Andean Sprouted Pseudocereals to Produce Healthier Extrudates: Impact in Nutritional and Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quinoa and Cañihua Germination
2.3. Pilot-Scale Extrusion-Cooking
2.4. Simulated Gastrointestinal Digestion
2.5. Nutritional Characterization
2.6. Total Soluble Phenolic Compounds (TSPC)
2.7. γ-Aminobutyric Acid (GABA)
2.8. Antioxidant Activity
2.9. Physicochemical Properties
2.10. Experimental Design and Optimization of Flours Blend Ratio
2.11. Statistical Analysis
3. Results and Discussion
3.1. SQF and SCF Had Higher Nutritional and Bioactive Value Than CG
3.2. Incorporation of Sprouted Pseudocereal Flours in the Formulation of Extrudates Increased PA, TSPC, GABA and ORAC
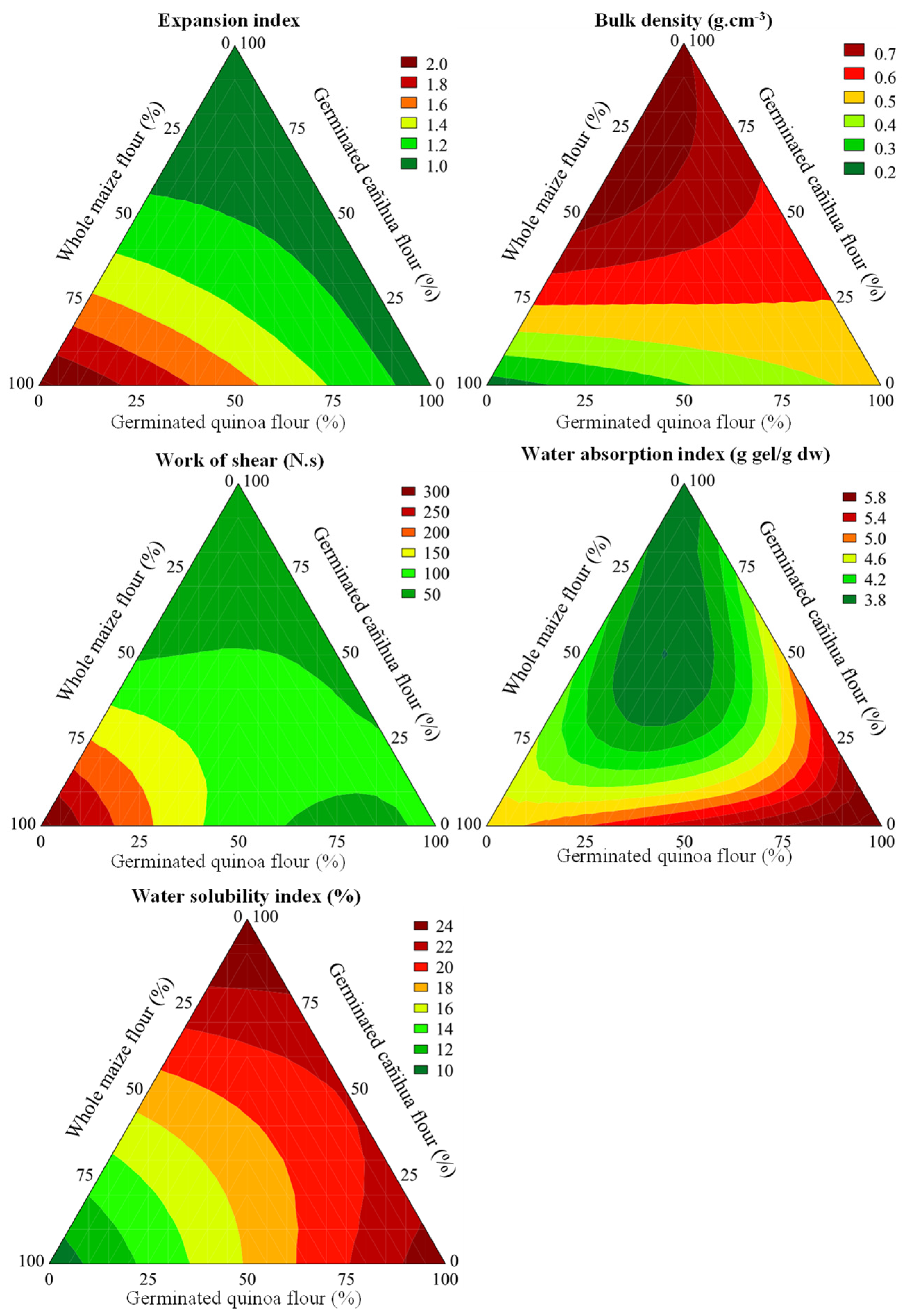
3.3. Effect of CG Replacement by Sprouted Pseudocereal Flours on Physical Properties of Extrudates
3.4. Optimal Formulation Improved the Nutritional Quality, Texture and Color of Corn-Sprouted Pseudocereal Flour Extrudates
3.5. Gastric and Intestinal Digestates of Optimized Extrudates Had Greater PA, GABA, TSPC and ORAC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Ready-to-eat snack products: The role of extrusion technology in developing consumer acceptable and nutritious snacks. Int. J. Food Sci. Technol. 2013, 48, 893–902. [Google Scholar] [CrossRef]
- Nørgaard, M.K.; Sørensen, B.T.; Brunsø, K. A concept test of novel healthy snacks among adolescents: Antecedents of preferences and buying intentions. Food Qual. Prefer. 2014, 33, 17–26. [Google Scholar] [CrossRef]
- Emin, M.A.; Wittek, P.; Schwegler, Y. Numerical analysis of thermal and mechanical stress profile during the extrusion processing of plasticized starch by non-isothermal flow simulation. J. Food Eng. 2021, 294, 110407. [Google Scholar] [CrossRef]
- Sajid Mushtaq, B.; Zhang, W.; Al-Ansi, W.; Ul Haq, F.; Rehman, A.; Omer, R.; Mahmood Khan, I.; Niazi, S.; Ahmad, A.; Ali Mahdi, A.; et al. A Critical Review on the Development, Physicochemical Variations and Technical Concerns of Gluten Free Extrudates in Food Systems. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Yi, C.; Qiang, N.; Zhu, H.; Xiao, Q.; Li, Z. Extrusion processing: A strategy for improving the functional components, physicochemical properties, and health benefits of whole grains. Food Res. Int. 2022, 160, 111681. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- FAO; CIRAD. State of the Art Report of Quinoa in the World in 2013; FAO: Rome, Italy, 2015. [Google Scholar]
- Graziano, S.; Agrimonti, C.; Marmiroli, N.; Gullì, M. Utilisation and limitations of pseudocereals (quinoa, amaranth, and buckwheat) in food production: A review. Trends Food Sci. Technol. 2022, 125, 154–165. [Google Scholar] [CrossRef]
- Robin, F.; Théoduloz, C.; Srichuwong, S. Properties of extruded whole grain cereals and pseudocereals flours. Int. J. Food Sci. Technol. 2015, 50, 2152–2159. [Google Scholar] [CrossRef]
