Evaluation of Nano-Wall Material for Production of Novel Lyophilized-Probiotic Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Growth of Probiotic Strains and Culture Conditions
2.3. Determination of Cholesterol Removal Activity
2.4. DPPH Radical Scavenging Activity
2.5. Biofilm Formation of Probiotic Strain
2.6. Screening of Gamma Aminobutyric Acid (GABA)-Producing Probiotic Strains Using Thin Layer Chromatography (TLC)
2.7. Production of Freeze-Dried Probiotic Product
2.7.1. Preparation of Cell Pellets
2.7.2. Preparation of Gum Arabic
2.7.3. Preparation of Complex Medium
2.7.4. Production of Lyophilized Product
2.8. Probiotic Strains Adherence on Medium Using Scanning Electron Microscopy (SEM)
2.9. Determination of Cell Viability, Storage and Microbial Safety
2.10. Statistical Analysis
3. Results and Discussion
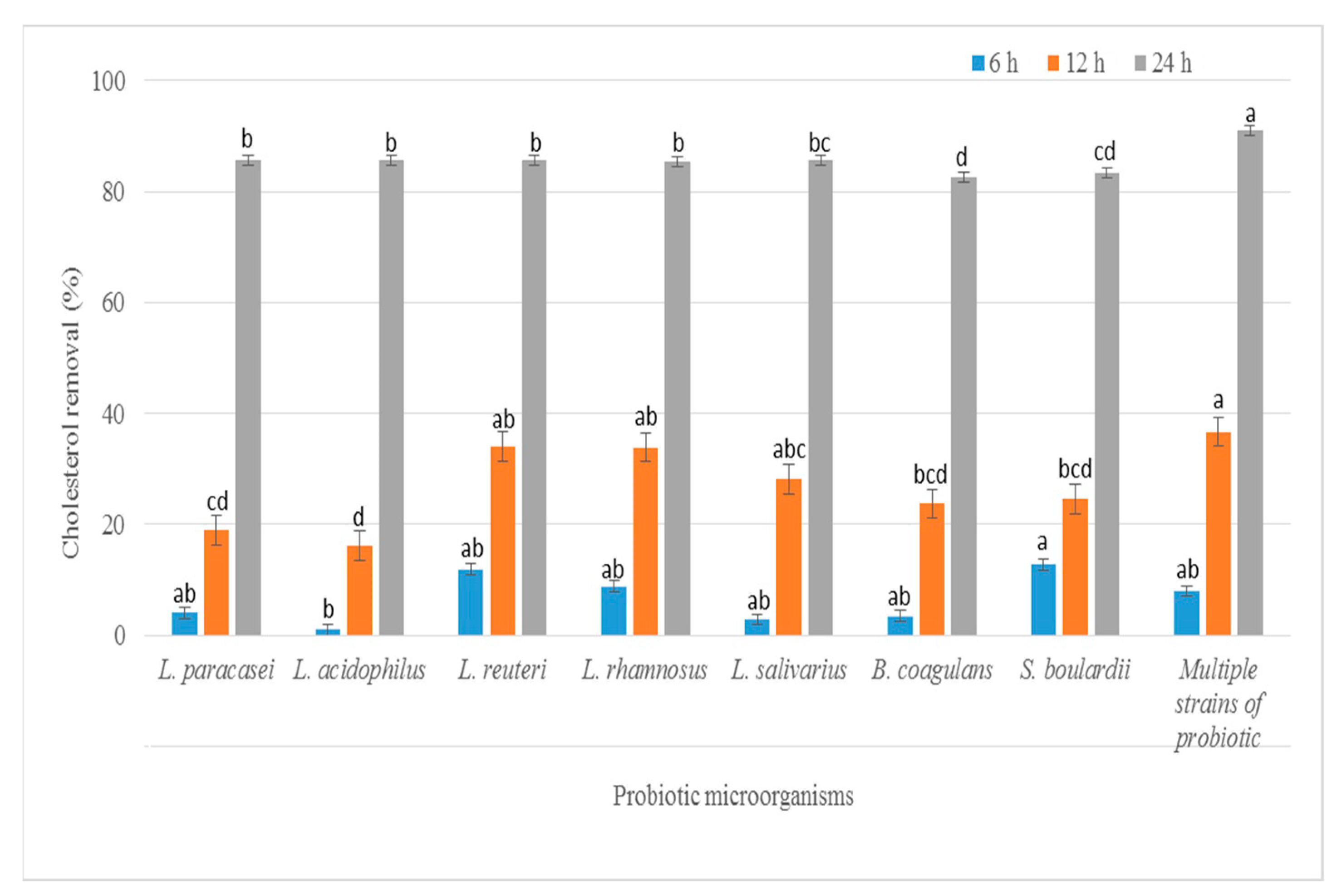
3.1. Determination of Cholesterol Removal
3.2. Antioxidant Activity: DPPH Radical Scavenging Assay
3.3. Biofilm Formation of Probiotic Strains
3.4. GABA-Producing Probiotic Strains Using TLC
3.5. Production of Lyophilized Probiotic Product
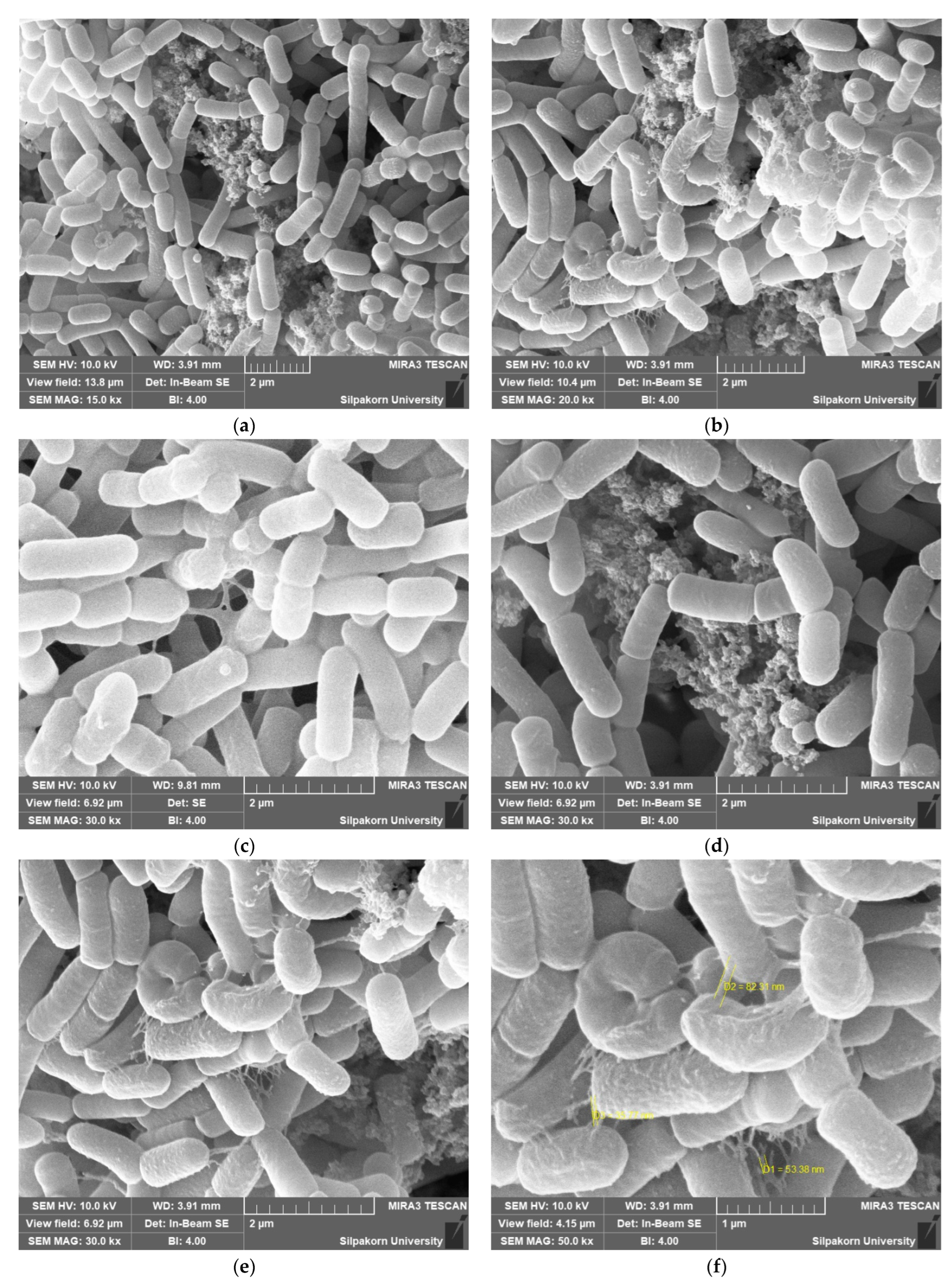
3.6. Adhesion of Probiotic Cell as Determined Using SEM
3.7. Microbial Safety
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Specifications for identity and purity of certain food additives. FAO Food Nutr. Pap. 1992, 52, 345–355. [Google Scholar]
- Abd-Razig, N.M.; Sabahelkhier, M.K.; Idris, O.F. Effect of gum arabic (Acacia senegal, L. Willd) on lipid profile and performance of laying hens. J. Appl. Biosci. 2010, 32, 2002–2007. [Google Scholar]
- Azeez, O. Decolourization of gum Arabic using activated charcoal. Leonardo J. Sci. 2005, 4, 23–32. [Google Scholar]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Elderbi, M.A.; Mohamed, A. Effect of gum arabic on coagulation system of albino rats. Int. J. PharmTech Res. 2010, 2, 1762–1766. [Google Scholar]
- Ali, K.; Daffalla, H. Physicochemical and functional properties of the gum arabic from Acacia senegal. Ann. Food Sci. Technol. 2018, 19, 27–34. [Google Scholar]
- Bisar, G.H.; El-Saadany, K.; Khattab, A.; El Kholy, W.M. The possibility of using fibers as a prebiotic in making of probiotic based on-some dairy products. Br. Microbiol. Res. J. 2014, 4, 678. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Harish, K.; Varghese, T. Probiotics in humans–evidence-based review. Calicut Med. J. 2006, 4, e3. [Google Scholar]
