Aconitic Acid Recovery from Renewable Feedstock and Review of Chemical and Biological Applications
Abstract
1. Introduction
2. Industrial Applications
2.1. Aconitic Acid Esters for Tissue Engineering
2.2. Aconitic Acid Esters as Plasticizers
2.3. Trans-Aconitic Acid as a Cross-Linking Agent
2.4. Role in Microparticles and Grafting Agents
2.5. Additional Aconitic Acid Uses in Green Chemistry
3. Biological Roles of Aconitic Acid with Applications in Biological Engineering and Sustainable Agriculture
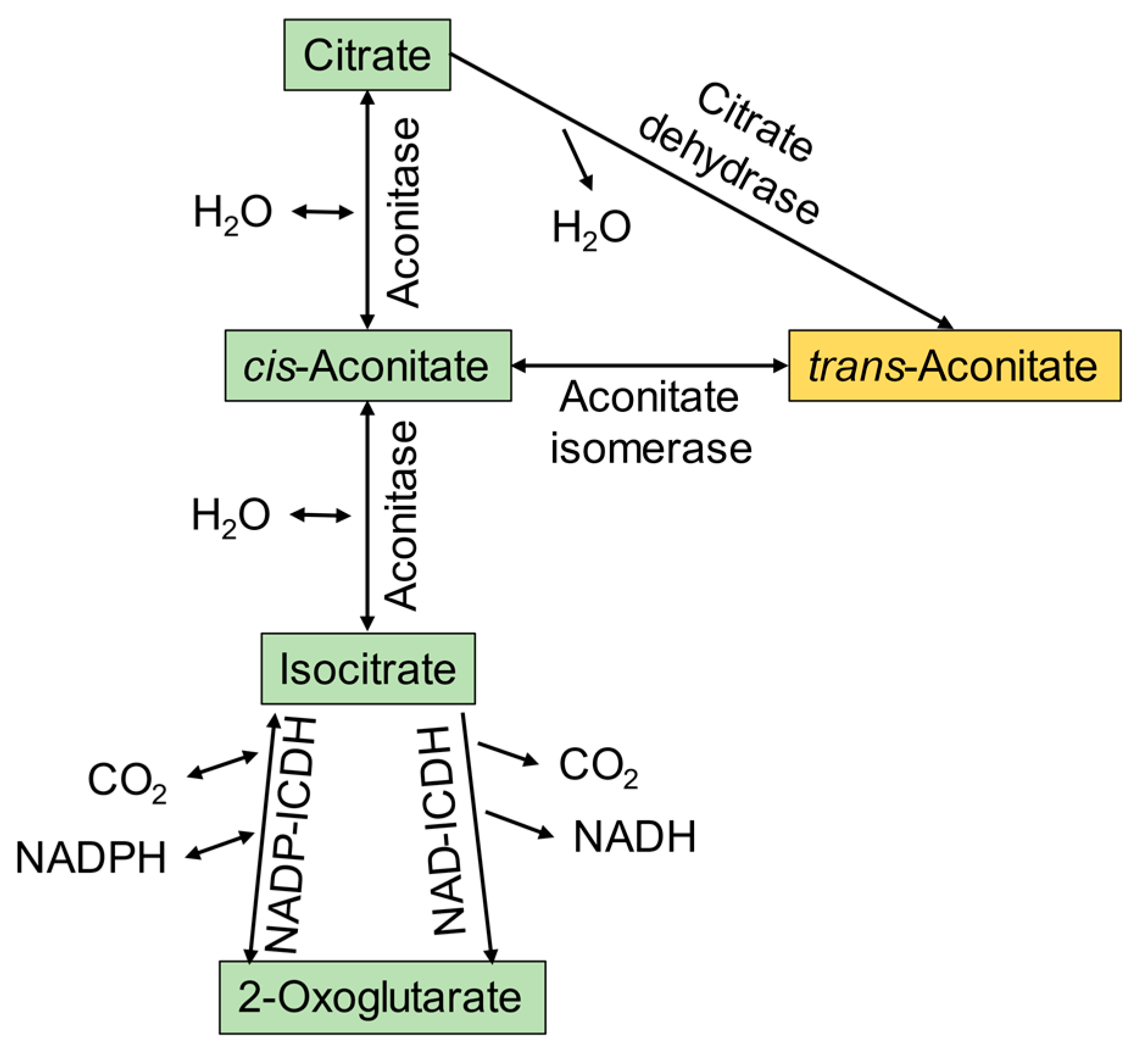
3.1. Microbial Conversion of Aconitic Acid to Itaconic Acid
3.2. Microbial Use as a Carbon Source
3.3. Aconitic Acid as a Fermentation Inhibitor
3.4. Nematocidal Activity of Trans-Aconitic Acid
3.5. Anti-Leishmanial Activity of Trans-Aconitic Acid
3.6. Aconitic Acid Production Confers Survival Advantages
3.6.1. Antifungal Defense
3.6.2. Antifeedant
3.6.3. Defense against Aluminum Toxicity
3.7. Biofilm Inhibition
3.8. Anti-Inflammatory Treatment
3.9. Antioxidant Activity
4. Aconitic Acid in Sugar Cane and Sweet Sorghum and Its Recovery
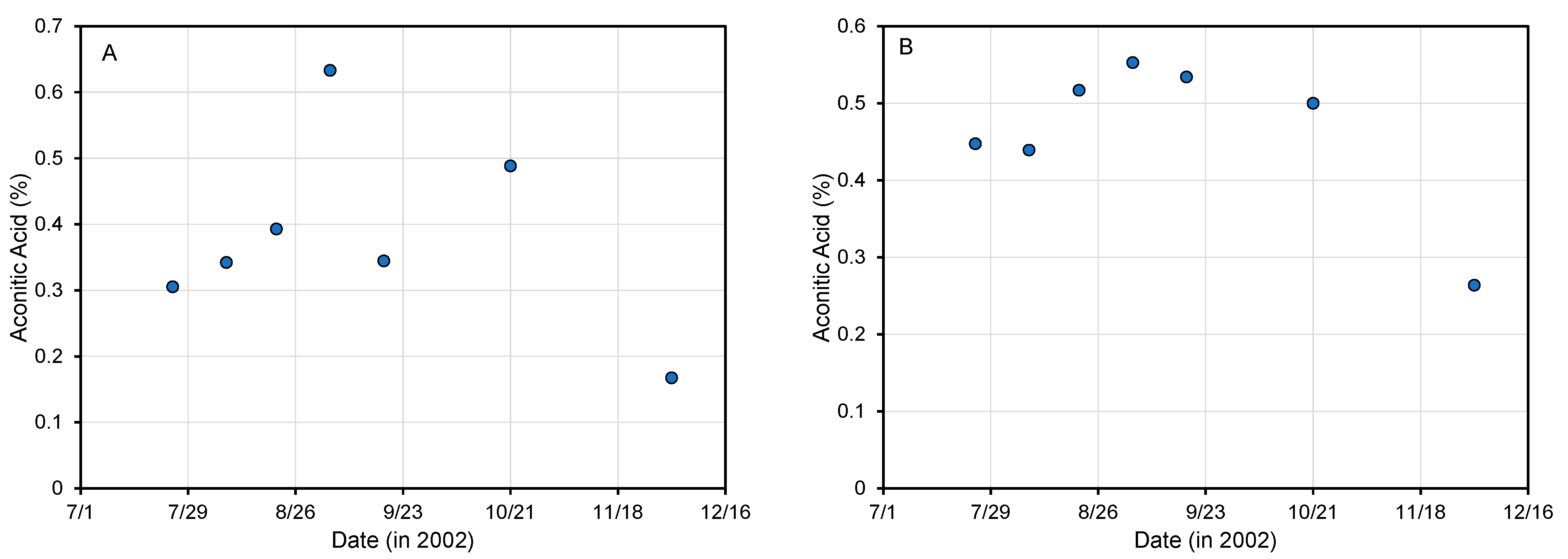
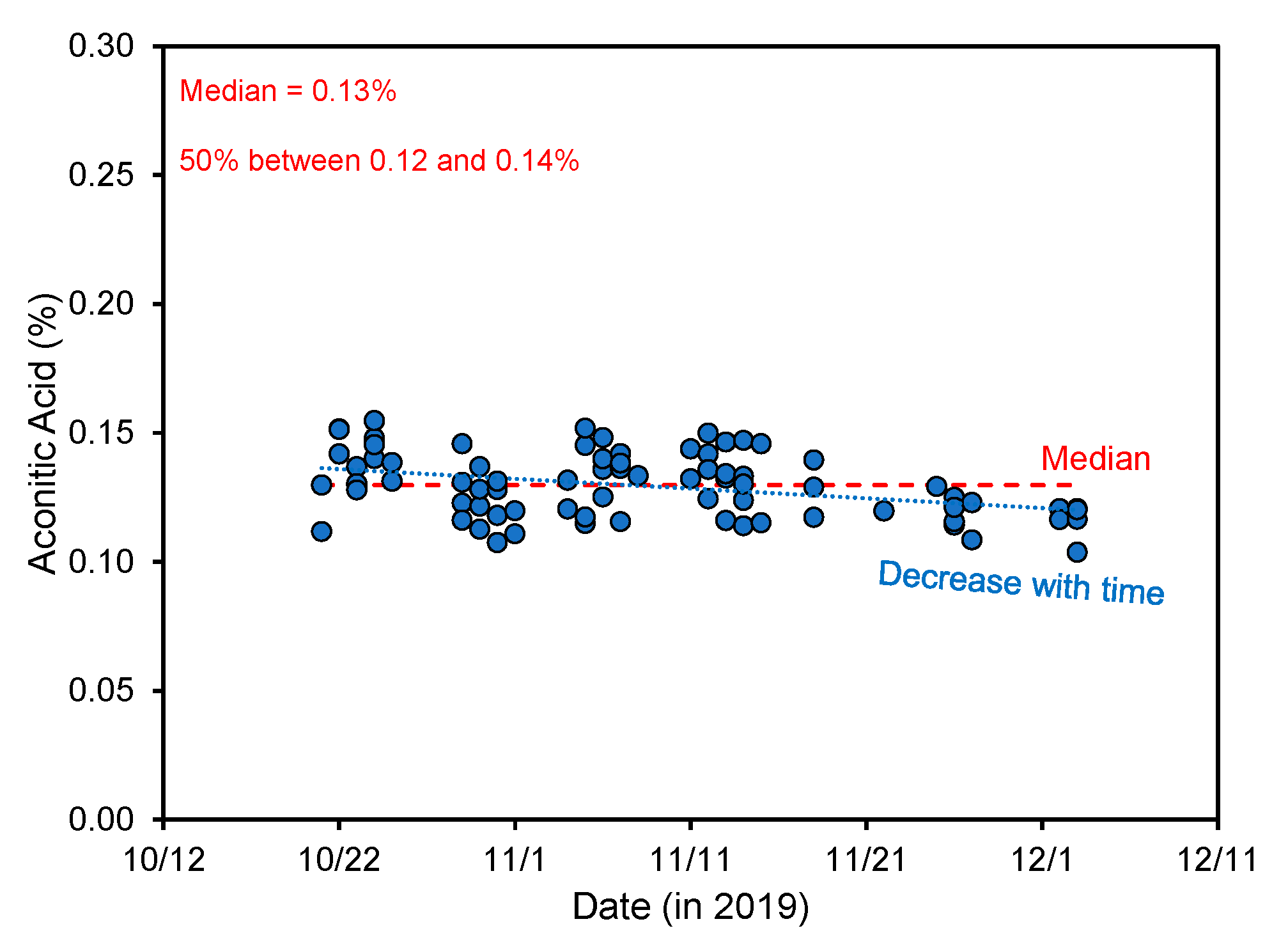
4.1. Aconitic Acid Changes during Plant Development
4.2. Impact of Plant Cultivar and Growth Location on Aconitic Acid Content
4.3. Fate of Aconitic Acid during Sugar Processing
4.4. The Recovery of Aconitic Acid from Sugar Crops
4.5. Aconitic Acid Recovery as Part of Fermentation of Sugars
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass, Volume I: Results from Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwst National Laboratory and the National Renewable Energy Laboratory: Washington, DC, USA, 2004. [Google Scholar]
- Kanitkar, A.; Aita, G.; Madsen, L. The recovery of polymerization grade aconitic acid from sugarcane molasses. J. Chem. Technol. Biotechnol. 2013, 88, 2188–2192. [Google Scholar] [CrossRef]
- King, W.D.; Kester, D.R. A general approach for calculating polyprotic acid speciation and buffer capacity. J. Chem. Educ. 1990, 67, 932–933. [Google Scholar] [CrossRef]
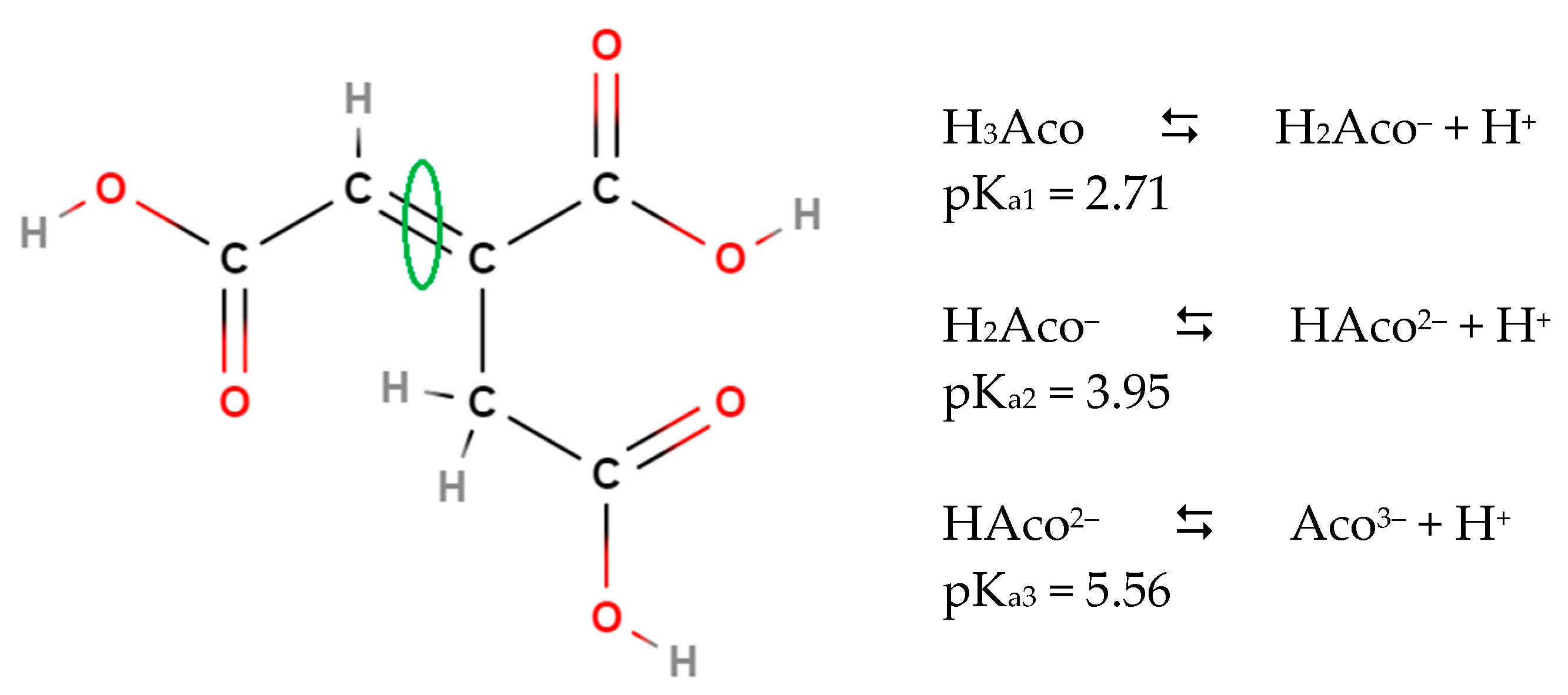
- Pfendt, L.; Drazic, B.; Popovic, G.; Drakulic, B.; Vitnik, Z.; Juranic, I. Determination of all pKa values of some di- and tri-carboxylic unsaturated and epoxy acids and their polylinear correlation with the carboxylic group atomic charges. J. Chem. Res. (S) 2003, 2003, 247–248. [Google Scholar] [CrossRef]
- Udén, P. Plant organic acids in fresh and ensiled forage plants. Grass Forage Sci. 2018, 73, 583–587. [Google Scholar] [CrossRef]
- Pintro, J.; Barloy, J.; Fallavier, P. Effects of low aluminum activity in nutrient solutions on the organic acid concentrations in maize plants. J. Plant Nutr. 1997, 20, 601–611. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic acids: The pools of fixed carbon involved in redox regulation and energy balance in higher plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef] [PubMed]
- Umbdenstock, R.R.; Bruins, P.F. Aconitic acid from citric acid by catalytic dehydration. Ind. & Eng. Chem. 1945, 37, 963–967. [Google Scholar]
- Collier, D.W. Process of treating aconitic acid-containing plant extracts. U.S. Patent 2,513,287, 16 September 1948. [Google Scholar]
- Klasson, K.T.; Qureshi, N.; Powell, R.; Heckemeyer, M.; Eggleston, G. Fermentation of sweet sorghum syrup to butanol in the presence of natural nutrients and inhibitors. Sugar Tech 2018, 20, 224–234. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, P.; Yin, H.; Liu, Z.; Zhao, H.; Tian, P. CRISPR interference-guided modulation of glucose pathways to boost aconitic acid production in Escherichia coli. Microbial Cell Fact. 2020, 19. [Google Scholar] [CrossRef]