- Li, H.; Xiong, Z.; Li, X. Optimization of the extrusion process for the development of extruded snacks using peanut, buckwheat, and rice blend. J. Food Process. Preserv. 2019, 43, e14264. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Mushtaq, B.S.; Al-Ansi, W.; Dhungle, A.; Haq, F.u.; Mahdi, A.A.; Walayat, N.; Manzoor, M.S.; Nawaz, A.; Fan, M.; Qian, H.; et al. Influence of pretreatments combined with extrusion on γ-amino butyric acid, nutritional composition and physicochemical properties of foxtail millet (Setaria italica). J. Cereal Sci. 2021, 102, 103359. [Google Scholar] [CrossRef]
- Krapf, J.; Arysanto, A.; Walther, G.; Flöter, E. Effect of sprouting conditions on the properties of direct expanded extruded wheat. J. Food Process Eng. 2019, 42, e13123. [Google Scholar] [CrossRef]
- Krapf, J.; Ding, L.; Brühan, J.; Lorimer, L.; Walther, G.; Flöter, E. Effect of sprouting temperature on selected properties of wheat flour and direct expanded extrudates. J. Food Process Eng. 2020, 43, e13365. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; Smolders, E.; Delcour, J.A. Mineral bio-accessibility and intrinsic saccharides in breakfast flakes manufactured from sprouted wheat. LWT 2021, 143, 111079. [Google Scholar] [CrossRef]
- Lavelli, V. Circular food supply chains—Impact on value addition and safety. Trends Food Sci. Technol. 2021, 114, 323–332. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Abderrahim, F.; Huanatico, E.; Repo-Carrasco-Valencia, R.; Arribas, S.M.; Gonzalez, M.C.; Condezo-Hoyos, L. Effect of germination on total phenolic compounds, total antioxidant capacity, Maillard reaction products and oxidative stress markers in canihua (Chenopodium pallidicaule). J. Cereal Sci. 2012, 56, 410–417. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- AACC. Approved Methods of AACC, 9th ed.; Method 08-03, 30-10; The American Association of Cereal Chemists: St. Paul, MN, USA, 2010. [Google Scholar]
- Pico, J.; Pismag, R.Y.; Laudouze, M.; Martinez, M.M. Systematic evaluation of the Folin–Ciocalteu and Fast Blue BB reactions during the analysis of total phenolics in legumes, nuts and plant seeds. Food Funct. 2020, 11, 9868–9880. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed Wheat Ingredients: Characterization, Bioaccessibility of Phenolic Compounds, and Bioactivity During in vitro Digestion. Front. Plant Sci. 2021, 12, 790898. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Brennan, M.A.; Monro, J.A.; Brennan, C.S. Effect of inclusion of soluble and insoluble fibres into extruded breakfast cereal products made with reverse screw configuration. Int. J. Food Sci. Technol. 2008, 43, 2278–2288. [Google Scholar] [CrossRef]
- Anderson, R. Gelatinization of corn grits by roll-and extrusion-cooking. Cereal Sci. Today 1969, 14, 4–12. [Google Scholar]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 403. [Google Scholar]
- Galvan, D.; Effting, L.; Cremasco, H.; Conte-Junior, C.A. Recent Applications of Mixture Designs in Beverages, Foods, and Pharmaceutical Health: A Systematic Review and Meta-Analysis. Foods 2021, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Kesre, C.; Masatcioglu, M.T. Physical characteristics of corn extrudates supplemented with red lentil bran. LWT 2022, 153, 112530. [Google Scholar] [CrossRef]