- Macfarlane, G.T.; Cummings, J.H. Probiotics and prebiotics: Can regulating the activities of intestinal bacteria benefit health? BMJ 1999, 318, 999–1003. [Google Scholar] [CrossRef]
- Kurtmann, L.; Carlsen, C.U.; Skibsted, L.H.; Risbo, J. Water activity-temperature state diagrams of freeze-dried Lactobacillus acidophilus (La-5): Influence of physical state on bacterial survival during storage. Biotechnol. Prog. 2009, 25, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Vogel, R.; Gänzle, M.G. Influence of oligosaccharides on the viability and membrane properties of Lactobacillus reuteri TMW1. 106 during freeze-drying. Cryobiology 2007, 55, 108–114. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Nireesha, G.; Divya, L.; Sowmya, C.; Venkateshan, N.; Lavakumar, V. Lyophilization/freeze drying: A review. Int. J. Novel Trends Pharm. Sci. 2013, 3, 87–98. [Google Scholar]
- Peighambardoust, S.; Tafti, A.G.; Hesari, J. Application of spray drying for preservation of lactic acid starter cultures: A review. Trends Food Sci. Technol. 2011, 22, 215–224. [Google Scholar] [CrossRef]
- Freitas, S.; Merkle, H.P.; Gander, B. Ultrasonic atomisation into reduced pressure atmosphere—Envisaging aseptic spray-drying for microencapsulation. J. Control. Release 2004, 95, 185–195. [Google Scholar] [CrossRef]
- Costantino, H.R.; Pikal, M.J. Lyophilization of Biopharmaceuticals; Springer Science & Business Media: Berlin/Heidelberg, Germany; AAPS Press: Arlington, VA, USA, 2004; pp. 1–74. ISBN 0-9711767-6-0. [Google Scholar]
- Meng, X.; Stanton, C.; Fitzgerald, G.; Daly, C.; Ross, R. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-Drying of Lactic Acid Bacteria. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257, pp. 477–488. ISBN 978-1-4939-2193-5. [Google Scholar]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Elansary, H.O.; Salem, M.Z.; Ashmawy, N.A.; Yacout, M.M. Chemical composition, antibacterial and antioxidant activities of leaves essential oils from Syzygium cumini L., Cupressus sempervirens L. and Lantana camara L. from Egypt. J. Agric. Sci. 2012, 4, 144. [Google Scholar] [CrossRef]
- Watnick, P.I.; Kolter, R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 1999, 34, 586–595. [Google Scholar] [CrossRef]