- Kobayashi, K.; Maruebi, J.; Kirimura, K. Bioproduction of trans-aconitic acid from citric acid by whole-cell reaction of Escherichia coli heterologously expressing the aconitate isomerase gene from Pseudomonas sp. WU-0701. ChemistrySelect 2016, 1, 1467–1471. [Google Scholar] [CrossRef]
- Cao, H.; Zheng, Y.; Zhou, J.; Wang, W.; Pandit, A. A novel hyperbranched polyester made from aconitic acid (B3) and di(ethylene glycol) (A2). Polym. Int. 2011, 60, 630–634. [Google Scholar] [CrossRef]
- Kanitkar, A.; Chen, C.; Smoak, M.; Hogan, K.; Scherr, T.; Aita, G.; Hayes, D. In vitro characterization of polyesters of aconitic acid, glycerol, and cinnamic acid for bone tissue engineering. J. Biomater. Appl. 2015, 29, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Kanitkar, A. Synthesis and Characterization of Novel Polyester Scaffolds from Sugarcane Industry by-Products for Use in Skin and Bone Tissue Engineering; Louisiana Stae University: Baton Rouge, LA, USA, 2014. [Google Scholar]
- Zhang, L.; Spector, M. Tissue engineering of muscoskeletal tissue. In Tissue Engineering: From Lab to Clinic; Pallua, N., Suschek, C.V., Eds.; Springer: New York, NY, USA, 2011; pp. 597–624. [Google Scholar]
- Zhang, Z.; Jiang, P.P.; Liu, D.; Feng, S.; Zhang, P.; Wang, Y.; Fu, J.; Agus, H. Research progress of novel bio-based plasticizers and their applications in poly(vinyl chloride). J. Mater. Sci. 2021, 56, 10155–10182. [Google Scholar] [CrossRef]
- Gilfillan, W.N.; Doherty, W.O.S. Starch composites with aconitic acid. Carbohydr. Polym. 2016, 141, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.Q.; Davies, J.; Nguyen, D.; Carranza, A.; Vincent, M.; Pojman, J.A. Microparticles and latexes prepared via suspension polymerization of a biobased vegetable oil and renewable carboxylic acid. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Ainali, N.M.; Xanthopoulou, E.; Michailidou, G.; Zamboulis, A.; Bikiaris, D.N. Microencapsulation of fluticasone propionate and salmeterol xinafoate in modified chitosan microparticles for release optimization. Molecules 2020, 25, 3888. [Google Scholar] [CrossRef]
- Hu, L.; Bui, V.T.; Huang, L.; Singh, R.P.; Lin, H. Facilely cross-linking polybenzimidazole with polycarboxylic acids to improve H2/CO2 separation performance. ACS Appl. Mater. Interfaces 2021, 13, 12521–12530. [Google Scholar] [CrossRef]
- Bohre, A.; Ali, M.A.; Ocepek, M.; Grilc, M.; Zabret, J.; Likozar, B. Copolymerization of biomass-derived carboxylic acids for biobased acrylic emulsions. Ind. Eng. Chem. Res. 2019, 58, 19825–19831. [Google Scholar] [CrossRef]
- Noordzij, G.J.; Wilsens, C.H.R.M. Cascade aza-Michael addition-cyclizations; toward renewable and multifunctional carboxylic acids for melt-polycondensation. Front. Chem. 2019, 7, 729. [Google Scholar] [CrossRef]
- Tzereme, A.; Christodoulou, E.; Kyzas, G.Z.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Chitosan grafted adsorbents for diclofenac pharmaceutical compound removal from single-component aqueous solutions and mixtures. Polymers 2019, 11, 497. [Google Scholar] [CrossRef]
- Okada, Y.; Banno, T.; Toshima, K.; Matsumura, S. Synthesis and properties of polycarboxylate-type green surfactants with S- or N-linkages. J. Oleo Sci. 2009, 58, 519–528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wypych, P. Handbook of Plasticizers, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017. [Google Scholar]
- Gil Zapata, N.J. Aconitic Acid from Sugarcane: Production and Industrial Application (A Dissertation); Louisiana State University: Baton Rouge, LA, USA, 2007. [Google Scholar]
