Nutrient Composition and In Vitro Fermentation Characteristics of Sorghum Depending on Variety and Year of Cultivation in Northern Italy
Abstract
1. Introduction
2. Materials and Methods
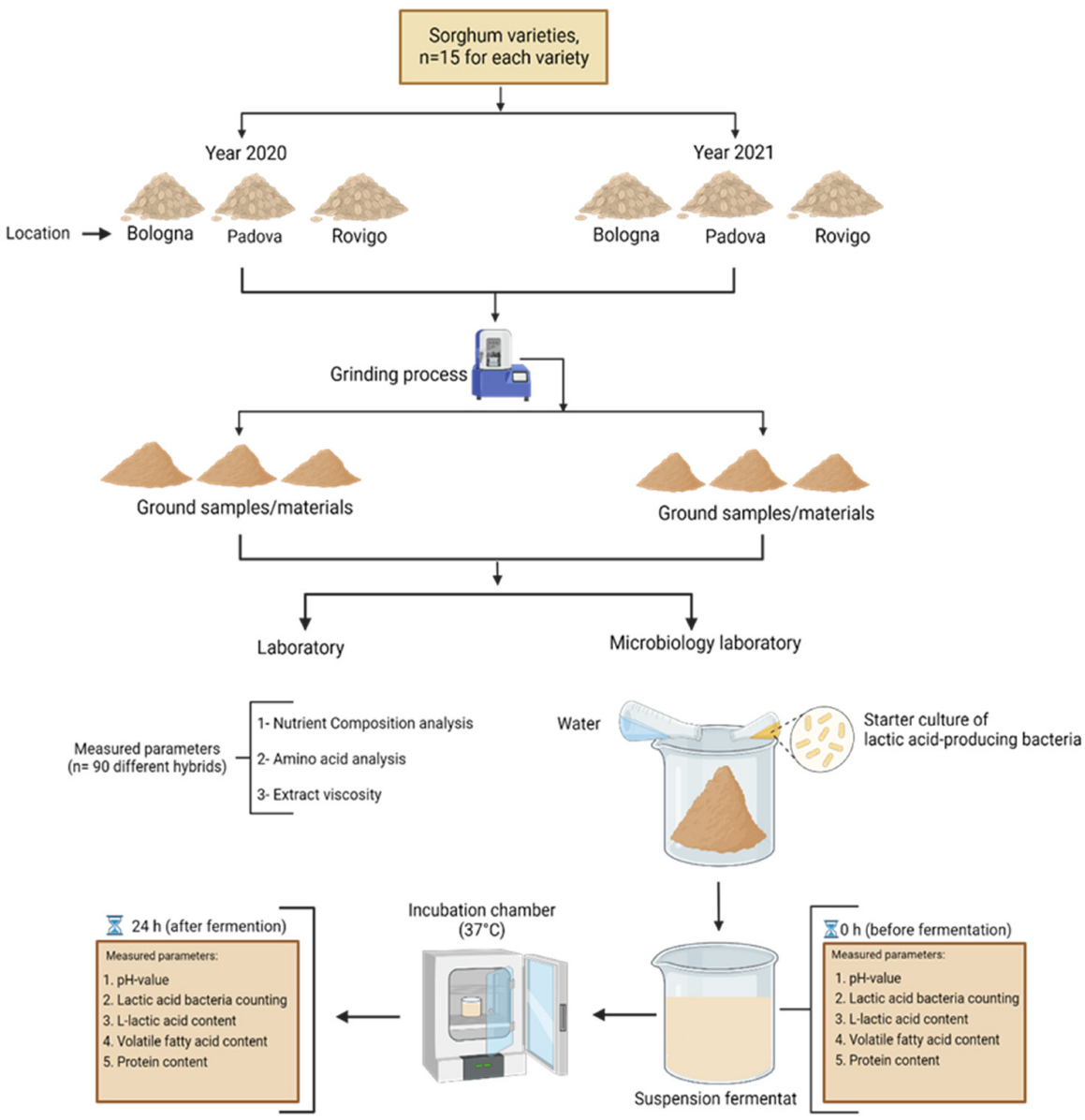
2.1. Experimental Design and Description of Samples
2.2. Grinding and Nutrient Composition
2.3. Extract Viscosity
2.4. In Vitro Fermentation
2.4.1. pH Values
2.4.2. Lactic Acid Bacteria Counts
2.4.3. L-Lactic Acid Content
2.4.4. Short Chain Fatty Acids
2.5. Statistical Analysis
3. Results
3.1. Nutrient Composition
Amino Acid Content
3.2. Viscosity
3.3. In Vitro Fermentation Characteristics of Different Sorghum Hybrids and Years from Location Bologna
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananda, G.K.; Myrans, H.; Norton, S.L.; Gleadow, R.; Furtado, A.; Henry, R.J. Wild sorghum as a promising resource for crop improvement. Front. Plant Sci. 2020, 11, 1108. [Google Scholar] [CrossRef]
- Taylor, J.; Emmambux, M. Gluten-free foods and beverages from millets. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., Bello, F.D., Eds.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Bertoft, E.; Nilsson, L. Starch: Analytical and structural aspects. In Carbohydrates in Food; CRC Press: Boca Raton, FL, USA, 2017; pp. 399–500. [Google Scholar]
- Mutisya, J.; Sathish, P.; Sun, C.; Andersson, L.; Ahlandsberg, S.; Baguma, Y.; Palmqvist, S.; Odhiambo, B.; Åman, P.; Jansson, C. Starch branching enzymes in sorghum (Sorghum bicolor) and barley (Hordeum vulgare): Comparative analyses of enzyme structure and gene expression. J. Plant Physiol. 2003, 160, 921–930. [Google Scholar] [CrossRef]
- Peng, W.; Berry, E.M. The concept of food security. Encycl. Food Secur. Sustain. 2019, 2, 1–7. [Google Scholar]
- Mendoza-Grimón, V.; Amorós, R.; Fernández-Vera, J.R.; Hernádez-Moreno, J.M.; Palacios-Díaz, M.d.P. Effect of Different Water Quality on the Nutritive Value and Chemical Composition of Sorghum bicolor Payenne in Cape Verde. Agronomy 2021, 11, 1091. [Google Scholar] [CrossRef]
- Keerthi, C.; Ranjitha, K.B.; Senthil, K.T. Sorghum mitigates climate variability and change on crop yield and quality. Planta 2021, 253, 113. [Google Scholar]
- Shinohara, T.; Hampton, J.G.; Hill, M.J. Location of deterioration within garden pea (Pisum sativum) cotyledons is associated with the timing of exposure to high temperature. N. Z. J. Crop Hortic. Sci. 2006, 34, 299–309. [Google Scholar] [CrossRef]
- Egli, D.; TeKrony, D.; Heitholt, J.; Rupe, J. Air temperature during seed filling and soybean seed germination and vigor. Crop Sci. 2005, 45, 1329–1335. [Google Scholar] [CrossRef]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Assefa, Y.; Staggenborg, S.A.; Prasad, V.P. Grain sorghum water requirement and responses to drought stress: A review. Crop Manag. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Rooney, L.; Miller, F.; Mertin, J. Variation in the structure and kernel characteristics of sorghum. In Proceedings of the International Symposium on Sorghum Grain Quality, Patancheru, India, 28–31 October 1981; pp. 143–162. [Google Scholar]
