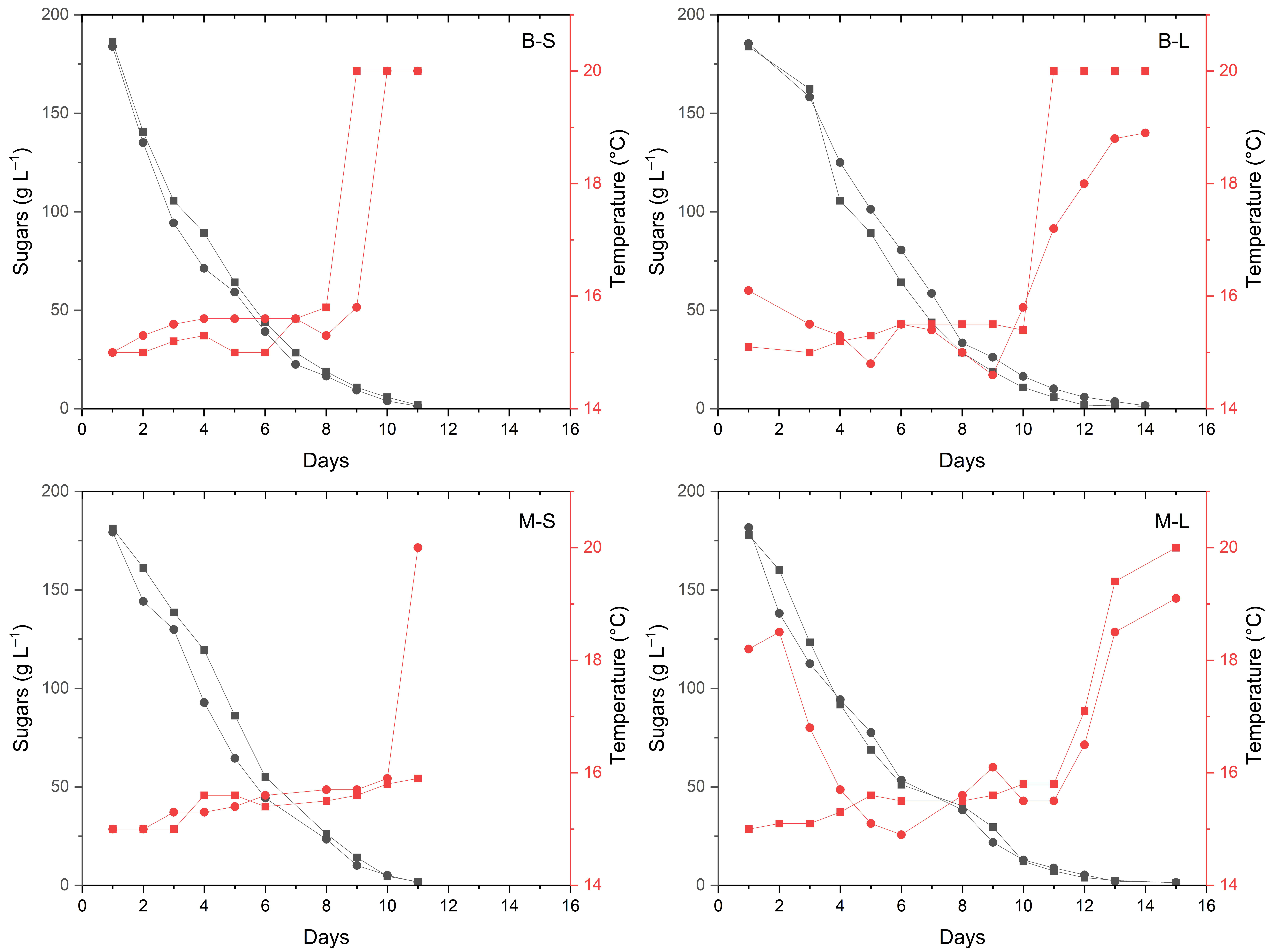
3.1. Wine Chemical Profiles
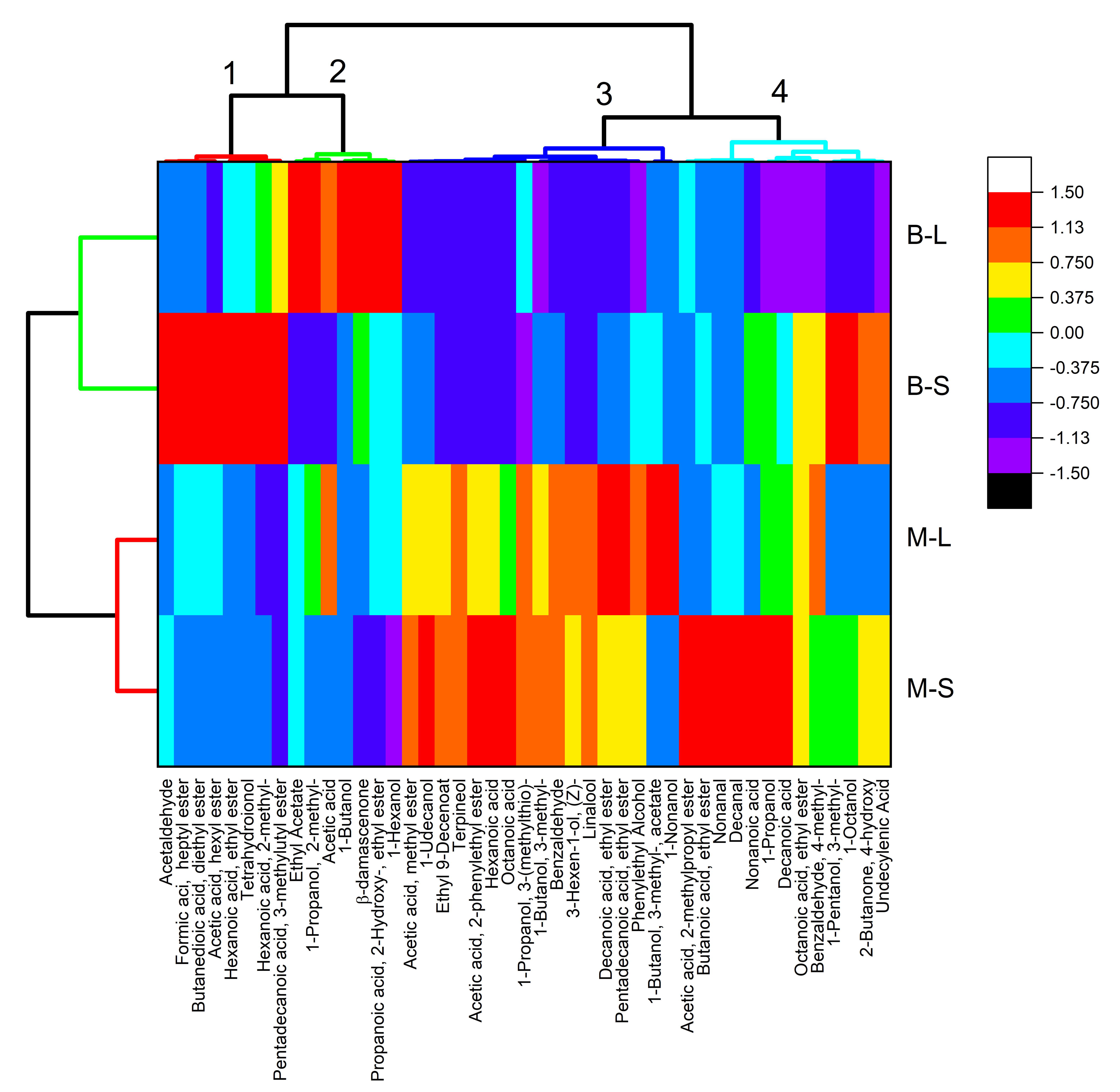
3.2. Wine Volatile Compounds
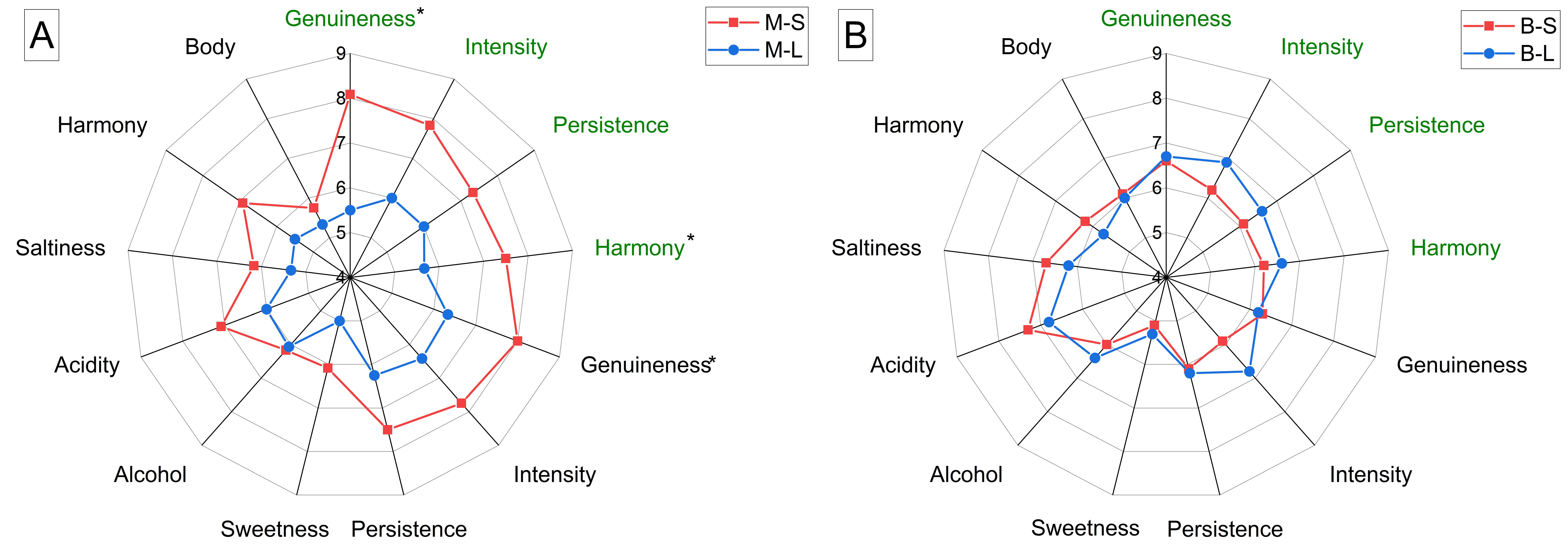
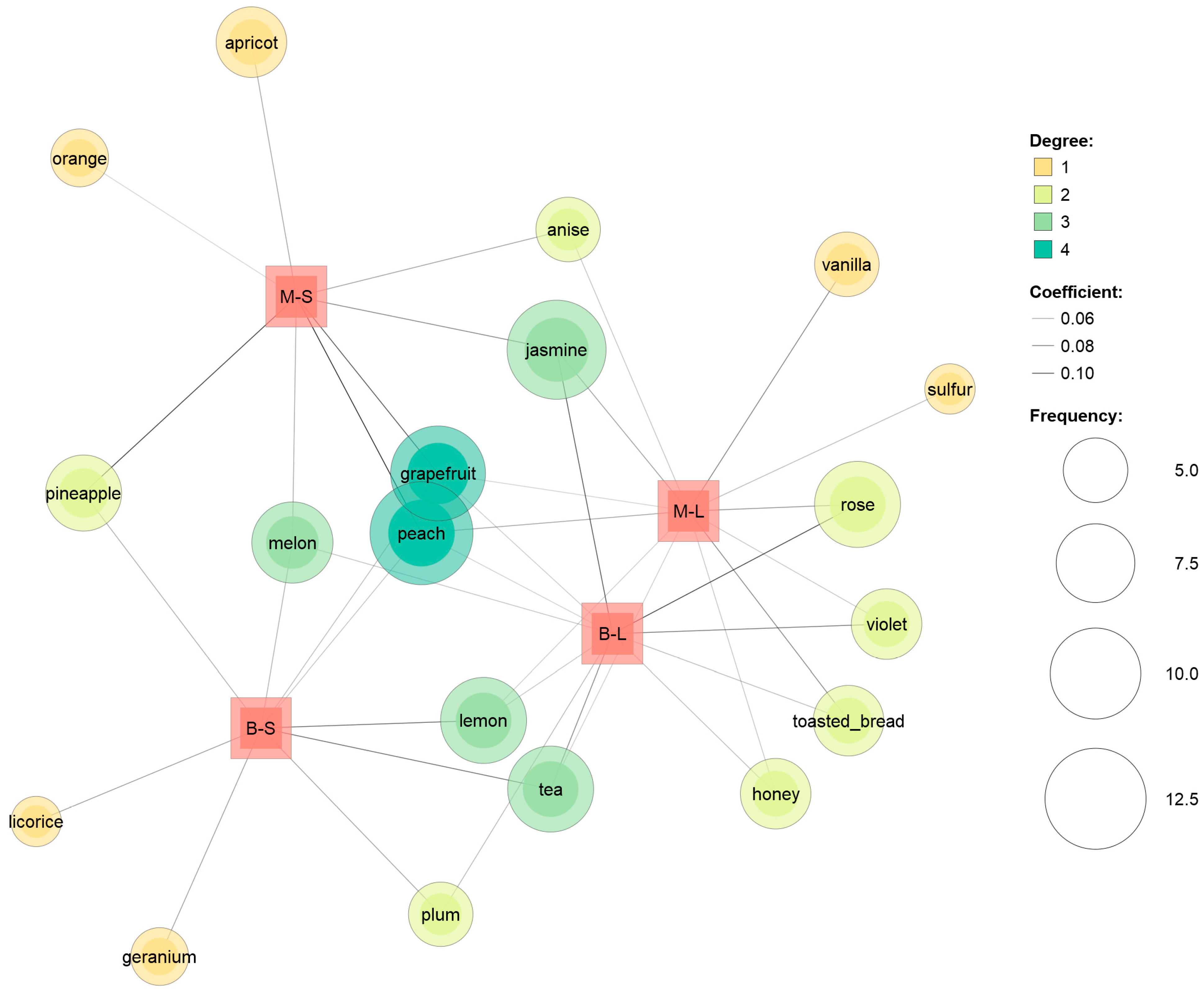
3.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- Pons, A.; Allamy, L.; Schüttler, A.; Rauhut, D.; Thibon, C.; Darriet, P. What is the expected impact of climate change on wine aroma compounds and their precursors in grape? Oeno One 2017, 51, 141–146. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- La Notte, P.; Civita, F.; Raimondi, S.; Schneider, A. Atlante dei Vitigni Tradizionali di Puglia; CRSFA Basile Caramia di Locorotondo: Locorotondo, Italy, 2018; ISBN 9788894358629. [Google Scholar]
- Istituto Nazionale di Statistica I.Stat. Available online: http://dati.istat.it/Index.aspx (accessed on 21 May 2022).