- Smalley, D. PVC industry structure dynamic. In PVC Handbook; Wilkes, C.E., Summers, J.W., Daniels, C.A., Eds.; Hanser Publications: Cincinnati, OH, USA, 2005; pp. 679–700. [Google Scholar]
- Wang, Y.; Wang, F.; Xiang, L.; Gu, C.; Redmile-Gordon, M.; Sheng, H.; Wang, Z.; Fu, Y.; Bian, Y.; Jiang, X. Risk assessment of agricultural plastic films based on release kinetics of phthalate acid esters. Environ. Sci. Technol. 2021, 55, 3676–3685. [Google Scholar] [CrossRef]
- Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate release from plastic fragments and degradation in seawater. Environ. Sci. Technol. 2019, 53, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Wu, L.H.; Chen, L.; Zhang, H.B.; Teng, Y.; Luo, Y.M. Phthalate esters contamination in soils and vegetables of plastic film greenhouses of suburb Nanjing, China and the potential human health risk. Environ. Sci. Pollut. Res. 2015, 22, 12018–12028. [Google Scholar] [CrossRef]
- Kong, S.; Ji, Y.; Liu, L.; Chen, L.; Zhao, X.; Wang, J.; Bai, Z.; Sun, Z. Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ. Pollut. 2012, 170, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, L.; Wang, H.; Wang, X.; Martin, F.L.; Zhang, J.; Huang, Q.; Shen, H. Phthalates induce androgenic effects at exposure levels that can be environmentally relevant in humans. Environ. Sci. Technol. Lett. 2018, 5, 232–236. [Google Scholar] [CrossRef]
- Swan, S.H. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008, 108, 177–184. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Latifi, A.M.; Orjuela, A.; Rodríguez, G.; Gil, I.D. Modeling, analysis and multi-objective optimization of an industrial batch process for the production of tributyl citrate. Comput. Chem. Eng. 2020, 132, 106603. [Google Scholar] [CrossRef]
- Magne, F.C.; Mod, R.R. Plasticizers from aconitic and triearballylic acids. Ind. Eng. Chem. 1953, 45, 1546–1547. [Google Scholar] [CrossRef]
- Gil, N.; Saska, M.; Negulescu, I. Evaluation of the effects of biobased plasticizers on the thermal and mechanical properties of poly(vinyl chloride). J. Appl. Polym. Sci. 2006, 102, 1366–1373. [Google Scholar] [CrossRef]
- Cox, F.W. Copolymers of Alkyl Aconitates and Vinyl Chloride. U.S. Patent 2,419,122, 29 June 1943. [Google Scholar]
- Hanson, A.W.; Goggin, W.C. Stabilized Vinylidene Chloride Composition. U.S. Patent 2,273,262, 19 September 1940. [Google Scholar]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Orozco, F.; Andrade, A.; Delgado, L.M.; Rojas, G. Rapid microwave controlled polyesterification of aconitic acid and ethylene glycol. Polym. Int. 2020, 69, 577–583. [Google Scholar] [CrossRef]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.M. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Chun, H.L.; Lee, S.Y.; Lee, S.H.; Lee, C.S.; Park, H.H. Enzymatic reaction mechanism of cis-aconitate decarboxylase based on the crystal structure of IRG1 from Bacillus subtilis. Sci. Rep. 2020, 10, 11305. [Google Scholar] [CrossRef] [PubMed]
- Kanamasa, S.; Dwiarti, L.; Okabe, M.; Park, E.Y. Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl. Microbiol. Biotechnol. 2008, 80, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef]
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of microbial itaconic acid production. Frontiers in Microbiology 2013, 4, 23. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bölker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb. Biotechnol. 2016, 9, 116–126. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Engel, M.; Kleineberg, W.; Büttner, L.; Sarikaya, E.; Hartog, T.D.; Klankermayer, J.; Leitner, W.; Bölker, M.; et al. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab. Eng. 2016, 38, 427–435. [Google Scholar] [CrossRef]