- Dicko, M.H.; Gruppen, H.; Traoré, A.S.; Voragen, A.G.; Van Berkel, W.J. Sorghum grain as human food in Africa: Relevance of content of starch and amylase activities. Afr. J. Biotechnol. 2006, 5, 384–395. [Google Scholar]
- Truong, H.H.; Liu, S.Y.; Selle, P.H. Starch utilisation in chicken-meat production: The foremost influential factors. Anim. Prod. Sci. 2015, 56, 797–814. [Google Scholar] [CrossRef]
- Casa, A.M.; Pressoir, G.; Brown, P.J.; Mitchell, S.E.; Rooney, W.L.; Tuinstra, M.R.; Franks, C.D.; Kresovich, S. Community resources and strategies for association mapping in sorghum. Crop Sci. 2008, 48, 30–40. [Google Scholar] [CrossRef]
- Khempaka, S.; Thongkratok, R.; Okrathok, S.; Molee, W. An evaluation of cassava pulp feedstuff fermented with A. oryzae, on growth performance, nutrient digestibility and carcass quality of broilers. J. Poult. Sci. 2014, 51, 71–79. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I. Haematological and biochemical parameters of broilers fed cassava pulp fermented with filamentous fungi isolated from the Indonesian fermented dried cassava. Livest. Res. Rural Dev. 2016, 28, 1–6. [Google Scholar]
- Kil, H.Y.; Seong, E.S.; Ghimire, B.K.; Chung, I.-M.; Kwon, S.S.; Goh, E.J.; Heo, K.; Kim, M.J.; Lim, J.D.; Lee, D. Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem. 2009, 115, 1234–1239. [Google Scholar] [CrossRef]
- Windpassinger, S.M. Breeding Strategies for the Adaptation of Sorghum (Sorghum Bicolor L. Moench) as a Novel Crop for Temperate Europe; Verlag Nicht Ermittelbar: Frankfurt, Germany, 2016. [Google Scholar]
- Pan, L.; An, D.; Zhu, W. Low-tannin sorghum grain could be used as an alternative to corn in diet for nursery pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 890–897. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Puntigam, R.; Brugger, D.; Slama, J.; Inhuber, V.; Boden, B.; Krammer, V.; Schedle, K.; Wetscherek-Seipelt, G.; Wetscherek, W. The effects of a partial or total replacement of ground corn with ground and whole-grain low-tannin sorghum (Sorghum bicolor (L.) Moench) on zootechnical performance, carcass traits and apparent ileal amino acid digestibility of broiler chickens. Livest. Sci. 2020, 241, 104187. [Google Scholar] [CrossRef]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar] [CrossRef]
- Yip, R.; Ramakrishnan, U. Experiences and Challenges in Developing Countries. J. Nutr. 2002, 132, 827S–830S. [Google Scholar] [CrossRef]
- Black, R.E. Zinc Deficiency, Infectious Disease and Mortality in the Developing World. J. Nutr. 2003, 133, 1485S–1489S. [Google Scholar] [CrossRef]
- Shali, T.; Singh, C.; Goindi, G. Prevalence of anemia amongst pregnant mothers and children in Delhi. Indian J. Pediatr. 2004, 71, 946. [Google Scholar] [CrossRef]
- Lee, S.M.; Pan, B.S. Effect of dietary sorghum distillery residue on hematological characteristics of cultured grey mullet (Mugil cephalus)—An animal model for prescreening antioxidant and blood thinning activities. J. Food Biochem. 2003, 27, 1–18. [Google Scholar] [CrossRef]
