Conversion of Food Waste into 2,3-Butanediol via Thermophilic Fermentation: Effects of Carbohydrate Content and Nutrient Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes and Reagents
2.2. Food Waste Collection, Pretreatment, and Hydrolysis
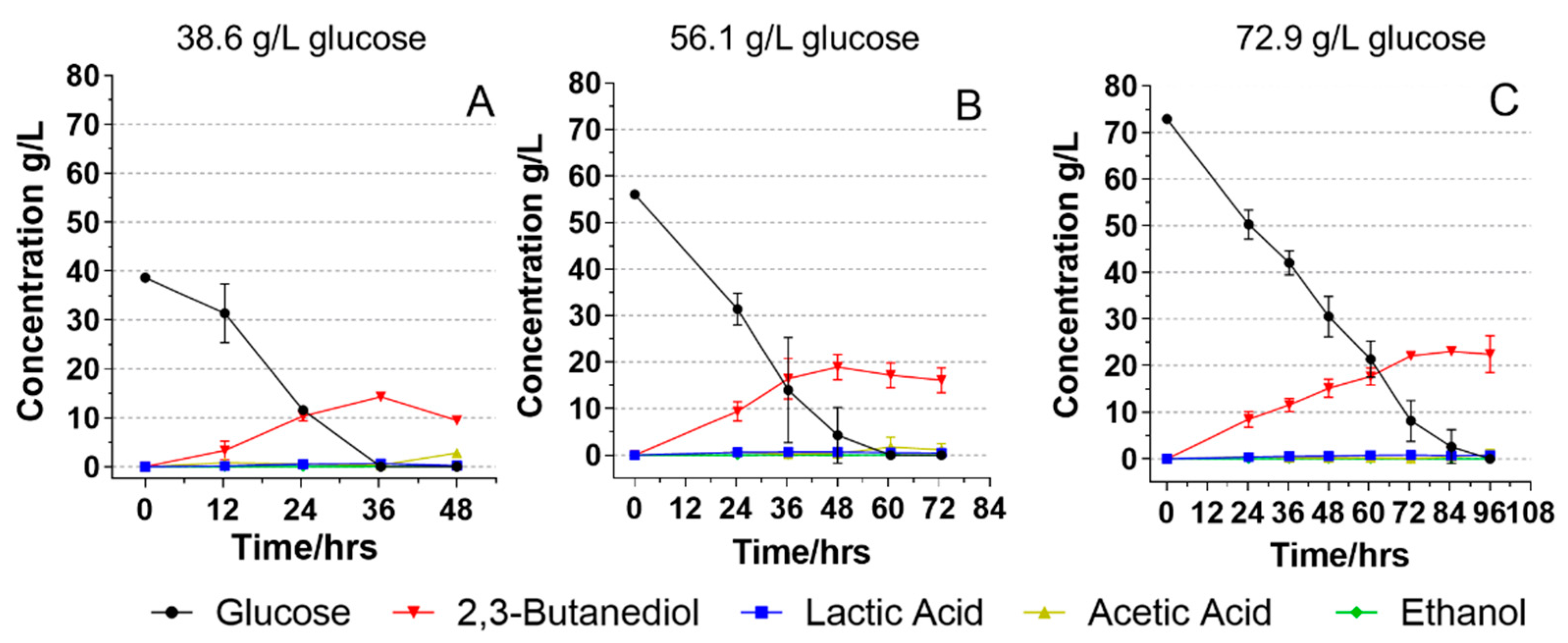
2.3. Initial Glucose Concentrations and Their Effects on Glucose Fermentation
2.4. Yeast Extract and Peptone Concentrations and Their Effects on Glucose and Food Waste Fermentation
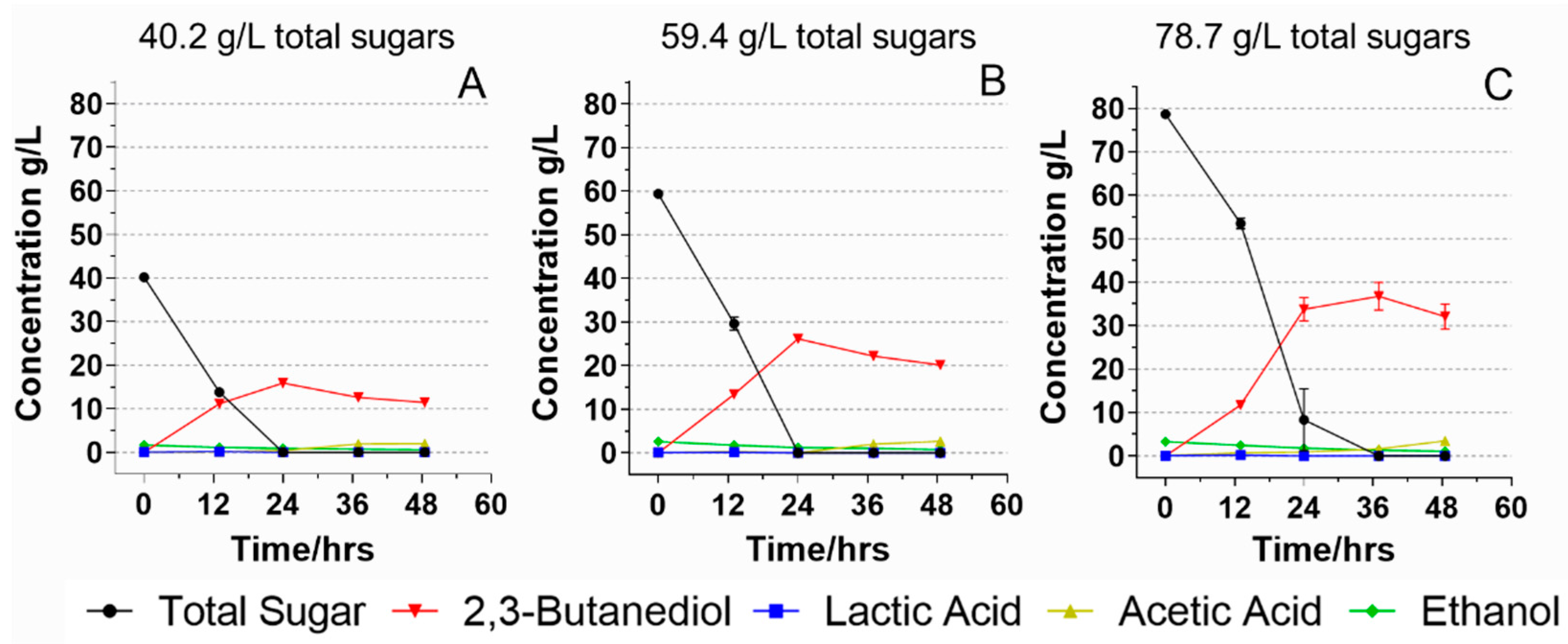
2.5. Initial Sugar Concentrations and Their Effects on Food Waste Hydrolysate Fermentation
2.6. Fermentation Sample Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fermentation on Glucose Media with Different Initial Glucose Concentrations
3.2. Fermentation of Glucose and Food Waste Hydrolysate Media with Different Yeast Extract and Peptone Concentrations
3.3. Fermentation on Food Waste Hydrolysate with Different Starting Total Sugar Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OHair, J.; Jin, Q.; Yu, D.; Wu, J.; Wang, H.; Zhou, S.; Huang, H. Non-sterile fermentation of food waste using thermophilic and alkaliphilic Bacillus licheniformis YNP5-TSU for 2,3-butanediol production. Waste Manag. 2021, 120, 248–256. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Poe, N.E.; Yu, D.; Jin, Q.; Ponder, M.A.; Stewart, A.C.; Ogejo, J.A.; Wang, H.; Huang, H. Compositional variability of food wastes and its effects on acetone-butanol-ethanol fermentation. Waste Manag. 2020, 107, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qureshi, N.; Chen, M.-H.; Liu, W.; Singh, V. Ethanol Production from Food Waste at High Solids Content with Vacuum Recovery Technology. J. Agric. Food Chem. 2015, 63, 2760–2766. [Google Scholar] [CrossRef]
- Huang, H.; Singh, V.; Qureshi, N. Butanol production from food waste: A novel process for producing sustainable energy and reducing environmental pollution. Biotechnol. Biofuels 2015, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef]
- Jin, Q.; An, Z.; Damle, A.; Poe, N.; Wu, J.; Wang, H.; Wang, Z.; Huang, H. High acetone-butanol-ethanol production from food waste by recombinant Clostridium saccharoperbutylacetonicum in batch and continuous immobilized-cell fermentation. ACS Sustain. Chem. Eng. 2020, 8, 9822–9832. [Google Scholar] [CrossRef]
- Jin, Q.; Neilson, A.P.; Stewart, A.C.; O’Keefe, S.F.; Kim, Y.-T.; McGuire, M.; Wilder, G.; Huang, H. Integrated approach for the valorization of red grape pomace: Production of oil, polyphenols, and acetone–butanol–ethanol. ACS Sustain. Chem. Eng. 2018, 6, 16279–16286. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food waste to energy: An overview of sustainable approaches for food waste management and nutrient recycling. BioMed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- Erian, A.M.; Freitag, P.; Gibisch, M.; Pflügl, S. High rate 2,3-butanediol production with Vibrio natriegens. Bioresour. Technol. Rep. 2020, 10, 100408. [Google Scholar] [CrossRef]