- Yuhara, K.; Yonehara, H.; Hattori, T.; Kobayashi, K.; Kirimura, K. Enzymatic characterization and gene identification of aconitate isomerase, an enzyme involved in assimilation of trans-aconitic acid, from Pseudomonas sp. WU-0701. FEBS J. 2015, 282, 4257–4267. [Google Scholar] [CrossRef]
- Amorim, H.V. Challenges to produce ethanol from sweet sorghum in Brazil. In Proceedings of the Sweet Sorghum Association 2015 Annual Conference, Orlando, FL, USA, 27–29 January 2015. [Google Scholar]
- Klasson, K.T. Impact of potential fermentation inhibitors present in sweet sorghum sugar solutions. Sugar Tech 2017, 19, 95–101. [Google Scholar] [CrossRef]
- Klasson, K.T. The inhibitory effects of aconitic acid on bioethanol production. Sugar Tech 2018, 20, 88–94. [Google Scholar] [CrossRef]
- Du, C.; Cao, S.; Shi, X.; Nie, X.; Zheng, J.; Deng, Y.; Ruan, L.; Peng, D.; Sun, M. Genetic and biochemical characterization of a gene operon for trans-aconitic acid, a novel nematicide from Bacillus thuringiensis. J. Biol. Chem. 2017, 292, 3517–3530. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Sanyal, T.; Sarkar, D.; Bhattacharya, P.K.; Ghosh, D.K. Evaluation of antileishmanial activity of trans-aconitic acid. Biochem. Med. Metab. Biol. 1989, 42, 171–178. [Google Scholar] [CrossRef]
- Kar, S.; Kar, K.; Bhattacharya, P.K.; Ghosh, D.K. Experimental visceral leishmaniasis: Role of trans-aconitic acid in combined chemotherapy. Antimicrob. Agents Chemother. 1993, 37, 2459–2465. [Google Scholar] [CrossRef]
- Gawron, O.; Jones, L. Structural basis for aconitase activity inactivation by butanedione and binding of substrates and inhibitors. Biochimica Biophysica Acta (BBA) Enzymol. 1977, 484, 453–464. [Google Scholar] [CrossRef]
- Quijano, C.; Trujillo, M.; Castro, L.; Trostchansky, A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016, 8, 28–42. [Google Scholar] [CrossRef]
- Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Aconitate and methyl aconitate are modulated by silicon in powdery mildew-infected wheat plants. J. Plant Physiol. 2009, 166, 1413–1422. [Google Scholar] [CrossRef]
- Kim, M.; Hen-Sik, K.; Tokio, O.; Hiroshi, F.; Shoziro, S. Isolation and identification of trans-aconitic acid as the antifeedant in barnyard grass against the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl. Entomol. Zool. 1976, 11, 53–57. [Google Scholar] [CrossRef]
- Nagata, T.; Hayakawa, T. Antifeeding activity of aconitic acids and oxalic acid on brown planthopper, Nilaparvata lugens (Stål) and green rice leafhopper, Nephotettix cincticeps (Uhler). Jap. J. Appl. Entomol. Zool. 1998, 42, 115–121. [Google Scholar] [CrossRef]
- Watanabe, K.; Katsuharar, M.; Nakao, H.; Sato, M. Detection and molecular analysis of plant- and insect-associated bacteria harboring aconitate isomerase involved in biosynthesis of trans-aconitic acid as antifeedant in brown planthoppers. Curr. Microbiol. 1997, 35, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M. Probing behavior of the brown planthopper, Nilaparvata lugens Stål (Homoptera: Delphacidae) on a non-host barnyard grass, and resistant and susceptible varieties of rice. Appl. Entomol. Zool. 2001, 36, 83–89. [Google Scholar] [CrossRef][Green Version]
- Oliveira, D.P.; Moreira, T.D.V.; Batista, N.V.; Souza Filho, J.D.; Amaral, F.A.; Teixeira, M.M.; Pádua, R.M.; Braga, F.C. Esterification of trans-aconitic acid improves its anti-inflammatory activity in LPS-induced acute arthritis. Biomed Pharmacother 2018, 99, 87–95. [Google Scholar] [CrossRef]