- Taylor, J.R.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Ikemefuna, C.; Obizoba, J.; Atii, J. Effects of soaking, sprouting, fermentation and cooking on nutrient composition and some antinutritional factors of sorghum (Guinesia) seeds. Plant Foods Hum. Nutr. 1991, 41, 203–212. [Google Scholar]
- Adebo, O.A. African sorghum-based fermented foods: Past, current and future prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef]
- Puntigam, R.; Slama, J.; Brugger, D.; Leitner, K.; Schedle, K.; Wetscherek-Seipelt, G.; Wetscherek, W. Fermentation of whole grain sorghum (Sorghum bicolor (L.) moench) with different dry matter concentrations: Effect on the apparent total tract digestibility of energy, crude nutrients and minerals in growing pigs. Animals 2021, 11, 1199. [Google Scholar] [CrossRef]
- Niba, A.; Beal, J.; Kudi, A.; Brooks, P. Bacterial fermentation in the gastrointestinal tract of non-ruminants: Influence of fermented feeds and fermentable carbohydrates. Trop. Anim. Health Prod. 2009, 41, 1393–1407. [Google Scholar] [CrossRef]
- Zentek, J.; Boroojeni, F.G. (Bio) Technological processing of poultry and pig feed: Impact on the composition, digestibility, anti-nutritional factors and hygiene. Anim. Feed Sci. Technol. 2020, 268, 114576. [Google Scholar] [CrossRef]
- Olstorpe, M.; Lyberg, K.; Lindberg, J.E.; Schnürer, J.; Passoth, V. Population diversity of yeasts and lactic acid bacteria in pig feed fermented with whey, wet wheat distillers’ grains, or water at different temperatures. Appl. Environ. Microbiol. 2008, 74, 1696–1703. [Google Scholar] [CrossRef]
- Osman, A.; Hartung, C.B.; Lingens, J.B.; Rohn, K.; Schreiner, T.; Ahmed, M.F.E.; Hankel, J.; Abd El-Wahab, A.; Visscher, C. Fermentation Characteristics of Rye and Sorghum Depending on Water: Feed Ratio. Fermentation 2022, 8, 155. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Kayitesi, E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- Masebe, K.M.; Adebo, O.A. Production and quality characteristics of a probiotic beverage from watermelon (Citrullus lanatus). In Proceedings of the 17th Johannesburg International Conference on Science, Engineering, Technology and Waste Management (SETWM-19), Johannesburg, South Africa, 18–19 November 2019; pp. 18–19. [Google Scholar]
- Haggard, P.; de Boer, L. Oral somatosensory awareness. Neurosci. Biobehav. Rev. 2014, 47, 469–484. [Google Scholar] [CrossRef]
- Fenghour, A.; Wakeham, W.A.; Vesovic, V. The viscosity of carbon dioxide. J. Phys. Chem. Ref. Data 1998, 27, 31–44. [Google Scholar] [CrossRef]
- Malleshi, N.; Desikachar, H. Reducing the paste viscosity (dietary bulk) of roller dried weaning foods using malt flour or fungal amylase. J. Food Sci. Technol. 1988, 25, 1–3. [Google Scholar]
- Mahgoub, S. Production and evaluation of weaning foods based on sorghum and legumes. Plant Foods Hum. Nutr. 1999, 54, 29–42. [Google Scholar] [CrossRef]
- Naumann, C.; Bassler, R. Methoden der landwirtschaftlichen Forschungs-und Untersuchungsanstalt, Biochemische Untersuchung von Futtermitteln. In Methodenbuch III (Einschließlich Der Achten Ergänzungen); DLUFA-Geschäftsstelle: Darmstadt, Germany, 2012. [Google Scholar]