- da Silva, E.M.M.; Ascheri, J.L.R.; de Carvalho, C.W.P.; Takeiti, C.Y.; Berrios, J.D.J. Physical characteristics of extrudates from corn flour and dehulled carioca bean flour blend. LWT-Food Sci. Technol. 2014, 58, 620–626. [Google Scholar] [CrossRef][Green Version]
- Paucar-Menacho, L.M.; Simpalo-López, W.D.; Castillo-Martínez, W.E.; Esquivel-Paredes, L.J.; Martínez-Villaluenga, C. Improving Nutritional and Health Benefits of Biscuits by Optimizing Formulations Based on Sprouted Pseudocereal Grains. Foods 2022, 11, 1533. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Simpalo-López, W.D.; Castillo-Martínez, W.E.; Esquivel-Paredes, L.J.; Martínez-Villaluenga, C. Reformulating Bread Using Sprouted Pseudo-cereal Grains to Enhance Its Nutritional Value and Sensorial Attributes. Foods 2022, 11, 1541. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch digestibility: Past, present, and future. J. Sci. Food Agric. 2020, 100, 5009–5016. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.C.; Haros, C.M. Combined Effect of Chia, Quinoa and Amaranth Incorporation on the Physico-Chemical, Nutritional and Functional Quality of Fresh Bread. Foods 2020, 9, 1859. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Manickavasagan, A.; Shobana, S.; Mohan, V. Glycemic index of pulses and pulse-based products: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1567–1588. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Al-Jumayi, H.A.O.; Elhendy, H.A. Effect of germination on the nutritional profile of quinoa (Cheopodium quinoa Willd.) seeds and its anti-anemic potential in Sprague–Dawley male albino rats. Cereal Chem. 2021, 98, 315–327. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Peñas, E.; Dueñas, M.; Frias, J.; Martínez-Villaluenga, C. Optimizing germination conditions to enhance the accumulation of bioactive compounds and the antioxidant activity of kiwicha (Amaranthus caudatus) using response surface methodology. LWT-Food Sci. Technol. 2017, 76, 245–252. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Optimization of germination time and temperature to maximize the content of bioactive compounds and the antioxidant activity of purple corn (Zea mays L.) by response surface methodology. LWT-Food Sci. Technol. 2017, 76, 236–244. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Castillo-Martínez, W.E.; Simpalo-Lopez, W.D.; Verona-Ruiz, A.; Lavado-Cruz, A.; Martínez-Villaluenga, C.; Peñas, E.; Frias, J.; Schmiele, M. Performance of Thermoplastic Extrusion, Germination, Fermentation, and Hydrolysis Techniques on Phenolic Compounds in Cereals and Pseudocereals. Foods 2022, 11, 1957. [Google Scholar] [CrossRef]
- Pramai, P.; Thanasukarn, P.; Thongsook, T.; Jannoey, P.; Chen, F.; Jiamyangyuen, S. Glutamate Decarboxylase (GAD) Extracted from Germinated Rice: Enzymatic Properties and Its Application in Soymilk. J. Nutr. Sci. Vitaminol. 2019, 65, S166–S170. [Google Scholar] [CrossRef]