- Pinto de Oliveira, D.; Guimarães Augusto, G.; Vieira Batista, N.; de Oliveira, V.L.S.; Santos Ferreira, D.; Castro, E.S.M.A.; Fernandes, C.; Almeida Amaral, F.; Martins Teixeira, M.; Maia de Pádua, R.; et al. Encapsulation of trans-aconitic acid in mucoadhesive microspheres prolongs the anti-inflammatory effect in LPS-induced acute arthritis. Eur. J. Pharm. Sci. 2018, 119, 112–120. [Google Scholar] [CrossRef]
- Garcia, E.D.F.; De Oliveira, M.A.; Dourado, L.P.A.; De Souza, D.G.; Teixeira, M.M.; Braga, F.C. In vitro TNF- α inhibition elicited by extracts from Echinodorus grandiflorus leaves and correlation with their phytochemical composition. Planta Med. 2016, 82, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Syabana, M.A.; Yuliana, N.D.; Batubara, I.; Fardiaz, D. Characterization of antioxidant compound from Syzygium polyanthum leaves extract using UHPLC-HRMS. Molekul 2021, 16, 38–45. [Google Scholar] [CrossRef]
- Piang-Siong, W.; de Caro, P.; Marvilliers, A.; Chasseray, X.; Payet, B.; Shum Cheong Sing, A.; Illien, B. Contribution of trans-aconitic acid to DPPHrad scavenging ability in different media. Food Chem. 2017, 214, 447–452. [Google Scholar] [CrossRef]
- Yarce, C.J.; Alhajj, M.J.; Sanchez, J.D.; Oñate-Garzón, J.; Salamanca, C.H. Development of antioxidant-loaded nanoliposomes employing lecithins with different purity grades. Molecules 2020, 25, 5344. [Google Scholar] [CrossRef]
- Bortolo, T.D.S.C.; Marchiosi, R.; Viganó, J.; Siqueira-Soares, R.D.C.; Ferro, A.P.; Barreto, G.E.; Bido, G.D.S.; Abrahão, J.; dos Santos, W.D.; Ferrarese-Filho, O. Trans-aconitic acid inhibits the growth and photosynthesis of Glycine max. Plant Physiol. Biochem. 2018, 132, 490–496. [Google Scholar] [CrossRef]
- Pestana-Nobles, R.; Leyva-Rojas, J.A.; Yosa, J. Searching hit potential antimicrobials in natural compounds space against biofilm formation. Molecules 2020, 25, 5334. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; van Luijk, N.; ter Beek, M.; Caspers, M.; Punt, P.; van der Werf, M. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet. Biol. 2011, 48, 602–611. [Google Scholar] [CrossRef]
- Deng, S.; Dai, Z.; Swita, M.; Pomraning, K.R.; Hofstad, B.; Panisko, E.; Baker, S.; Magnuson, J. Deletion analysis of the itaconic acid biosynthesis gene cluster components in Aspergillus pseudoterreus ATCC32359. Appl. Microbiol. Biotechnol. 2020, 104, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Steiger, M.G.; Punt, P.J.; Ram, A.F.J.; Mattanovich, D.; Sauer, M. Characterizing MttA as a mitochondrial cis-aconitic acid transporter by metabolic engineering. Metab. Eng. 2016, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Hartmann, S.K.; Schmitz, L.M.; Büttner, L.; Hosseinpour Tehrani, H.; Geiser, E.; Beudels, M.; Venc, D.; Wandrey, G.; Büchs, J.; et al. Promoters from the itaconate cluster of Ustilago maydis are induced by nitrogen depletion. Fung. Biol. Biotechnol. 2017, 4, 11. [Google Scholar] [CrossRef]
- Rampazzo, P.E.; Marcos, F.C.C.; Cipriano, M.A.P.; Marchiori, P.E.R.; Freitas, S.S.; Machado, E.C.; Nascimento, L.C.; Brocchi, M.; Ribeiro, R.V. Rhizobacteria improve sugarcane growth and photosynthesis under well-watered conditions. Ann. Appl. Biol. 2018, 172, 309–320. [Google Scholar] [CrossRef]
- Day, D.F.; Sarkar, D. Fuel alcohol from sweet sorghum: Microbial aspects. Dev. Ind. Microbiol. 1982, 23, 361–366. [Google Scholar]
- Wu, X.; Staggenborg, S.; Propheter, J.L.; Rooney, W.L.; Yu, J.; Wang, D. Features of sweet sorghum juice and their performance in ethanol fermentation. Ind. Crops Prod. 2010, 31, 164–170. [Google Scholar] [CrossRef]
- Gibbons, W.R.; Westby, C.A. Cofermentation of sweet sorghum juice and grain for production of fuel ethanol and distillers’ wet grain. Biomass 1989, 18, 43–57. [Google Scholar] [CrossRef]