- Dusel, G.; Kluge, H.; Glaser, K.; Simon, O.; Hartmann, G.; Lengerken, J.; Jeroch, H. An investigation into the variability of extract viscosity of wheat-relationship with the content of non-starch-polysaccharide fractions and metabolisable energy for broiler chickens. Arch. Anim. Nutr. 1997, 50, 121–135. [Google Scholar] [CrossRef]
- Bunte, S.; Grone, R.; Keller, B.; Keller, C.; Galvez, E.; Strowig, T.; Kamphues, J.; Hankel, J. Intestinal microbiota of fattening pigs offered non-fermented and fermented liquid feed with and without the supplementation of non-fermented coarse cereals. Microorganisms 2020, 8, 638. [Google Scholar] [CrossRef]
- Pontieri, P.; Del Giudice, L. Sorghum: A novel and healthy food. Encycl. Food Health 2016, 33–42. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Taleon, V.; Dykes, L.; Rooney, W.; Rooney, L. Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J. Cereal Sci. 2012, 56, 470–475. [Google Scholar] [CrossRef]
- Awika, J.M. Sorghum: Its unique nutritional and health-promoting attributes. In Gluten-Free Ancient Grains; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–54. [Google Scholar]
- Pontieri, P.; Troisi, J.; Di Fiore, R.; Di Maro, A.; Bean, S.R.; Tuinstra, M.R.; Roemer, E.; Boffa, A.; Giudice, A.D.; Pizzolante, G. Mineral contents in grains of seven food-grade sorghum hybrids grown in a Mediterranean environment. Aust. J. Crop Sci. 2014, 8, 1550–1559. [Google Scholar]
- Gerrano, A.; Labuschagne, M.; Van Biljon, A.; Shargie, N. Quantification of mineral composition and total protein content in sorghum [Sorghum bicolor (L.) Moench] genotypes. Cereal Res. Commun. 2016, 44, 272–285. [Google Scholar] [CrossRef]
- Jambunathan, R. Improvement of the nutritional quality of sorghum and pearl millet. Food Nutr. Bull. 1980, 2, 11–16. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Effect of soaking, cooking, germination and fermentation processing on proximate analysis and mineral content of three white sorghum varieties (Sorghum bicolor L. Moench). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 92–98. [Google Scholar]
- White, C.; Lopez, F.; McDowell, L. Effect of virginiamycin and fat on the utilization of grain sorghum-soyabean meal diets fed to pig. Int. J. Anim. Sci. 2003, 17, 13–24. [Google Scholar]
- Serna-Saldivar, S.; Clegg, C.; Rooney, L. Effects of parboiling and decortication on the nutritional value of sorghum (Sorghum bicolor L. Moench) and pearl millet (Pennisetum glaucum L.). J. Cereal Sci. 1994, 19, 83–89. [Google Scholar] [CrossRef]
- Szentmihályi, K.; Kéry, Á.; Then, M.; Lakatos, B.; Sándor, Z.; Vinkler, P. Potassium–sodium ratio for the characterization of medicinal plant extracts with diuretic activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1998, 12, 163–166. [Google Scholar] [CrossRef]
- Saxholt, E.; Christensen, A.T.; Møller, A.; Hartkopp, H.B.; Hess Ygil, K.; Hels, O.H. Danish Food Composition Databank, Revision 7; Department of Nutrition, National Food Institute, Technical University of Denmark: Lyngby, Denmark, 2008. [Google Scholar]