- Qi, G.; Kang, Y.; Li, L.; Xiao, A.; Zhang, S.; Wen, Z.; Xu, D.; Chen, S. Deletion of meso-2,3-butanediol dehydrogenase gene bud C for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnol. Biofuels 2014, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Wang, A.; Qin, J.; Li, L.; Ai, X.; Jiang, T.; Tang, H.; Xu, P. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 2009, 82, 49–57. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Du, J.; Zhu, J.G.; Ren, L.J.; Hu, N.; Li, S. Enhanced 2,3-butanediol production by Klebsiella oxytoca using a two-stage agitation speed control strategy. Bioresour. Technol. 2009, 100, 3410–3414. [Google Scholar] [CrossRef]
- O’Hair, J.; Jin, Q.; Yu, D.; Poe, N.; Li, H.; Thapa, S.; Zhou, S.; Huang, H. Thermophilic and alkaliphilic Bacillus licheniformis YNP5-TSU as an ideal candidate for 2,3-butanediol production. ACS Sustain. Chem. Eng. 2020, 8, 11244–11252. [Google Scholar] [CrossRef]
- Ge, L.; Wu, X.; Chen, J.; Wu, J. A new method for industrial production of 2,3-butanediol. Citeseer 2011, 2, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Song, C.W.; Rathnasingh, C.; Song, H. CRISPR-Cas9 mediated metabolic engineering of a mucoid Bacillus licheniformis isolate for mass production of 2,3-butanediol. Biochem. Eng. J. 2021, 175, 108141. [Google Scholar] [CrossRef]
- Petrov, K.; Petrova, P. High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl. Microbiol. Biotechnol. 2009, 84, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, M.; Lv, G.; Zhou, L.; Li, X.; Bertoluzzi, L.; Liu, C.; Zhu, S.; Zhu, J. Interfacial solar steam generation enables fast-responsive, energy-efficient, and low-cost off-grid sterilization. Adv. Mater. 2018, 30, 1805159. [Google Scholar] [CrossRef]
- OHair, J.; Jin, Q.; Li, H.; Yu, D.; He, Y.; Thapa, S.; Bhatti, S.; Huang, H.; Zhou, S. Biochemical and genomic identification of novel thermophilic Bacillus licheniformis strains YNP1-TSU, YNP2-TSU, and YNP3-TSU with potential in 2,3-butanediol production from non-sterile food waste fermentation. Food Bioprod. Process. 2021, 129, 34–45. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, X.; Huang, Y.; Huo, F.; Zhu, X.; Xi, L.; Lu, J.R. Thermophilic fermentation of acetoin and 2,3-butanediol by a novel Geobacillus strain. Biotechnol. Biofuels 2012, 5, 88. [Google Scholar] [CrossRef]