- Lee, B.-J.; Kim, J.-S.; Kang, Y.M.; Lim, J.-H.; Kim, Y.-M.; Lee, M.-S.; Jeong, M.-H.; Ahn, C.-B.; Je, J.-Y. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Chumphon, T.; Sriprasertsak, P.; Promsai, S. Development of rice as potential carriers for probiotic Lactobacillus amylovorus. Int. J. Food Sci. Technol. 2016, 51, 1260–1267. [Google Scholar] [CrossRef]
- Savedboworn, W.; Wanchaitanawong, P. Viability and probiotic properties of Lactobacillus plantarum TISTR 2075 in spray-dried fermented cereal extracts. Maejo Int. J. Sci. Technol. 2015, 9, 382. [Google Scholar] [CrossRef]
- Shehata, M.; El Sohaimy, S.; El-Sahn, M.A.; Youssef, M. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016, 61, 65–75. [Google Scholar] [CrossRef]
- Klaver, F.; Van der Meer, R. The assumed assimilation of cholesterol by lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl. Environ. Microbiol. 1993, 59, 1120–1124. [Google Scholar] [CrossRef]
- Gilliland, S.; Nelson, C.; Maxwell, C. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1985, 49, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Thibessard, A.; Borges, F.; Fernandez, A.; Gintz, B.; Decaris, B.; Leblond-Bourget, N. Identification of Streptococcus thermophilus CNRZ368 genes involved in defense against superoxide stress. Appl. Environ. Microbiol. 2004, 70, 2220–2229. [Google Scholar] [CrossRef]
- Arasu, M.V.; Kim, D.H.; Kim, P.I.; Jung, M.W.; Ilavenil, S.; Jane, M.; Lee, K.D.; Al-Dhabi, N.A.; Choi, K.C. In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann. Microbiol. 2014, 64, 1333–1346. [Google Scholar] [CrossRef]
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L. A review of natural and synthetic antioxidants important for health and longevity. Curr. Med. Chem. 2010, 17, 3262–3288. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, B.-H.; Kim, Y.-H.; Nam, S.-W.; Jeon, S.-J. Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol. Bioprocess Eng. 2007, 12, 707–712. [Google Scholar] [CrossRef]
- Villegas, J.M.; Brown, L.; de Giori, G.S.; Hebert, E.M.J.L.-F.S. Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT-Food Sci. Technol. 2016, 67, 22–26. [Google Scholar] [CrossRef]
- Savini, M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Pilot-scale production and viability analysis of freeze-dried probiotic bacteria using different protective agents. Nutrients 2010, 2, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Lakhtin, V.; Aleshkin, V.; Lakhtin, M.; Afanas’ev, S.; Pospelova, V.; Shenderov, B. Lectins, adhesins, and lectin-like substances of lactobacilli and bifidobacteria. Vestn. Ross. Akad. Med. Nauk. 2006, 1, 28–34. [Google Scholar]