- Kumar, A.A.; Reddy, B.V.; Sahrawat, K.; Ramaiah, B. Combating micronutrient malnutrition: Identification of commercial sorghum cultivars with high grain iron and zinc. J. SAT Agric. Res. 2010, 8, 1–5. [Google Scholar]
- Argyrakopoulou, G.; Simati, S.; Dimitriadis, G.; Kokkinos, A. How important is eating rate in the physiological response to food intake, control of body weight, and glycemia? Nutrients 2020, 12, 1734. [Google Scholar] [CrossRef]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef] [PubMed]
- Smits, C.H.; Annison, G. Non-starch plant polysaccharides in broiler nutrition–towards a physiologically valid approach to their determination. World’s Poult. Sci. J. 1996, 52, 203–221. [Google Scholar] [CrossRef]
- Bedford, M.R.; Classen, H.L. Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. J. Nutr. 1992, 122, 560–569. [Google Scholar] [PubMed]
- Van Krimpen, M.; Torki, M.; Schokker, D. Effects of rye inclusion in grower diets on immune competence-related parameters and performance in broilers. Poult. Sci. 2017, 96, 3324–3337. [Google Scholar] [CrossRef]
- Choct, M.; Annison, G. Anti-nutritive activity of wheat pentosans in broiler diets. Br. Poult. Sci. 1990, 31, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef]
- Prakash, J. Safety of fermented cereals and legumes. In Regulating Safety of Traditional and Ethnic Foods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 283–310. [Google Scholar]
- Correia, I.; Nunes, A.; Guedes, S.; Barros, A.S.; Delgadillo, I. Screening of lactic acid bacteria potentially useful for sorghum fermentation. J. Cereal Sci. 2010, 52, 9–15. [Google Scholar] [CrossRef]
- Taylor, J.R.; Duodu, K.G. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agric. 2015, 95, 225–237. [Google Scholar] [CrossRef]
- Correia, I.; Nunes, A.; Barros, A.S.; Delgadillo, I. Comparison of the effects induced by different processing methods on sorghum proteins. J. Cereal Sci. 2010, 51, 146–151. [Google Scholar] [CrossRef]
- Scholten, R.H.; van der Peet-Schwering, C.D.; den Hartog, L.A.; Balk, M.; Schrama, J.; Verstegen, M. Fermented wheat in liquid diets: Effects on gastrointestinal characteristics in weanling piglets. J. Anim. Sci. 2002, 80, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Scholten, R.H.; Tricarico, J.M.; Brooks, P.H.; Verstegen, M.W. Fermentation of wheat: Effects of backslopping different proportions of pre-fermented wheat on the microbialand chemical composition. Arch. Anim. Nutr. 2006, 60, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Gbashi, S.; Phoku, J.Z.; Kayitesi, E. Fermented pulse-based food products in developing nations as functional foods and ingredients. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; TECNALIA Research & Innovation: Donostia-San Sebastian, Spain, 2017; pp. 77–109. [Google Scholar]