- Xiao, Z.; Gu, R.; Hou, X.; Zhao, J.; Zhu, H.; Lu, J.R. Non-sterilized fermentative production of acetoin with 2,3-butanediol as a main byproduct from maize hydrolysate by a newly isolated thermophilic Bacillus strain. J. Chem. Technol. Biotechnol. 2017, 92, 2845–2852. [Google Scholar] [CrossRef]
- Song, C.W.; Rathnasingh, C.; Park, J.M.; Lee, J.; Song, H. Isolation and evaluation of Bacillus strains for industrial production of 2,3-butanediol. J. Microbiol. Biotechnol. 2018, 28, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chen, T.; Zhao, X.; Chamu, J. Metabolic engineering of thermophilic Bacillus licheniformis for chiral pure D-2,3-butanediol production. Biotechnol. Bioeng. 2012, 109, 1610–1621. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Reddy, M.V.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-stage polyhydroxyalkanoates (PHA) production from cheese whey using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Li, K.; Wang, Y.; Gao, C.; Han, B.; Ma, C.; Xu, P. A newly isolated Bacillus licheniformis strain thermophilically produces 2,3-butanediol, a platform and fuel bio-chemical. Biotechnol. Biofuels 2013, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Liu, C.; Li, D.; Gu, Z. Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max [L.] Merr). Ind. Crops Prod. 2013, 50, 743–749. [Google Scholar] [CrossRef]
- Wang, X.; Lv, M.; Zhang, L.; Li, K.; Gao, C.; Ma, C.; Xu, P. Efficient bioconversion of 2,3-butanediol into acetoin using Gluconobacter oxydans DSM 2003. Biotechnol. Biofuels 2013, 6, 155. [Google Scholar] [CrossRef] [Green Version]
- Tsigoriyna, L.; Ganchev, D.; Petrova, P.; Petrov, K. Highly efficient 2,3-butanediol production by Bacillus licheniformis via complex optimization of nutritional and technological parameters. Ferment. 2021, 7, 118. [Google Scholar] [CrossRef]
- Białkowska, A.M.; Gromek, E.; Krysiak, J.; Sikora, B.; Kalinowska, H.; Jędrzejczak-Krzepkowska, M.; Kubik, C.; Lang, S.; Schütt, F.; Turkiewicz, M. Application of enzymatic apple pomace hydrolysate to production of 2,3-butanediol by alkaliphilic Bacillus licheniformis NCIMB 8059. J. Ind. Microbiol. Biotechnol. 2015, 42, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Jurchescu, I.-M.; Hamann, J.; Zhou, X.; Ortmann, T.; Kuenz, A.; Prüße, U.; Lang, S. Enhanced 2,3-butanediol production in fed-batch cultures of free and immobilized Bacillus licheniformis DSM 8785. Appl. Microbiol. Biotechnol. 2013, 97, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- O’hair, J.A.; Li, H.; Rangu, M.; Thapa, S.; Yang, Y.; Fish, T.; Bhatti, S.; Thannhauser, T.W.; Zhou, S. Proteomic effects of magnesium stress on biofilm associated proteins isolated from cellulolytic Bacillus licheniformis YNP5-TSU. J. Proteom. Bioinform. 