- Schneider, A.; Raimondi, S.; Pirolo, C.S.; Marinoni, D.T.; Ruffa, P.; Venerito, P.; La Notte, P. Genetic characterization of grape cultivars from Apulia (southern Italy) and synonymies in other Mediterranean regions. Am. J. Enol. Vitic. 2014, 65, 244–249. [Google Scholar] [CrossRef]
- Zulini, L.; Russo, M.; Peterlunger, E. Genotyping wine and table grape cultivars from Apulia (southern Italy) using microsatellite markers. Vitis 2002, 41, 183–187. [Google Scholar]
- Gatta, B.L.; Arace, E.; Gambacorta, G.; Notte, E.L.; Notte, P. La Phenolic characterization of some native varieties of grapes and wines from Southern Italy. Acta Hortic. 2007, 754, 65–72. [Google Scholar] [CrossRef]
- Tamborra, P.; Piracci, A.; Coletta, A.; Esti, M. Winemaking technique optimization for enancing aroma in Fiano wine. In Atti delle Tornate dell’accademia Ital. della Vite e del Vino; Accademia Italiana della Vite e del Vino: Firenze-Conegliano, Italy, 2009; p. 25. [Google Scholar]
- Costacurta, A.; Catalano, V.; Carraro, R.; Crespan, M.; Giust, M.; Calò, A. Chiarimenti sull’identità dei Fiani in Puglia. In Atti delle Tornate dell’accademia Ital. della Vite e del Vino; Accademia Italiana della Vite e del Vino: Firenze-Conegliano, Italy, 2006; p. 69. [Google Scholar]
- Teslić, N.; Patrignani, F.; Ghidotti, M.; Parpinello, G.P.; Ricci, A.; Tofalo, R.; Lanciotti, R.; Versari, A. Utilization of ‘early green harvest’ and non-Saccharomyces cerevisiae yeasts as a combined approach to face climate change in winemaking. Eur. Food Res. Technol. 2018, 244, 1301–1311. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Oenological potential of non-Saccharomyces yeasts to mitigate effects of climate change in winemaking: Impact on aroma and sensory profiles of Treixadura wines. FEMS Yeast Res. 2019, 19, foz065. [Google Scholar] [CrossRef]
- Vejarano, R.; Gil-Calderón, A. Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The Combined Use of Schizosaccharomyces pombe and Lachancea thermotolerans—Effect on the Anthocyanin Wine Composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Gonzalo-Diago, A.; Iribarren, M.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. Bioprotection as a tool to free additives winemaking: Effect on sensorial, anthocyanic and aromatic profile of young red wines. LWT 2018, 98, 458–464. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Pargoletti, E.; Sanarica, L.; Ceruti, M.; Elli, F.; Pisarra, C.; Cappelletti, G. A comprehensive study on the effect of bentonite fining on wine charged model molecules. Food Chem. 2021, 338, 127840. [Google Scholar] [CrossRef] [PubMed]