- Escobedo, A.; Mojica, L. Pulse-based snacks as functional foods: Processing challenges and biological potential. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4678–4702. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Singh, B.; Dar, B.N. Effect of extrusion variables (temperature, moisture) on the antinutrient components of cereal brans. J. Food Sci. Technol. 2015, 52, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Chalermchaiwat, P.; Jangchud, K.; Jangchud, A.; Charunuch, C.; Prinyawiwatkul, W. Antioxidant activity, free gamma-aminobutyric acid content, selected physical properties and consumer acceptance of germinated brown rice extrudates as affected by extrusion process. LWT-Food Sci. Technol. 2015, 64, 490–496. [Google Scholar] [CrossRef]
- Zhu, L.; Adedeji, A.A.; Alavi, S. Effect of Germination and Extrusion on Physicochemical Properties and Nutritional Qualities of Extrudates and Tortilla from Wheat. J. Food Sci. 2017, 82, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Xiong, H.; Tui, W.; Zhu, Y.; Zhang, J. Effect of Extrusion or Fermentation on Physicochemical and Digestive Properties of Barley Powder. Front. Nutr. 2021, 8, 794355. [Google Scholar] [CrossRef]
- Huang, L.; Dong, J.-L.; Zhang, K.-Y.; Zhu, Y.-Y.; Shen, R.-L.; Qu, L.-B. Thermal processing influences the physicochemical properties, in vitro digestibility and prebiotics potential of germinated highland barley. LWT 2021, 140, 110814. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097–1110. [Google Scholar] [CrossRef]
- Félix-Medina, J.V.; Gutiérrez-Dorado, R.; López-Valenzuela, J.A.; López-Ángulo, G.; Quintero-Soto, M.F.; Perales-Sánchez, J.X.K.; Montes-Ávila, J. Nutritional, antioxidant and phytochemical characterization of healthy ready-to-eat expanded snack produced from maize/common bean mixture by extrusion. LWT 2021, 142, 111053. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.A.-M.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci. Technol. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Machado Pereira, A.; Schmiele, M.; Dierings de Souza, E.J.; Pio Ávila, B.; Hirsch Ramos, A.; da Rosa Zavareze, E.; Arocha Gularte, M. Extrudate gluten-free breakfast cereals from rice and corn flours with different amylose content: Technological and sensory properties. Int. J. Food Sci. Technol. 2021, 56, 4182–4190. [Google Scholar] [CrossRef]
- Salvador-Reyes, R.; Sampaio, U.M.; de Menezes Alves Moro, T.; Brito, A.D.C.D.; Behrens, J.; Campelo, P.H.; Pedrosa Silva Clerici, M.T. Andean purple maize to produce extruded breakfast cereals: Impact on techno-functional properties and sensory acceptance. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef] [PubMed]
- Meza, S.L.R.; Sinnecker, P.; Schmiele, M.; Massaretto, I.L.; Chang, Y.K.; Marquez, U.M.L. Production of innovative gluten-free breakfast cereals based on red and black rice by extrusion processing technology. J. Food Sci. Technol. 2019, 56, 4855–4866. [Google Scholar] [CrossRef] [PubMed]