2019, 12, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Hakizimana, O.; Matabaro, E.; Lee, B.H. The current strategies and parameters for the enhanced microbial production of 2,3-butanediol. Biotechnol. Rep. 2020, 25, e00397. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Jiang, T.; Li, L.; Ma, C.; Xu, P. Production of 2,3-butanediol from corncob molasses, a waste by-product in xylitol production. Appl. Microbiol. Biotechnol. 2010, 87, 965–970. [Google Scholar] [CrossRef]
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; An, A.K.; Chaiprapat, S.; Leu, S.Y. Whole sugar 2,3-butanediol fermentation for oil palm empty fruit bunches biorefinery by a newly isolated Klebsiella pneumoniae PM2. Bioresour. Technol. 2021, 333, 125206. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, K.; Wang, K.; Chen, C.; Gao, C.; Ma, C.; Xu, P. Efficient production of 2,3-butanediol from corn stover hydrolysate by using a thermophilic Bacillus licheniformis strain. Bioresour. Technol. 2014, 170, 256–261. [Google Scholar] [CrossRef]
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.J.; Koutinas, A.; Venus, J. Volumetric oxygen transfer coefficient as fermentation control parameter to manipulate the production of either acetoin or D-2,3-butanediol using bakery waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef]
- He, Y.; OHair, J.A.; Jin, Q.; Xu, Z.; Wu, J.; Huang, H. Co-Production of Protein Hydrolysates and 2,3-Butanediol from Brewer’s Spent Grain. ACS Sustain. Chem. Eng. 2021, 9, 15166–15174. [Google Scholar] [CrossRef]
- Amraoui, Y.; Prabhu, A.A.; Narisetty, V.; Coulon, F.; Chandel, A.K.; Willoughby, N.; Jacob, S.; Koutinas, A.; Kumar, V. Enhanced 2,3-Butanediol production by mutant Enterobacter ludwigii using Brewers’ spent grain hydrolysate: Process optimization for a pragmatic biorefinery loom. Chem. Eng. J. 2022, 427, 130851. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Ujor, V.; Ezeji, T.C. Production of 2,3-Butanediol from non-detoxified wheat straw hydrolysate: Impact of microbial inhibitors on Paenibacillus polymyxa DSM 365. Ind. Crops Prod. 2021, 159, 113047. [Google Scholar] [CrossRef]


| Strain | Carbon Source | Total Sugar (g/L) | Fermentation Type | Media Supplementation | Titer (g/L) | Yield (g/g) | Productivity (g/L/h) | Refs |
|---|---|---|---|---|---|---|---|---|
| Bacillus licheniformis YNP5-TSU | Cabbage | 14.2 | Shaking Flask | Yeast extract 0.5 (w/v); Peptone 0.5 (w/v) | 6.8 | 0.48 | 0.28 | [1] |
| Bacillus licheniformis YNP5-TSU | Brewer’s spent grain hydrolysate | 48.2 | Shaking Flask | Yeast extract 10 g/L; Peptone 5 g/L | 20.4 | 0.45 | 0.28 | [39] |