- Wagner, R.D.; Johnson, S.J. Probiotic bacteria prevent Salmonella–induced suppression of lymphoproliferation in mice by an immunomodulatory mechanism. BMC Microbiol. 2017, 17, 77. [Google Scholar] [CrossRef]
- Gopal, P.K.; Prasad, J.; Smart, J.; Gill, H.S. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 2001, 67, 207–216. [Google Scholar] [CrossRef]

| Strain | Amount of Crystal Violet (µg) | Antioxidant Activity (mg Gallic Acid/ mL Extract) |
|---|---|---|
| L. reuteri KUKPS6103 | 4.61 ± 1.17 c,* | 0.096 ± 0.041 a |
| L. rhamnosus KUKPS6007 L. paracasei KUKPS6201 L. acidophilus KUKPS6107 L. salivarius KUKPS6202 B. coagulans KPSTF02 S. boulardii KUKPS6005 Multi-strain | 2.45 ± 0.47 a,b,c 4.45 ± 0.02 c 3.74 ± 1.35 c 3.82 ± 1.48 c 0.98 ± 0.52 a 1.25 ± 0.20 a,b 3.36 ± 1.45 b,c | 2.246 ± 0.190 b 2.038 ± 0.219 b 1.893 ± 0.292 b 2.403 ± 0.298 b 0.220 ± 0.140 a 0.311 ± 0.090 a 2.310 ± 0.138 b |
| Time (Week) | Viability of Cell Count (CFU/g) | Survival Rate (%) |
|---|---|---|
| 0 1 2 3 4 5 6 7 8 | 4.90 ± 1.90 × 109 * 8.26 ± 0.71 × 108 6.56 ± 0.78 × 109 1.75 ± 0.41 × 109 4.66 ± 0.86 × 109 3.76 ± 0.73 × 109 1.18 ± 0.08 × 109 9.56 ± 0.65 × 108 7.53 ± 0.65 × 108 | 100.00 16.86 133.88 35.71 95.10 76.73 24.08 19.51 15.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swe, Z.M.; Chumphon, T.; Panya, M.; Pangjit, K.; Promsai, S. Evaluation of Nano-Wall Material for Production of Novel Lyophilized-Probiotic Product. Foods 2022, 11, 3113. https://doi.org/10.3390/foods11193113
Swe ZM, Chumphon T, Panya M, Pangjit K, Promsai S. Evaluation of Nano-Wall Material for Production of Novel Lyophilized-Probiotic Product. Foods. 2022; 11(19):3113. https://doi.org/10.3390/foods11193113
Chicago/Turabian StyleSwe, Zin Myo, Thapakorn Chumphon, Marutpong Panya, Kanjana Pangjit, and Saran Promsai. 2022. "Evaluation of Nano-Wall Material for Production of Novel Lyophilized-Probiotic Product" Foods 11, no. 19: 3113. https://doi.org/10.3390/foods11193113
APA StyleSwe, Z. M., Chumphon, T., Panya, M., Pangjit, K., & Promsai, S. (2022). Evaluation of Nano-Wall Material for Production of Novel Lyophilized-Probiotic Product. Foods, 11(19), 3113. https://doi.org/10.3390/foods11193113