- Bhati, D.; Singh, B.; Singh, A.; Sharma, S.; Pandiselvam, R. Assessment of physicochemical, rheological, and thermal properties of Indian rice cultivars: Implications on the extrusion characteristics. J. Texture Stud. 2022. [Google Scholar] [CrossRef] [PubMed]
- Finnie, S.; Brovelli, V.; Nelson, D. 6—Sprouted grains as a food ingredient. In Sprouted Grains; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Washington, DC, USA, 2019; pp. 113–142. [Google Scholar] [CrossRef]
- Schmiele, M.; Sampaio, U.M.; Gomes, P.T.G.; Clerici, M.T.P.S. Chapter 6—Physical Modifications of Starch. In Starches for Food Application; Silva Clerici, M.T.P., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Rivero Meza, S.L.; Louro Massaretto, I.; Sinnecker, P.; Schmiele, M.; Chang, Y.K.; Noldin, J.A.; Lanfer Marquez, U.M. Impact of thermoplastic extrusion process on chemical, nutritional, technological and sensory properties of gluten-free breakfast cereals from pigmented rice. Int. J. Food Sci. Technol. 2021, 56, 3218–3226. [Google Scholar] [CrossRef]
- Muñoz-Pabon, K.S.; Parra-Polanco, A.S.; Roa-Acosta, D.F.; Hoyos-Concha, J.L.; Bravo-Gomez, J.E. Physical and Paste Properties Comparison of Four Snacks Produced by High Protein Quinoa Flour Extrusion Cooking. Front. Sustain. Food Syst. 2022, 6, 54. [Google Scholar] [CrossRef]
- Lakshmi, S.; Goudar, G.; Singh, M.; Dhaliwal, H.S.; Sharma, P.; Longvah, T. Variability in resistant starch, vitamins, carotenoids, phytochemicals and in-vitro antioxidant properties among diverse pigmented grains. J. Food Meas. Charact. 2021, 15, 2774–2789. [Google Scholar] [CrossRef]
- Gomez Cahuata, J.F.; Rosas-Quina, Y.E.; Pachari Vera, E. Cañihua ( Aellen) a promising superfood in food industry: A review. Nutr. Food Sci. 2022, 52, 917–928. [Google Scholar] [CrossRef]
- Wong, D.W.S. Colorants. In Mechanism and Theory in Food Chemistry, 2nd ed.; Wong, D.W.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 169–218. [Google Scholar] [CrossRef]
- Korczak, R.; Slavin, J.L. Definitions, regulations, and new frontiers for dietary fiber and whole grains. Nutr. Rev. 2020, 78, 6–12. [Google Scholar] [CrossRef]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can sprouting reduce phytate and improve the nutritional composition and nutrient bioaccessibility in cereals and legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef]
- de Bie, T.H.; Balvers, M.G.J.; de Vos, R.C.H.; Witkamp, R.F.; Jongsma, M.A. The influence of a tomato food matrix on the bioavailability and plasma kinetics of oral gamma-aminobutyric acid (GABA) and its precursor glutamate in healthy men. Food Funct. 2022, 13, 8399–8410. [Google Scholar] [CrossRef]
- Dust, J.M.; Gajda, A.M.; Flickinger, E.A.; Burkhalter, T.M.; Merchen, N.R.; Fahey, G.C. Extrusion Conditions Affect Chemical Composition and in Vitro Digestion of Select Food Ingredients. J. Agric. Food Chem. 2004, 52, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Cao, Y.; Augustin, M. Extrusion of apple pomace increases antioxidant activity upon in vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef] [PubMed]