- The Internationation Organisation of Vine and Wine. Guidelines on Infrared Analysers in Oenology; The Internationation Organisation of Vine and Wine: Paris, France, 2010. [Google Scholar]
- Cardinale, M.; Trinchera, R.; Natrella, G.; Difonzo, G.; De Benedittis, C.; D’amato, I.; Mascellani, M.; Paradiso, V.M.; Rustioni, L. Dynamics of the fermentation process and chemical profiling of pomegranate (Punica granatum L.) wines obtained by different cultivar×yeast combinations. Foods 2021, 10, 1913. [Google Scholar] [CrossRef]
- The Internationation Organisation of Vine and Wine. OIV Standard for International Wine Competitions and Spiritous Beverages of Vitivinicultural Origin (OIV-Concours 332A-2009); The Internationation Organisation of Vine and Wine: Paris, France, 2009; p. 27. [Google Scholar]
- Pizarro, C.; Esteban-Díez, I.; Rodríguez-Tecedor, S.; González-Sáiz, J.M. A sensory approach for the monitoring of accelerated red wine aging processes using multi-block methods. Food Qual. Prefer. 2013, 28, 519–530. [Google Scholar] [CrossRef]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G. Influence of autochthonous Saccharomyces cerevisiae strains on volatile profile of Negroamaro wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Baiano, A.; Varva, G. Evolution of physico-chemical and sensory characteristics of Minutolo white wines during aging in amphorae: A comparison with stainless steel tanks. LWT 2019, 103, 78–87. [Google Scholar] [CrossRef]
- Baiano, A.; Mentana, A.; Quinto, M.; Centonze, D.; Longobardi, F.; Ventrella, A.; Agostiano, A.; Varva, G.; De Gianni, A.; Terracone, C.; et al. The effect of in-amphorae aging on oenological parameters, phenolic profile and volatile composition of Minutolo white wine. Food Res. Int. 2015, 74, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Varva, G.; De Gianni, A.; Terracone, C.; Viggiani, I.; Del Nobile, M.A. Effects of different vinification technologies on physico-chemical properties and antioxidant activity of “Falanghina” and “Bombino bianco” wines. Eur. Food Res. Technol. 2013, 237, 831–842. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Bely, M.; Masneuf-Pomarede, I.; Jiranek, V.; Albertin, W. The evolution of Lachancea thermotolerans is driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE 2017, 12, e0184652. [Google Scholar] [CrossRef] [PubMed]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Kong, C.L.; Ma, N.; Yin, J.; Zhao, H.Y.; Tao, Y.S. Fine tuning of medium chain fatty acids levels increases fruity ester production during alcoholic fermentation. Food Chem. 2021, 346. [Google Scholar] [CrossRef]
- Suriano, S.; Basile, T.; Tarricone, L.; Di Gennaro, D.; Tamborra, P. Effects of skin maceration time on the phenolic and sensory characteristics of Bombino Nero rosé wines. Ital. J. Agron. 2015, 10, 21. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Tomasino, E.; Bolman, S. The potential effect of β-ionone and β-damascenone on sensory perception of pinot noir wine aroma. Molecules 2021, 26, 1288. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Zhang, B.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Distinctive chemical and aromatic composition of red wines produced by Saccharomyces cerevisiae co-fermentation with indigenous and commercial non-Saccharomyces strains. Food Biosci. 2021, 41, 100925. [Google Scholar] [CrossRef]
- Chin, S.T.; Eyres, G.T.; Marriott, P.J. Identification of potent odourants in wine and brewed coffee using gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 7487–7498. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zhang, J.-R.; Zhang, R.; Qin, Y.; Yang, X.; Jin, G.; Tao, Y.-S. Oxidation-reduction potential affects medium-chain fatty acid ethyl ester production during wine alcohol fermentation. Food Res. Int. 2022, 157, 111369. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Kato, E.; Ohe, Y.; Blandford, D. Examining the opinions of potential consumers about plant-derived cosmetics: An approach combining word association, co-occurrence network, and multivariate probit analysis. J. Sens. Stud. 2019, 34, e12484. [Google Scholar] [CrossRef]
- De Palma, L.; Tarricone, L.; Costacurta, A.; Carparelli, P.; Novello, V. Il Fiano Aromatico di Puglia, dal DNA al vino. In Atti delle Tornate dell’Accademia Italiana della Vite e del Vino; Accademia Italiana della Vite e del Vino: Firenze-Conegliano, Italy, 2009; Volume 26. [Google Scholar]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, Á. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef]
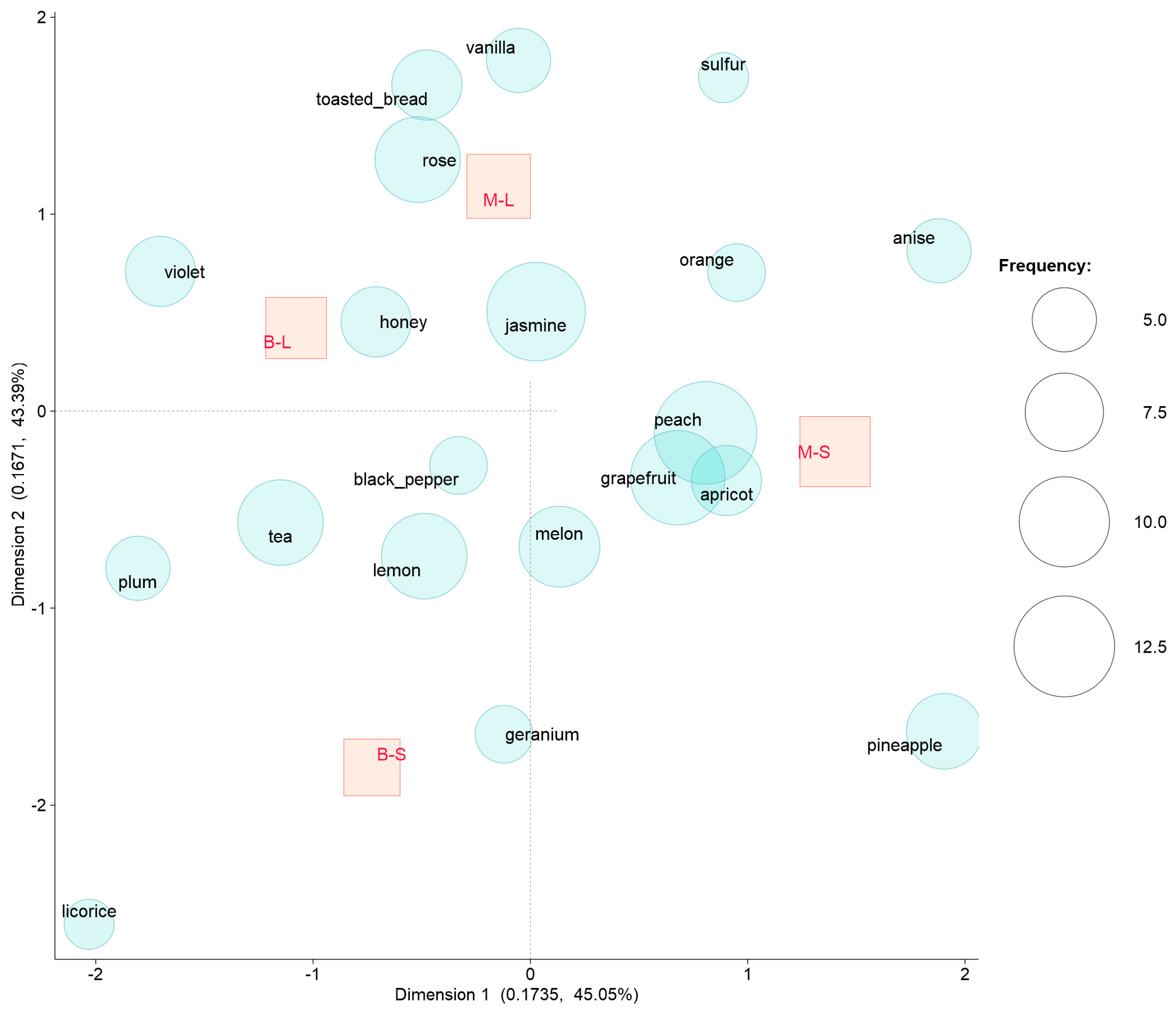
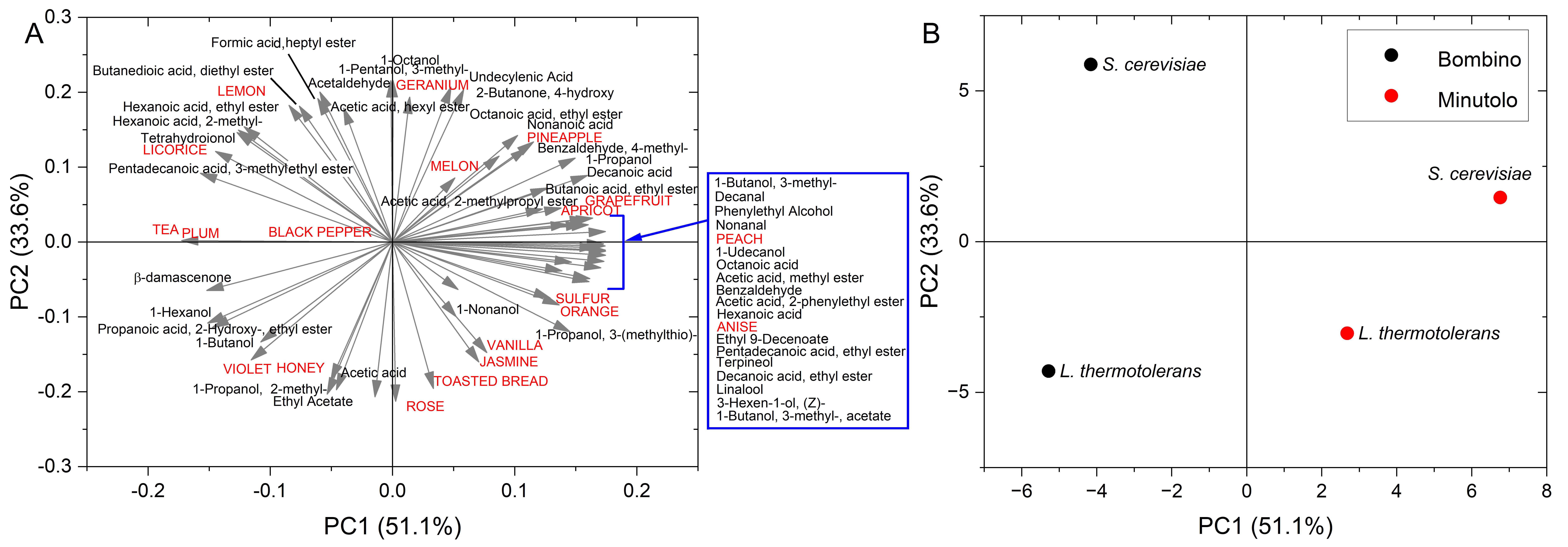