- Ghaemi, R.; Pourjam, E.; Safaie, N.; Verstraeten, B.; Mahmoudi, S.B.; Mehrabi, R.; De Meyer, T.; Kyndt, T. Molecular insights into the compatible and incompatible interactions between sugar beet and the beet cyst nematode. BMC Plant Biol. 2020, 20, 483. [Google Scholar] [CrossRef]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Stout, P.R.; Brownell, J.; Burau, R.G. Occurrences of trans-aconitate in range forage species. Agron. J. 1967, 59, 21–24. [Google Scholar] [CrossRef]
- Nelson, E.K.; Mottern, H.H. Some organic acids in barley, maize, oats and rye plants. J. Am. Chem. Soc. 1931, 53, 3046–3048. [Google Scholar] [CrossRef]
- Cai, H.; Strouse, J.; Dumlao, D.; Jung, M.E.; Clarke, S. Distinct reactions catalyzed by bacterial and yeast trans-aconitate methyltransferases. Biochemistry 2001, 40, 2210–2219. [Google Scholar] [CrossRef]
- Cai, H.; Dumlao, D.; Katz, J.E.; Clarke, S. Identification of the gene and characterization of the activity of the trans-aconitate methyltransferase from Saccharomyces cerevisiae. Biochemistry 2001, 40, 13699–13709. [Google Scholar] [CrossRef]
- Sugimoto, T.; Kato, T.; Park, E.Y. Functional analysis of cis-aconitate decarboxylase and trans-aconitate metabolism in riboflavin-producing filamentous Ashbya gossypii. J. Biosci. Bioeng. 2014, 117, 563–568. [Google Scholar] [CrossRef]
- Orioli, G.A.; Thompson, J.F. Aconitate accumulation in wheat seedlings. Botanical Gazette 1990, 151, 30–37. [Google Scholar] [CrossRef]
- Lv, J.; Xiao, J.; Guo, Z.; Dong, K.; Dong, Y. Nitrogen supply and intercropping control of Fusarium wilt in faba bean depend on organic acids exuded from the roots. Sci. Rep. 2021, 11, 9589. [Google Scholar] [CrossRef]
- Rustamani, M.A.; Kanehisa, K.; Tsumuki, H. Aconitic acid content of some cereals and its effect on aphids. Appl. Entomol. Zool. 1992, 27, 79–87. [Google Scholar] [CrossRef]
- Uchimiya, M.; Knoll, J.E. Rapid data analytics to relate sugarcane aphid [(Melanaphis sacchari (Zehntner)] population and damage on sorghum (Sorghum bicolor (L.) Moench). Sci. Rep. 2019, 9, 370. [Google Scholar] [CrossRef]
- Knoll, J.E.; Uchimiya, M.; Harris-Shultz, K. Juice chemical properties of 24 sorghum cultivars under varying levels of sugarcane aphid (Melanaphis sacchari) infestation. Arthropod-Plant Interact. 2021, 15, 707–719. [Google Scholar] [CrossRef]
- Antoniani, D.; Bocci, P.; Maciag, A.; Raffaelli, N.; Landini, P. Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl. Microbiol. Biotechnol. 2010, 85, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Tryon, R.G.; Kim, J.H. Screening for diguanylate cyclase (DGC) inhibitors mitigating bacterial biofilm formation. Front. Chem. 2020, 8, 264. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Yoder, P.A. Notes on the determination of acids in sugar cane juice. Ind. Eng. Chem. 1911, 3, 640–646. [Google Scholar] [CrossRef][Green Version]
- Behr, A. Ueber das vorkommen von aconitsäure im zuckerohrsaft und colonialzucker. Berichte der Deutschen Chemischen Gesellschaft 1877, 10, 351–355. [Google Scholar] [CrossRef]
- Parsons, H.B. Aconitic acid in the scale from sorghum sugar pans. Am. Chem. J. 1882, 4, 39–42. [Google Scholar]
- Clarke, M.A.; Brannan, M.A. Rapid analyses of lactic acid, an indicator of sugar cane deterioration, and aconitic acid, an indicator of sugar cane maturity, by high performance liquid chromatography. J. Am. Soc. Sugar Cane Technol. 1983, 2, 88. [Google Scholar]
- Haines, H.W., Jr.; Joyner, L.G. Calcium magesium aconitate. Ind. Eng. Chem. 1955, 47, 178–186. [Google Scholar] [CrossRef]
- McCalip, M.A.; Seibert, A.H. Aconitic acid from sugar cane products. Ind. Eng. Chem. 1941, 33, 637–640. [Google Scholar] [CrossRef]