- Yousif, N.E.; El Tinay, A.H. Effect of fermentation on sorghum protein fractions and in vitro protein digestibility. Plant Foods Hum. Nutr. 2001, 56, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Duodu, K.; Taylor, J.; Belton, P.; Hamaker, B. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef]
- Osman, M.A. Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. J. Saudi Soc. Agric. Sci. 2011, 10, 1–6. [Google Scholar] [CrossRef]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of natural and Lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Biosci. 2013, 2, 46–52. [Google Scholar]
- Alavi, S.; Mazumdar, S.D.; Taylor, J.R. Modern convenient sorghum and millet food, beverage and animal feed products, and their technologies. In Sorghum and Millets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–329. [Google Scholar]
- Evers, A.D.; Rosentrater, K.A. Kent’s Technology of Cereals: An Introduction for Students of Food Science and Agriculture; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Niba, A.T.; Kouchika, H.; Kudi, A.C.; Beal, J.D.; Brooks, P.H. Effect of micro-organism and particle size on fermentation of sorghum and maize for poultry feed. Afr. J. Biotechnol. 2013, 12, 4147–4157. [Google Scholar]
| Year | Parameter | Unit | Bologna | Padova | Rovigo | p-Value |
|---|---|---|---|---|---|---|
| 2020 | Dry matter | (g/kg fresh basis) | 897 ± 7.18 | 899 ± 8.10 | 895 ± 7.80 | 0.7441 |
| Crude ash | (g/kg DM) | 16.4 ± 1.06 b | 18.7 ± 2.35 a | 16.2 ± 1.47 b | 0.0002 | |
| Crude protein | 96.5 ± 21.1 b | 124 ± 29.3 a | 109 ± 14.6 ab | 0.0044 | ||
| Crude fat | 42.7 ± 2.59 | 42.9 ± 3.10 | 42.1 ± 3.20 | 0.7563 | ||
| Crude fibre | 30.3 ± 2.10 | 32.3 ± 2.03 | 32.1± 3.75 | 0.1035 | ||
| NfE 1 | 814 ± 22.3 a | 782 ± 30.9 b | 800 ± 16.6 a | 0.0026 | ||
| Starch | 775 ± 20.4 a | 725 ± 17.2 c | 752 ± 11.9 b | ˂0.0001 | ||
| Sugar | 11.5 ± 1.41 | 11.7 ± 1.38 | 11.1 ± 2.67 | 0.7041 | ||
| Gross energy 2 | (MJ/kg DM) | 18.1 ± 0.33 | 18.0 ± 0.73 | 18.1 ± 0.43 | 0.6736 | |
| Calcium | (g/kg DM) | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.3371 | |
| Phosphorus | 3.11 ± 0.23 ab | 3.43 ± 0.49 a | 3.00 ± 0.64 b | 0.0503 | ||
| Magnesium | 1.25 ± 0.09 b | 1.45 ± 0.17 a | 1.36 ± 0.15 a | 0.0030 | ||
| Sodium | 0.09 ± 0.07 a | 0.07 ± 0.01 b | 0.07 ± 0.08 b | ˂0.0001 | ||
| Potassium | 4.03 ± 0.27 | 3.80 ± 0.25 | 3.94 ± 0.41 | 0.1127 | ||
| Copper | (mg/kg DM) | 3.50 ± 1.11 | 3.07 ± 0.51 | 3.31 ± 1.06 | 0.4644 | |
| Zinc | 18.0 ± 2.36 b | 23.2 ± 3.10 a | 21.2 ± 3.17 a | ˂0.0001 | ||
| Iron | 35.3 ± 6.92 | 39.4 ± 6.60 | 37.1 ± 4.30 | 0.1857 | ||
| Manganese | 5.65 ± 0.09 a | 5.62 ± 0.07 b | 5.62 ± 0.04 b | ˂0.0001 | ||
| 2021 | Dry matter | (g/kg fresh basis) | 912 ± 2.01 | 909 ± 2.22 | 910 ± 3.77 | 0.7351 |
| Crude ash | (g/kg DM) | 18.0 ± 2.65 | 17.3 ± 0.71 | 17.2 ± 4.02 | 0.8554 | |
| Crude protein | 116 ± 7.77 | 110 ± 10.2 | 117 ± 16.5 | 0.1228 | ||
| Crude fat | 45.0 ± 4.39 | 44.6 ± 3.11 | 41.2 ± 11.2 | 0.4114 | ||
| Crude fibre | 27.2 ± 3.01 | 26.3 ± 2.10 | 26.4 ± 3.50 | 0.4650 | ||
| NfE1 | 794 ± 12.7 | 804 ± 11.2 | 798 ± 20.9 | 0.2127 | ||
| Starch | 734 ± 12.6 | 746 ± 13.3 | 738 ± 22.1 | 0.1410 | ||
| Sugar | 13.1 ± 1.34 | 12.0 ± 1.62 | 13.1 ± 1.02 | 0.0284 | ||