| Parameter | Minutolo | Bombino | Cv. | Yeast | ||||
|---|---|---|---|---|---|---|---|---|
| S | L | S | L | M | B | S | L | |
| Ethanol (% vol) | 11.57 ± 0.03 a | 11.66 ± 0.08 a | 11.18 ± 0.07 b | 11.29 ± 0.06 b | A | B | ||
| pH | 3.21 ± 0.03 | 3.29 ± 0.13 | 3.37 ± 0.00 | 3.36 ± 0.11 | ||||
| Total acidity (g tartaric acid L−1) | 6.49 ± 0.41 | 5.94 ± 0.27 | 5.45 ± 0.18 | 5.98 ± 0.91 | ||||
| Volatile acidity (g acetic acid L−1) | 0.19 ± 0.00 b | 0.23 ± 0.00 ab | 0.06 ± 0.00 c | 0.28 ± 0.03 a | A | B | B | A |
| Malic acid (g·L−1) | 1.00 ± 0.14 | 1.00 ± 0.14 | 1.00 ± 0.00 | 0.80 ± 0.42 | ||||
| Lactic acid (g·L−1) | 0.30 ± 0.00 b | 0.40 ± 0.00 b | 0.40 ± 0.00 b | 1.50 ± 0.14 a | B | A | B | A |
| Residual sugars (g·L−1) | 0.95 ± 0.08 | 1.51 ± 0.42 | 0.97 ± 0.01 | 2.03 ± 1.89 | ||||
| Total dry extract (g·L−1) | 19.50 ± 0.16 | 20.54 ± 0.51 | 18.09 ± 0.12 | 20.09 ± 2.19 | ||||
| Net dry extract (g·L−1) | 18.56 ± 0.25 | 19.03 ± 0.93 | 17.12 ± 0.12 | 18.06 ± 0.30 | A | B | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradiso, V.M.; Sanarica, L.; Zara, I.; Pisarra, C.; Gambacorta, G.; Natrella, G.; Cardinale, M. Cultivar-Dependent Effects of Non-Saccharomyces Yeast Starter on the Oenological Properties of Wines Produced from Two Autochthonous Grape Cultivars in Southern Italy. Foods 2022, 11, 3373. https://doi.org/10.3390/foods11213373
Paradiso VM, Sanarica L, Zara I, Pisarra C, Gambacorta G, Natrella G, Cardinale M. Cultivar-Dependent Effects of Non-Saccharomyces Yeast Starter on the Oenological Properties of Wines Produced from Two Autochthonous Grape Cultivars in Southern Italy. Foods. 2022; 11(21):3373. https://doi.org/10.3390/foods11213373
Chicago/Turabian StyleParadiso, Vito Michele, Luigi Sanarica, Ignazio Zara, Chiara Pisarra, Giuseppe Gambacorta, Giuseppe Natrella, and Massimiliano Cardinale. 2022. "Cultivar-Dependent Effects of Non-Saccharomyces Yeast Starter on the Oenological Properties of Wines Produced from Two Autochthonous Grape Cultivars in Southern Italy" Foods 11, no. 21: 3373. https://doi.org/10.3390/foods11213373
APA StyleParadiso, V. M., Sanarica, L., Zara, I., Pisarra, C., Gambacorta, G., Natrella, G., & Cardinale, M. (2022). Cultivar-Dependent Effects of Non-Saccharomyces Yeast Starter on the Oenological Properties of Wines Produced from Two Autochthonous Grape Cultivars in Southern Italy. Foods, 11(21), 3373. https://doi.org/10.3390/foods11213373