- Uchimiya, M.; Knoll, J.E. Electroactivity of polyphenols in sweet sorghum (Sorghum bicolor (L.) Moench) cultivars. PLoS ONE 2020, 15, e0234509. [Google Scholar] [CrossRef]
- Uchimiya, M.; Spaunhorst, D.J. Influence of summer fallow on aromatic secondary products in sugarcane (Saccharum spp. hybrids). J. Agric. Food Res. 2020, 2. [Google Scholar] [CrossRef]
- Celestine-Myrtil, D.A.; Parfait, A. HPLC determination of organc acids in sugar cane and its industrial by-products. Int. Sugar J. 1988, 90, 28–32. [Google Scholar]
- Martin, L.F. The non-nitrogenous organic acid of sugarcane. In Principles of Sugar Technology, 1st ed.; Honig, P., Ed.; Elsevier Publishing Company: New York, NY, USA, 1953; pp. 128–156. [Google Scholar]
- Almodares, A.; Ranjbar, M.; Hadi, M.R. Effects of nitrogen treatments and harvesting stages on the aconitic acid, invert sugar and fiber in sweet sorghum cultivars. J. Environ. Biol. 2010, 31, 1001–1005. [Google Scholar] [PubMed]
- De Carvalho Gonçalves, J.F.; Cambraia, J.; Mosquim, P.R.; Araújo, E.F. Aluminum effect on organic acid production and accumulation in sorghum. J. Plant Nutr. 2005, 28, 507–520. [Google Scholar] [CrossRef]
- Uchimiya, M.; Knoll, J.E.; Anderson, W.F.; Harris-Shultz, K.R. Chemical analysis of fermentable sugars and secondary products in 23 sweet sorghum cultivars. J. Agric. Food Chem. 2017, 65, 7629–7637. [Google Scholar] [CrossRef]
- Uchimiya, M.; Knoll, J.E. Prediction of carboxylic and polyphenolic chemical feedstock quantities in sweet sorghum. Energy Fuels 2018, 32, 5252–5263. [Google Scholar] [CrossRef]
- Balch, R.T.; Broeg, C.B.; Ambler, J.A. Acontic acid in sugar cane products. Sugar 1945, 40, 32–35. [Google Scholar]
- Bachmann, R.; Horns, A.L.; Paasch, N.; Schrieck, R.; Weidner, M.; Fransson, I.; Schrör, J.P. Minor metabolites as chemical marker for the differentiation of cane, beet and coconut blossom sugar. From profiling towards identification of adulterations. Food Control 2022, 135, 108832. [Google Scholar] [CrossRef]
- Ventre, E.K.; Ambler, J.A.; Henry, H.C.; Byall, S.; Paine, H.S. Extraction of aconitic acid from sorgo. Ind. Eng. Chem. 1946, 38, 201–204. [Google Scholar] [CrossRef]
- Ventre, E.K.; Paine, H.S.; Wickard, C.R. Sugar from Sorghum Juices. U.S. Patent 2,280,085, 24 March 1941. [Google Scholar]
- Ventre, E.K.; Ambler, J.A.; Byall, S.; Henry, H.C. Process for the Extraction of Acontic Acid from Plant Juices. U.S. Patent 2,359,537, 10 August 1943. [Google Scholar]
- Ventre, E.K. Extraction of Aconitic Acid from Sugar Cane. U.S. Patent 2,469,090, 2 May 1946. [Google Scholar]
- Ventre, E.K. Method for Extracting Aconitic Acid from Sugarcane and Sorgo Juices, Sirups, and Molasses. U.S. Patent 2,712,552, 26 May 1952. [Google Scholar]
- Collier, D.W. Concentration of aqueous aconitic acid solutions. U.S. Patent 2,650,248, 15 March 1950. [Google Scholar]
- Wu-Tiu-Yen, J.; Lameloise, M.L.; Petit, A.; Lewandowski, R.; Broyart, B.; Fargues, C. Aconitic acid recovery from sugar-cane stillage: From the modeling of the anion-exchange step to the conception of a novel combined process. Sep. Sci. Technol. 2020, 56, 1752–1768. [Google Scholar] [CrossRef]
- Montoya, G.; Gutierrez, M.I.; Giraldo, J.D.; Jaramillo, L.D.; Ruiz-Sandoval, J.; Orozco, S.; Orozco, F.; Ward, J.; Rojas, G.; Villegas-Torres, M.F. Sustainable sugarcane vinasse biorefinement for trans-aconitic acid-based biopolymer synthesis and bioenergy generation. Bioresour. Technol. Rep. 2021, 15, 100786. [Google Scholar] [CrossRef]
- Klasson, K.T.; Sturm, M.P.; Cole, M.R. Acid hydrolysis of sucrose in sweet sorghum syrup followed by succinic acid production using a genetically engineered Escherichia coli. Biocatal. Agric. Biotechnol. 2022, 39, 102231. [Google Scholar] [CrossRef]