| Gross energy 2 | (MJ/kg DM) | 18.0 ± 0.22 | 18.0 ± 0.15 | 18.0 ± 0.50 | 0.7799 | |
| Calcium | (g/kg DM) | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.18 ± 0.26 | 0.3787 | |
| Phosphorus | 3.43 ± 0.37 | 3.52 ± 0.70 | 3.36 ± 0.66 | 0.5716 | ||
| Magnesium | 1.52 ± 0.36 | 1.46 ± 0.08 | 1.40 ± 0.21 | 0.3781 | ||
| Sodium | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.1632 | ||
| Potassium | 4.25 ± 0.32 | 4.11 ± 0.37 | 4.10 ± 0.00 | 0.1709 | ||
| Copper | (mg/kg DM) | 2.80 ± 0.01 b | 2.81 ± 0.06 b | 5.33 ± 1.43 a | ˂0.0001 | |
| Zinc | 22.2 ± 2.18 b | 19.4 ± 2.20 c | 24.5 ± 2.70 a | ˂0.0001 | ||
| Iron | 45.9 ± 6.47 a | 32.5 ± 5.22 b | 35.1 ± 6.02 b | ˂0.0001 | ||
| Manganese | 5.54 ± 0.01 c | 6.78 ± 1.63 b | 8.86 ± 2.23 a | ˂0.0001 |
| Year | Parameter | Bologna | Padova | Rovigo | p-Value |
|---|---|---|---|---|---|
| 2020 | Arginine | 4.12 ± 0.36 | 3.73 ± 0.89 | 3.74 ± 0.24 | 0.1187 |
| Histidine | 2.31 ± 0.15 a | 2.11 ± 0.14 b | 2.18 ± 0.14 b | 0.0024 | |
| Isoleucine | 3.24 ± 0.20 | 3.70 ± 0.25 | 3.78 ± 0.22 | 0.6550 | |
| Leucine | 12.1 ± 0.75 | 12.4 ± 0.90 | 12.1 ± 0.93 | 0.5297 | |
| Lysine | 2.47 ± 0.34 a | 2.00 ± 0.19 b | 2.32 ± 0.24 a | ˂0.0001 | |
| Methionine | 2.02 ± 0.51 | 1.96 ± 0.33 | 2.50 ± 0.50 | 0.3424 | |
| Phenylalanine | 4.93 ± 0.29 | 4.92 ± 0.34 | 4.83 ± 0.24 | 0.6100 | |
| Threonine | 3.10 ± 0.15 | 3.05 ± 0.24 | 3.14 ± 0.23 | 0.4756 | |
| Valine | 4.98 ± 0.23 | 4.75 ± 0.33 | 4.92 ± 0.30 | 0.1029 | |
| Alanine | 8.58 ± 0.69 | 8.67 ± 0.58 | 8.43 ± 0.47 | 0.5355 | |
| Aspartic acid | 6.74 ± 0.46 | 6.51 ± 0.45 | 6.42 ± 0.28 | 0.1065 | |
| Cysteine | 2.56 ± 0.69 | 2.04 ± 0.51 | 2.34 ± 0.63 | 0.0808 | |
| Glutamic acid | 19.6 ± 2.53 | 21.1 ± 2.13 | 19.7 ± 1.45 | 0.1068 | |
| Proline | 7.59 ± 0.38 | 8.09 ± 0.91 | 7.60 ± 0.47 | 0.0577 | |
| Serine | 4.31 ± 0.25 | 4.20 ± 0.27 | 4.31 ± 0.24 | 0.0749 | |
| Tyrosine | 3.60 ± 0.17 a | 3.39 ± 0.43 b | 3.54 ± 0.21 ab | 0.0411 | |
| Glycine | 3.51 ± 0.38 a | 3.00 ± 0.27 b | 3.22 ± 0.26 b | 0.0002 | |
| 2021 | Arginine | 3.69 ± 0.26 | 3.84 ± 0.25 | 3.81 ± 0.28 | 0.2779 |
| Histidine | 2.18 ± 0.10 | 2.26 ± 0.23 | 2.21 ± 0.16 | 0.5221 | |
| Isoleucine | 3.92 ± 0.19 | 3.87 ± 0.08 | 3.96 ± 0.24 | 0.4580 | |
| Leucine | 12.4 ± 0.42 b | 12.0 ± 0.32 b | 13.0 ± 0.90 a | 0.0002 | |
| Lysine | 2.13 ± 0.15 b | 2.32 ± 0.17 a | 2.30 ± 0.21 a | 0.0118 | |
| Methionine | 2.16 ± 0.23 | 2.07 ± 0.21 | 2.16 ± 0.33 | 0.5759 | |
| Phenylalanine | 4.89 ± 0.13 | 5.52 ± 0.75 | 5.00 ± 0.26 | 0.1641 | |
| Threonine | 3.18 ± 0.19 | 3.39 ± 0.11 | 3.20 ± 0.33 | 0.0407 | |
| Valine | 4.86 ± 0.10 b | 5.02 ± 0.12 a | 5.05 ± 0.28 a | 0.0262 | |
| Alanine | 8.85 ± 0.26 | 8.76 ± 0.20 | 9.05 ± 0.61 | 0.1583 | |
| Aspartic acid | 6.38 ± 0.19 c | 6.63 ± 0.13 b | 6.95 ± 0.35 a | ˂0.0001 | |
| Cysteine | 2.14 ± 0.37 | 2.00 ± 0.17 | 1.96 ± 0.19 | 0.1701 | |
| Glutamic acid | 20.8 ± 0.56 | 21.2 ± 0.55 | 21.4 ± 1.47 | 0.1640 | |
| Proline | 7.71 ± 0.20 b | 7.90 ± 0.29 b | 8.23 ± 0.23 a | 0.0015 | |
| Serine | 4.27 ± 0.10 | 4.39 ± 0.13 | 4.31 ± 0.24 | 0.1845 | |