| Test | Flour Blend Ratio | ||
|---|---|---|---|
| CG | SQF | SCF | |
| 1 | 100 | 0 | 0 |
| 2 | 0 | 100 | 0 |
| 3 | 0 | 0 | 100 |
| 4 | 50 | 50 | 0 |
| 5 | 50 | 0 | 50 |
| 6 | 0 | 50 | 50 |
| 7 | 66.67 | 16.67 | 16.67 |
| 8 | 16.67 | 66.67 | 16.67 |
| 9 | 16.67 | 16.67 | 66.67 |
| 10 | 33.33 | 33.33 | 33.33 |
| 11 | 33.33 | 33.33 | 33.33 |
| 12 | 33.33 | 33.33 | 33.33 |
| Parameters | CG | SQF | SCF |
|---|---|---|---|
| Moisture | 11.12 ± 0.74 b | 8.94 ± 0.26 a | 12.03 ± 0.13 b |
| Starch | 71.15 ± 0.12 c | 55.84± 0.52 b | 41.21 ± 1.47 a |
| TDF | 5.06 ± 0.74 a | 18.84 ± 1.20 b | 22.16 ± 0.83 c |
| IDF | 1.19 ± 0.33 a | 12.34 ± 1.06 b | 15.28 ± 0.95 c |
| SDF | 3.87 ± 1.08 a | 6.14 ± 0.14 b | 6.88 ± 0.12 b |
| Protein | 7.22 ± 0.05 a | 23.36 ± 4.38 b | 19.11 ± 0.27 b |
| Fat | 3.95 ± 0.02 a | 6.55 ± 0.11 b | 6.23 ± 0.25 b |
| Ash | 0.85 ± 0.09 a | 3.66 ± 0.11 c | 2.68 ± 0.05 b |
| PA | 0.37 ± 0.01 a | 0.58 ± 0.01 b | 0.56 ± 0.03 b |
| GABA | 4.02 ± 0.03 a | 55.43 ± 2.37 b | 51.55 ± 4.35 b |
| TSPC | 171.11 ± 8.95 a | 525.50 ± 38.14 b | 1545.09 ± 111.33 c |
| ORAC | 23.47 ± 1.32 a | 45.30 ± 3.96 b | 114.92 ± 14.17 c |
| Test | CG (x1) | SQF (x2) | SCF (x3) | PA (g/100 g) | GABA (mg/100 g) | TSPC (mg GAE/100 g) | ORAC (µmol TE/g) |
|---|---|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 0.36 ± 0.03 | 5.19 ± 0.18 | 141.1 ± 11.2 | 19.10 ± 1.48 |
| 2 | 0 | 100 | 0 | 0.53 ± 0.04 | 41.02 ± 0.46 | 910.4 ± 60.4 | 71.52 ± 3.51 |
| 3 | 0 | 0 | 100 | 0.62 ± 0.01 | 33.67 ± 0.62 | 1917.7 ± 149.1 | 122.67 ± 2.50 |
| 4 | 50 | 50 | 0 | 0.50 ± 0.01 | 25.56 ± 1.16 | 399.0 ± 10.3 | 33.88 ± 2.65 |
| 5 | 50 | 0 | 50 | 0.46 ± 0.01 | 22.81 ± 0.36 | 908.9 ± 57.3 | 75.27 ± 1.65 |
| 6 | 0 | 50 | 50 | 0.57 ± 0.01 | 27.13 ± 0.45 | 1320.97± 99.2 | 103.84 ± 3.43 |
| 7 | 66.67 | 16.67 | 16.67 | 0.35 ± 0.03 | 22.83 ± 1.03 | 265.6 ± 73.5 | 32.60 ± 0.80 |
| 8 | 16.67 | 66.67 | 16.67 | 0.52 ± 0.00 | 34.72 ± 2.94 | 839.56 ± 43.7 | 70.38 ± 2.86 |
| 9 | 16.67 | 16.67 | 66.67 | 0.47 ± 0.02 | 28.40 ± 0.12 | 1592.6 ± 139.5 | 81.79 ± 3.70 |
| 10 | 33.33 | 33.33 | 33.33 | 0.42 ± 0.05 | 36.88 ± 0.31 | 713.88 ± 66.1 | 52.00 ± 0.65 |
| 11 | 33.33 | 33.33 | 33.33 | 0.41 ± 0.03 | 36.66 ± 0.31 | 698.1 ± 35.3 | 48.19 ± 0.05 |
| 12 | 33.33 | 33.33 | 33.33 | 0.38 ± 0.03 | 37.10 ± 0.31 | 660.4 ± 26.7 | 44.99 ± 0.84 |
| Tests | CG (X1) | SQF (X2) | SCF (X3) | EI | BD (g/cm3) | SF (N) | SW (N·s) | WAI (g gel/g dw) | WSI (%) | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 0 | 0 | 2.10 ± 0.06 | 0.22 ± 0.02 | 32.48 ± 3.17 | 331.66 ± 27.53 | 4.48 ± 0.18 | 8.44 ± 0.16 | 61.23 ± 1.41 | 5.99 ± 0.21 | 37.65 ± 0.55 |
| 2 | 0 | 100 | 0 | 1.00 ± 0.08 | 0.46 ± 0.07 | 21.24 ± 2.56 | 67.05 ± 10.24 | 5.96 ± 0.14 | 24.75 ± 1.46 | 41.59 ± 0.29 | 8.80 ± 0.18 | 21.08 ± 0.40 |
| 3 | 0 | 0 | 100 | 0.94 ± 0.02 | 0.73 ± 0.09 | 10.16 ± 1.25 | 14.11 ± 2.25 | 4.06 ± 0.01 | 24.85 ± 0.09 | 34.89 ± 1.07 | 8.03 ± 0.20 | 20.63 ± 0.75 |