- Wright, M.; Klasson, K.T.; Kimura, K. Production of acetoin from sweet sorghum syrup and beet juice via fermentation. Sugar Tech 2020, 22, 354–359. [Google Scholar] [CrossRef]
- Klasson, K.T.; Cole, M.R.; Pancio, B.T.; Heckemeyer, M. Development of an enzyme cocktail to bioconvert untapped starch in sweet sorghum processing by-products: Part II. Application and economic potential. Ind. Crops Prod. 2022, 176, 114370. [Google Scholar] [CrossRef]
| Industrial Uses and Applications | References |
|---|---|
| formation of polyesters for tissue engineering | [13,14,15,16] |
| bio-derived plasticizer | [17,18] |
| hyperbranched ester polymers | [13] |
| chemical conversion to C5 itaconic acid | [1] |
| polymers to form microparticles for drug delivery | [19,20] |
| cross-linking of polybenzimidazole chains for H2/CO2 separation | [21] |
| cross-linking of starch polymers | [18] |
| production of methylacrylic acid | [22] |
| trans-tri-methyl aconitate in green click reactions | [23] |
| grafting agent to modify chitosan as an adsorbent | [24] |
| production of green surfactant | [25] |
| Biological Uses and Applications | Method or Approach | References |
|---|---|---|
| microbial production of itaconic acid | Aspergillus terreus decarboxylation of CAA | [43,44,45] |
| microbial production of itaconic acid | Ustilago maydis decarboxylation of TAA | [43,46,47,48] |
| Pseudomonas sp. use as sole carbon source | isomerization of TAA to CAA for TCA cycle | [49] |
| fermentation inhibitor | in Saccharomyces cerevisiae, pH-dependent | [50,51,52] |
| nematocidal activity | Meloidogyne incognita | [53] |
| anti-leishmanial activity | Leishmania donovani | [54,55] |
| regulation of TCA cycle | TAA-based inhibition of aconitase | [7,56,57] |
| antifungal defense in plants | methyl-TAA acts as a phytoalexin | [58] |
| antifeedant | involved in resistance of some plants to Nilaparvata lugens | [59,60,61,62] |
| defense against aluminum toxicity | organic acid chelation of Al | [5,6] |
| anti-inflammatory activity | inhibition of TNF-α release by monocytes | [63,64,65] |
| antioxidant activity | DPPH assay and nanoliposomes | [66,67,68] |
| inhibitor of Glycine max | Increased H2O2 in roots and reduced water uptake | [69] |
| inhibitor of quorum sensing | ligand inhibitor of PleD | [70] |
| Fermentation | Aconitic Acid before Fermentation | Aconitic Acid after Fermentation |
|---|---|---|
| Succinic acid using Escherichia coli AFP 184 [119] | 0.11% | 0.072% |
| Acetone/butanol/ethanol using Clostridium beijerinckii NCP 260 [10] | 0.075% (Syrup a) 0.082% (Syrup b) | 0.001% (Syrup a) 0.002% (Syrup b) |
| Acetoin using Bacillus subtillus NFRI 8291 and NFRI 8299 [120] | 0.304% | 0.325% |
| Ethanol using Baker’s yeast [121] | 0.28% (Clarifier mud) | 0.25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, G.O.; Klasson, K.T. Aconitic Acid Recovery from Renewable Feedstock and Review of Chemical and Biological Applications. Foods 2022, 11, 573. https://doi.org/10.3390/foods11040573
Bruni GO, Klasson KT. Aconitic Acid Recovery from Renewable Feedstock and Review of Chemical and Biological Applications. Foods. 2022; 11(4):573. https://doi.org/10.3390/foods11040573
Chicago/Turabian StyleBruni, Gillian O., and K. Thomas Klasson. 2022. "Aconitic Acid Recovery from Renewable Feedstock and Review of Chemical and Biological Applications" Foods 11, no. 4: 573. https://doi.org/10.3390/foods11040573
APA StyleBruni, G. O., & Klasson, K. T. (2022). Aconitic Acid Recovery from Renewable Feedstock and Review of Chemical and Biological Applications. Foods, 11(4), 573. https://doi.org/10.3390/foods11040573