| Tyrosine | 3.50 ± 0.11 | 3.44 ± 0.10 | 3.39 ± 0.24 | 0.1828 | |
| Glycine | 3.11 ± 0.14 b | 3.33 ± 0.23 a | 3.24 ± 0.28 ab | 0.0413 |
| Year | Bologna | Padova | Rovigo | p-Value |
|---|---|---|---|---|
| 2020 | 1.33± 0.33 | 1.30 ± 0.31 | 1.20 ± 0.13 | 0.3794 |
| 2021 | 1.22 ± 0.20 a | 1.08 ± 0.07 b | 1.10 ± 0.08 b | 0.0100 |
| Parameters | Time, h | Hybrid/Year | ||||
|---|---|---|---|---|---|---|
| Hybrid1 2020 | Hybrid2 2020 | Hybrid1 2021 | Hybrid2 2021 | p-Value | ||
| pH-value | 0 | 6.32 ± 0.03 a | 6.31 ± 0.01 a | 6.15 ± 0.03 c | 6.25 ± 0.00 b | ˂0.0001 |
| Count of LAB | 3.82 ± 0.07 a | 3.71 ± 0.16 ab | 3.68 ± 0.17 ab | 3.39 ± 0.25 b | 0.0281 | |
| L-lactic acid | 0.32 ± 0.03 | 0.31 ± 0.03 | 0.28 ± 0.01 | 0.29 ± 0.01 | 0.0861 | |
| Acetic acid | 1.59 ± 0.33 b | 2.19 ± 0.28 a | 1.42 ± 0.03 b | 1.57 ± 0.15 b | 0.0022 | |
| Propionic acid | 0.04 ± 0.00 b | 0.07 ± 0.01 a | 0.04 ± 0.01 b | 0.06 ± 0.00 ab | 0.0009 | |
| Butyric acid | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.13 ± 0.08 a | 0.02 ± 0.00 b | 0.0027 | |
| Crude protein | 147 ± 2.00 b | 123 ± 2.00 c | 183 ± 2.65 a | 147 ± 3.68 b | ˂0.0001 | |
| pH-value | 24 | 3.71 ± 0.01 b | 3.88 ± 0.01 b | 3.98 ± 0.02 a | 3.81 ± 0.03 c | ˂0.0001 |
| Count of LAB | 9.39 ± 0.16 | 9.53 ±0.34 | 9.22 ± 0.04 | 9.22 ± 0.12 | 0.1354 | |
| L-lactic acid | 16.6 ± 1.60 | 16.7 ± 1.51 | 16.1 ± 1.05 | 16.9 ± 1.18 | 0.8203 | |
| Acetic acid | 2.91 ± 0.19 a | 1.87 ± 0.20 b | 3.62 ± 1.01 a | 3.16 ± 0.45 a | 0.0063 | |
| Propionic acid | 0.11 ± 0.07 | 0.11 ± 0.04 | 0.11 ± 0.02 | 0.11 ± 0.03 | 0.9988 | |
| Butyric acid | 0.49 ± 0.09 b | 0.20 ± 0.11 b | 2.19 ± 0.75 a | 1.72 ± 0.50 a | 0.0001 | |
| Crude protein | 150 ± 0.60 b | 128 ± 2.47 c | 184 ± 2.99 a | 153 ± 0.97 b | ˂0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.; Abd El-Wahab, A.; Ahmed, M.F.E.; Buschmann, M.; Visscher, C.; Hartung, C.B.; Lingens, J.B. Nutrient Composition and In Vitro Fermentation Characteristics of Sorghum Depending on Variety and Year of Cultivation in Northern Italy. Foods 2022, 11, 3255. https://doi.org/10.3390/foods11203255
Osman A, Abd El-Wahab A, Ahmed MFE, Buschmann M, Visscher C, Hartung CB, Lingens JB. Nutrient Composition and In Vitro Fermentation Characteristics of Sorghum Depending on Variety and Year of Cultivation in Northern Italy. Foods. 2022; 11(20):3255. https://doi.org/10.3390/foods11203255
Chicago/Turabian StyleOsman, Ahmed, Amr Abd El-Wahab, Marwa Fawzy Elmetwaly Ahmed, Magdalena Buschmann, Christian Visscher, Clara Berenike Hartung, and Jan Berend Lingens. 2022. "Nutrient Composition and In Vitro Fermentation Characteristics of Sorghum Depending on Variety and Year of Cultivation in Northern Italy" Foods 11, no. 20: 3255. https://doi.org/10.3390/foods11203255
APA StyleOsman, A., Abd El-Wahab, A., Ahmed, M. F. E., Buschmann, M., Visscher, C., Hartung, C. B., & Lingens, J. B. (2022). Nutrient Composition and In Vitro Fermentation Characteristics of Sorghum Depending on Variety and Year of Cultivation in Northern Italy. Foods, 11(20), 3255. https://doi.org/10.3390/foods11203255