| 4 | 50 | 50 | 0 | 1.41 ± 0.07 | 0.19 ± 0.02 | 15.46 ± 1.69 | 88.86 ± 13.24 | 5.30 ± 0.10 | 15.38 ± 0.07 | 51.61 ± 0.56 | 6.96 ± 0.09 | 22.67 ± 0.52 |
| 5 | 50 | 0 | 50 | 1.04 ± 0.09 | 0.72 ± 0.10 | 37.36 ± 4.00 | 48.68 ± 5.96 | 4.01 ± 0.17 | 17.63 ± 0.82 | 41.78 ± 1.27 | 8.14 ± 0.19 | 23.50 ± 0.88 |
| 6 | 0 | 50 | 50 | 0.88 ± 0.11 | 0.60 ± 0.05 | 26.92 ± 5.78 | 27.93 ± 5.43 | 4.55 ± 0.11 | 21.78 ± 0.74 | 34.17 ± 0.46 | 10.73 ± 0.37 | 20.52 ± 0.42 |
| 7 | 66.67 | 16.67 | 16.67 | 1.47 ± 0.04 | 0.32 ± 0.05 | 23.31 ± 2.59 | 94.32 ± 16.85 | 4.77 ± 0.23 | 15.65 ± 1.65 | 50.35 ± 0.97 | 6.43 ± 0.08 | 25.73 ± 0.28 |
| 8 | 16.67 | 66.67 | 16.67 | 1.00 ± 0.05 | 0.40 ± 0.05 | 22.62 ± 1.89 | 42.80 ± 27.53 | 4.89 ± 0.07 | 17.83 ± 0.33 | 38.18 ± 0.95 | 9.22 ± 0.10 | 21.36 ± 0.43 |
| 9 | 16.67 | 16.67 | 66.67 | 1.11 ± 0.09 | 0.56 ± 0.08 | 49.96 ± 6.97 | 51.10 ± 5.73 | 3.07 ± 0.47 | 20.27 ± 0.19 | 36.92 ± 1.15 | 9.13 ± 0.16 | 22.52 ± 0.50 |
| 10 | 33.33 | 33.33 | 33.33 | 1.09 ± 0.03 | 0.66 ± 0.07 | 58.41 ± 8.13 | 78.34 ± 10.56 | 3.76 ± 0.01 | 16.55 ± 0.12 | 41.56 ± 0.51 | 7.46 ± 0.25 | 22.82 ± 0.81 |
| 11 | 33.33 | 33.33 | 33.33 | 1.05 ± 0.05 | 0.66 ± 0.07 | 57.52 ± 5.41 | 81.26 ± 8.72 | 3.76 ± 0.08 | 16.42 ± 0.22 | 41.70 ± 0.36 | 7.55 ± 0.12 | 23.17 ± 0.36 |
| 12 | 33.33 | 33.33 | 33.33 | 1.05 ± 0.07 | 0.67 ± 0.07 | 57.30 ± 7.35 | 76.93 ± 7.51 | 3.74 ± 0.06 | 16.50 ± 0.15 | 40.89 ± 0.35 | 7.73 ± 0.08 | 22.53 ± 0.32 |
| Independent Variables | Criteria | Lower Level | Upper Level | Importance Level | Solution 1 (OPM1) | Solution 2 (OPM2) | ||
|---|---|---|---|---|---|---|---|---|
| CG (%) | in range | 0 | 1 | 3 | 0 | 24 | ||
| SQF (%) | in range | 0 | 1 | 3 | 14 | 17 | ||
| SCF (%) | in range | 0 | 1 | 3 | 86 | 59 | ||
| Response Variables | Criteria | Lower Level | Upper Level | Importance level | Predicted Values | Experimental Values | Predicted Values | Experimental Values |
| PA | minimize | 0.35 | 0.62 | 5 | 0.54 | 0.33 | 0.47 | 0.23 |
| GABA | maximize | 5.19 | 41.02 | 5 | 29.48 | 37.10 | 30.80 | 41.81 |
| TPC | maximize | 196.30 | 1917.75 | 5 | 1817.17 | 1511.46 | 1168.84 | 972.7 |
| ORAC | maximize | 19.10 | 122.67 | 5 | 115.78 | 101.79 | 73.78 | 69.62 |
| EI | maximize | 0.88 | 2.10 | 1 | 0.96 | 0.88 | 0.96 | 1.03 |
| BD | minimize | 0.19 | 0.73 | 1 | 0.67 | 0.71 | 0.70 | 0.72 |
| SW | minimize | 14.11 | 331.66 | 5 | 25.78 | 33.18 | 39.31 | 88.68 |
| L* | in range | 47.41 | 76.30 | 5 | 50.93 | 39.16 | 53.61 | 39.46 |
| a* | maximize | 4.87 | 8.01 | 5 | 7.41 | 7.00 | 7.39 | 7.55 |
| b* | minimize | 14.06 | 36.40 | 5 | 17.12 | 17.94 | 19.46 | 19.99 |
| WAI | minimize | 3.07 | 5.96 | 5 | 3.91 | 4.18 | 3.61 | 3.41 |
| WSI | maximize | 8.44 | 24.85 | 5 | 22.95 | 22.71 | 19.27 | 17.84 |
| Desirability | 0.7420 | - | 0.6451 | - | ||||
| Parameters | Control | OPM1 | OPM2 |
|---|---|---|---|
| Undigested extrudates (time endpoint = 0 min) | |||
| Starch (g/100 g dw) | 71.14 ± 0.12 c | 33.93 ± 0.20 a | 44.05 ± 1.39 b |