| Bacillus licheniformis X10 | Corn stover hydrolysate | 80.0 | Shaking Flask | Yeast extract, 5 g/L; CSLP, 14.5 g/L; Triammonium citrate, 1 g/L; Sodium acetate, 6.5 g/L; K2HPO4·3H2O, 4 g/L; MgSO4·7H2O, 0.25 g/L | 31.2 | 0.39 | 2.6 | [37] |
| Bacillus amyloliquefaciens 18,025 | Bakery waste hydrolysate | 120.0 | Bioreactor | Yeast extract 15 g/L, KH2PO4 0.5 g/L, K2HPO4 2 g/L, KCl 0.3 g/L and MnSO4·H2O 0.025 g/L | 42.1 | 0.38 | 1.56 | [38] |
| Enterobacter ludwigii | Brewer’s spent grain hydrolysate | 40.0 | Bioreactor | (NH4)2HPO4 6 g/L; (NH4)2SO4 7.2 g/L; KOH 0.45 g/L; EDTA 0.51 g/L; MgSO4·7H2O 0.3 g/L; CaCl2·6H2O 0.09 g/L; FeSO4·7H2O 0.022 g/L; MnSO4·H2O 0.0038 g/L; ZnSO4·7H2O 0.0075 g/L | 16.4 | 0.41 | 1.03 | [40] |
| Klebsiella pneumoniae PM2 | Oil palm empty fruit bunches hydrolysate | 46.0 | Bioreactor | Tryptone 5 g/L; Yeast extract 5 g/L; K2HPO4·3H2O 7 g/L; KH2PO4 5.5 g/L; MgSO4·7H2O 0.25 g/L; Na₂MoO₄·2H₂O 0.12 g/L; CaCl2·2H2O 0.021 g/L | 19.0 | 0.48 | 0.53 | [36] |
| Paenibacillus polymyxa DSM 365 | Wheat straw hydrolysate | 98.9 | Shaking Flask | Yeast extract 5 g/L; Tryptone 3.5 g/L; (NH4)2SO4 3.0 g/L; KH2PO4 3.5 g/L; K2HPO4 2.75 g/L; MgSO4 0.2 g/L; NH4 acetate 1.5 g/L; CoCl2 0.05 g/L; 3-(N-morpholino) propanesulfonic acid (MOPS) 10 g/L; Trace element solution 3 mL (per liter) | 23.4 | 0.27 | 0.28 | [41] |
| Bacillus licheniformis YNP5-TSU | Bakery waste hydrolysate | 80.0 | Shaking Flask | Yeast extract 2 g/L; Peptone 1 g/L | 36.7 | 0.47 | 0.99 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; O’Hair, J.; Poe, N.; Jin, Q.; Pinton, S.; He, Y.; Huang, H. Conversion of Food Waste into 2,3-Butanediol via Thermophilic Fermentation: Effects of Carbohydrate Content and Nutrient Supplementation. Foods 2022, 11, 169. https://doi.org/10.3390/foods11020169
Yu D, O’Hair J, Poe N, Jin Q, Pinton S, He Y, Huang H. Conversion of Food Waste into 2,3-Butanediol via Thermophilic Fermentation: Effects of Carbohydrate Content and Nutrient Supplementation. Foods. 2022; 11(2):169. https://doi.org/10.3390/foods11020169
Chicago/Turabian StyleYu, Dajun, Joshua O’Hair, Nicholas Poe, Qing Jin, Sophia Pinton, Yanhong He, and Haibo Huang. 2022. "Conversion of Food Waste into 2,3-Butanediol via Thermophilic Fermentation: Effects of Carbohydrate Content and Nutrient Supplementation" Foods 11, no. 2: 169. https://doi.org/10.3390/foods11020169
APA StyleYu, D., O’Hair, J., Poe, N., Jin, Q., Pinton, S., He, Y., & Huang, H. (2022). Conversion of Food Waste into 2,3-Butanediol via Thermophilic Fermentation: Effects of Carbohydrate Content and Nutrient Supplementation. Foods, 11(2), 169. https://doi.org/10.3390/foods11020169