| TDF (g/100 g dw) | 9.88 ± 0.14 a | 32.95 ± 1.71 c | 26.69 ± 2.41 b |
| IDF (g/100 g dw) | 6.87 ± 0.18 a | 24.35 ± 2.41 c | 18.04 ± 0.46 b |
| SDF (g/100 g dw) | 3.02 ± 0.04 a | 8.59 ± 0.69 b | 8.65 ± 2.87 b |
| Protein (g/100 g dw) | 7.22 ± 0.05 a | 16.28 ± 0.19 c | 13.73 ± 0.50 b |
| Fat (g/100 g dw) | 3.95 ± 0.02 a | 6.69 ± 0.02 c | 4.81 ± 0.24 b |
| Ash (g/100 g dw) | 0.85 ± 0.09 a | 3.64 ± 1.23 c | 1.82 ± 0.10 b |
| PA (g/100 g dw) | 0.36 ± 0.03 b,C | 0.33 ± 0.03 b,C | 0.23 ± 0.07 a,B |
| GABA (mg/100 g) | 5.19 ± 0.18 a,B | 37.10 ± 0.40 b,A | 41.81 ± 0.53 c,A |
| TSPC (mg GAE/100 g) | 196.3 ± 5.0 a,A | 1511.5 ± 24.7 c,A | 973.3 ± 23.0 b,A |
| ORAC (μmol TE/g) | 19.85 ± 2.60 a,A | 101.79 ± 1.53 c,A | 69.62 ± 1.17 b,A |
| Gastric digestates (time endpoint = 120 min) | |||
| PA (g/100 g dw) | 0.19 ± 0.03 a,B | 0.17 ± 0.01 a,B | 0.21 ± 0.04 a,B |
| GABA (mg/100 g) | 2.13 ± 0.03 a,A | 36.88 ± 2.63 b,A | 42.72 ± 3.60 b,A |
| TSPC (mg GAE/100 g) | 270.5 ± 18.9 a,B | 2493.9 ± 59.7 c,C | 1816.2 ± 98.7 b,C |
| ORAC (μmol TE/g) | 57.11 ± 2.42 a,B | 118.09 ± 6.28 c,B | 82.82 ± 11.51 b,B |
| Intestinal digestates (time endpoint = 240 min) | |||
| PA (g/100 g dw) | 0.08 ± 0.03 a,A | 0.14 ± 0.10 b,A | 0.17 ± 0.01 b,A |
| GABA (mg/100 g) | 11.32 ± 0.38 a,C | 34.34 ± 1.60 b,A | 38.90 ± 0.47 b,A |
| TSPC (mg GAE/100 g) | 821.3 ± 49.8 a,C | 1921.9 ± 278.11 c,B | 1248.1 ± 27.4 b,B |
| ORAC (μmol TE/g) | 205.82 ± 25.61 a,C | 282.37 ± 10.57 b,C | 233.42 ± 7.14 a,C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar-Menacho, L.M.; Schmiele, M.; Lavado-Cruz, A.A.; Verona-Ruiz, A.L.; Mollá, C.; Peñas, E.; Frias, J.; Simpalo-Lopez, W.D.; Castillo-Martínez, W.E.; Martínez-Villaluenga, C. Andean Sprouted Pseudocereals to Produce Healthier Extrudates: Impact in Nutritional and Physicochemical Properties. Foods 2022, 11, 3259. https://doi.org/10.3390/foods11203259
Paucar-Menacho LM, Schmiele M, Lavado-Cruz AA, Verona-Ruiz AL, Mollá C, Peñas E, Frias J, Simpalo-Lopez WD, Castillo-Martínez WE, Martínez-Villaluenga C. Andean Sprouted Pseudocereals to Produce Healthier Extrudates: Impact in Nutritional and Physicochemical Properties. Foods. 2022; 11(20):3259. https://doi.org/10.3390/foods11203259
Chicago/Turabian StylePaucar-Menacho, Luz María, Marcio Schmiele, Alicia Anais Lavado-Cruz, Anggie Liseth Verona-Ruiz, Carmen Mollá, Elena Peñas, Juana Frias, Wilson Daniel Simpalo-Lopez, Williams Esteward Castillo-Martínez, and Cristina Martínez-Villaluenga. 2022. "Andean Sprouted Pseudocereals to Produce Healthier Extrudates: Impact in Nutritional and Physicochemical Properties" Foods 11, no. 20: 3259. https://doi.org/10.3390/foods11203259
APA StylePaucar-Menacho, L. M., Schmiele, M., Lavado-Cruz, A. A., Verona-Ruiz, A. L., Mollá, C., Peñas, E., Frias, J., Simpalo-Lopez, W. D., Castillo-Martínez, W. E., & Martínez-Villaluenga, C. (2022). Andean Sprouted Pseudocereals to Produce Healthier Extrudates: Impact in Nutritional and Physicochemical Properties. Foods, 11(20), 3259. https://doi.org/10.3390/foods11203259










