Effects of Potato Protein Isolated Using Ethanol on the Gelation and Anti-Proteolytic Properties in Pacific Whiting Surimi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Potato Fruit Juice (PFJ)
2.3. Potato Protein Isolation
2.4. Effect of Inhibitors on Protease Inhibition
2.5. Surimi Gel Preparation
2.6. Water Retention Ability Measurement
2.7. Texture Analysis
2.8. Determination of Whiteness
2.9. Sodium Dodecyl Sulfate-polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.10. Scanning Electron Microscopy (SEM)
2.11. Evaluation of Fractal Dimension (Df)
2.12. Statistical Analysis
3. Results and Discussion
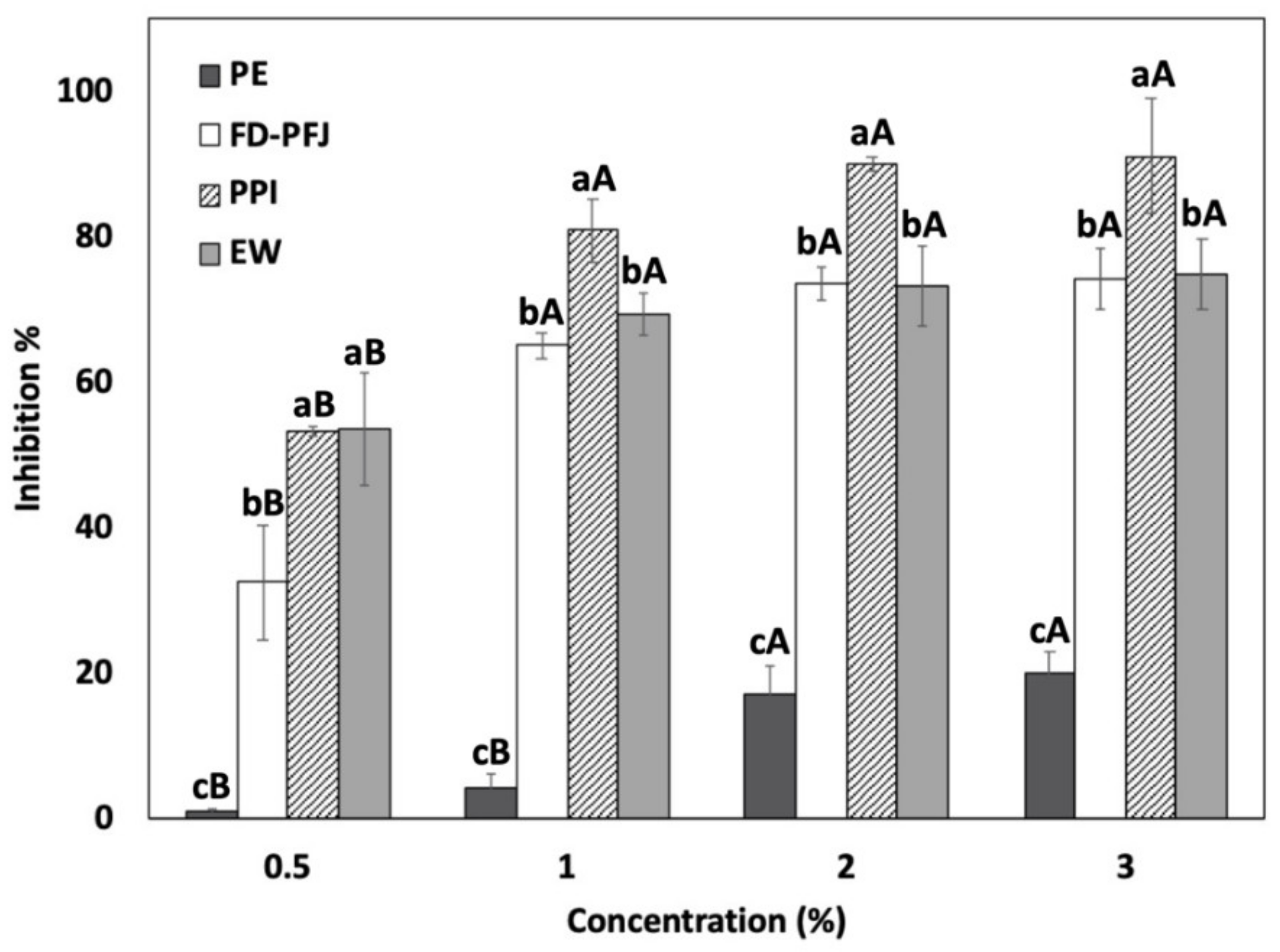
3.1. Effect of PPI on Autolysis of Surimi Paste
3.2. Surimi Gel Texture and Water Retention as Affected by Various Protease Inhibitors
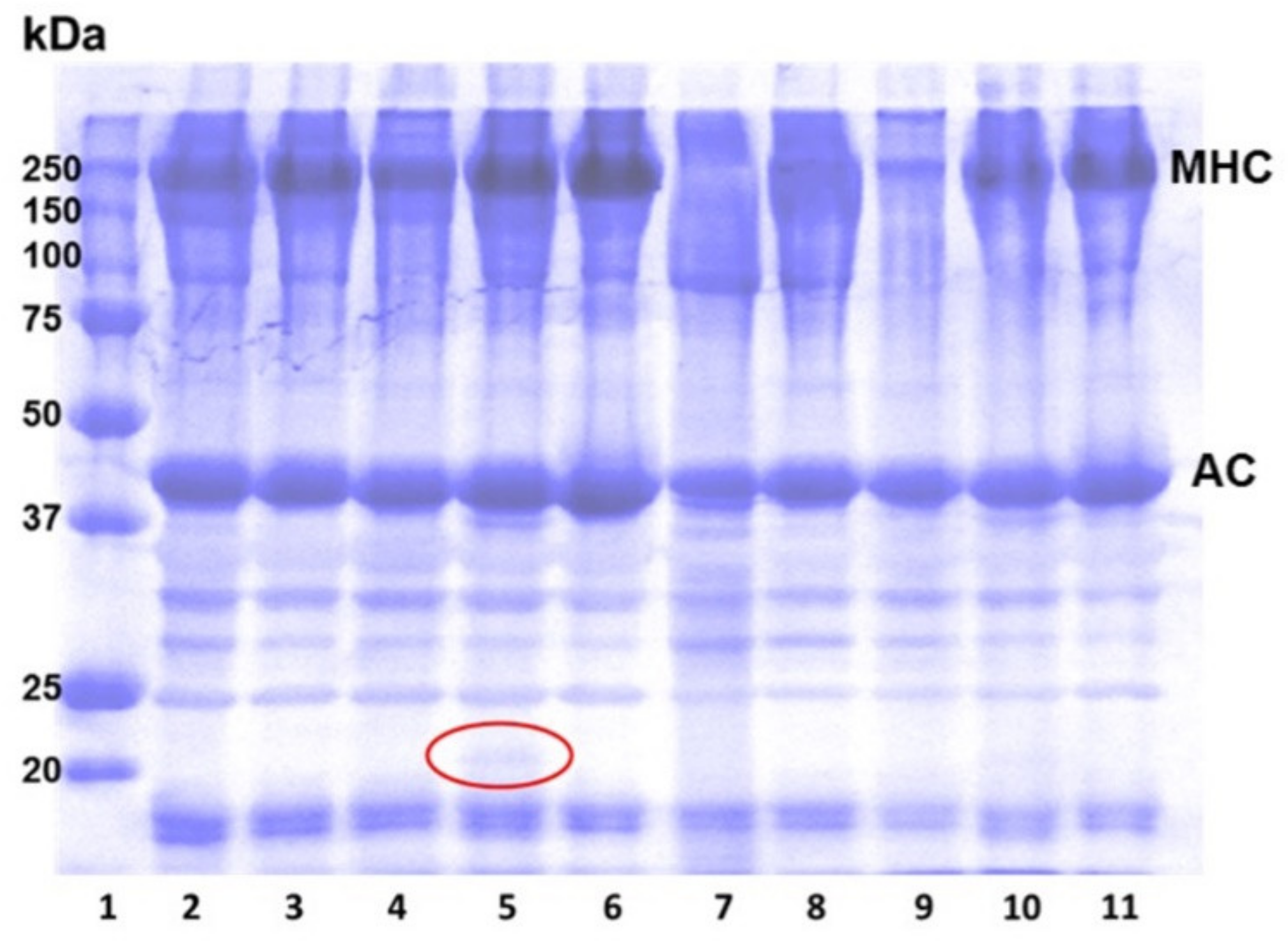
3.3. Protein Patterns of Surimi Gels as Affected by Protease Inhibitors
3.4. Whiteness of Surimi Gels
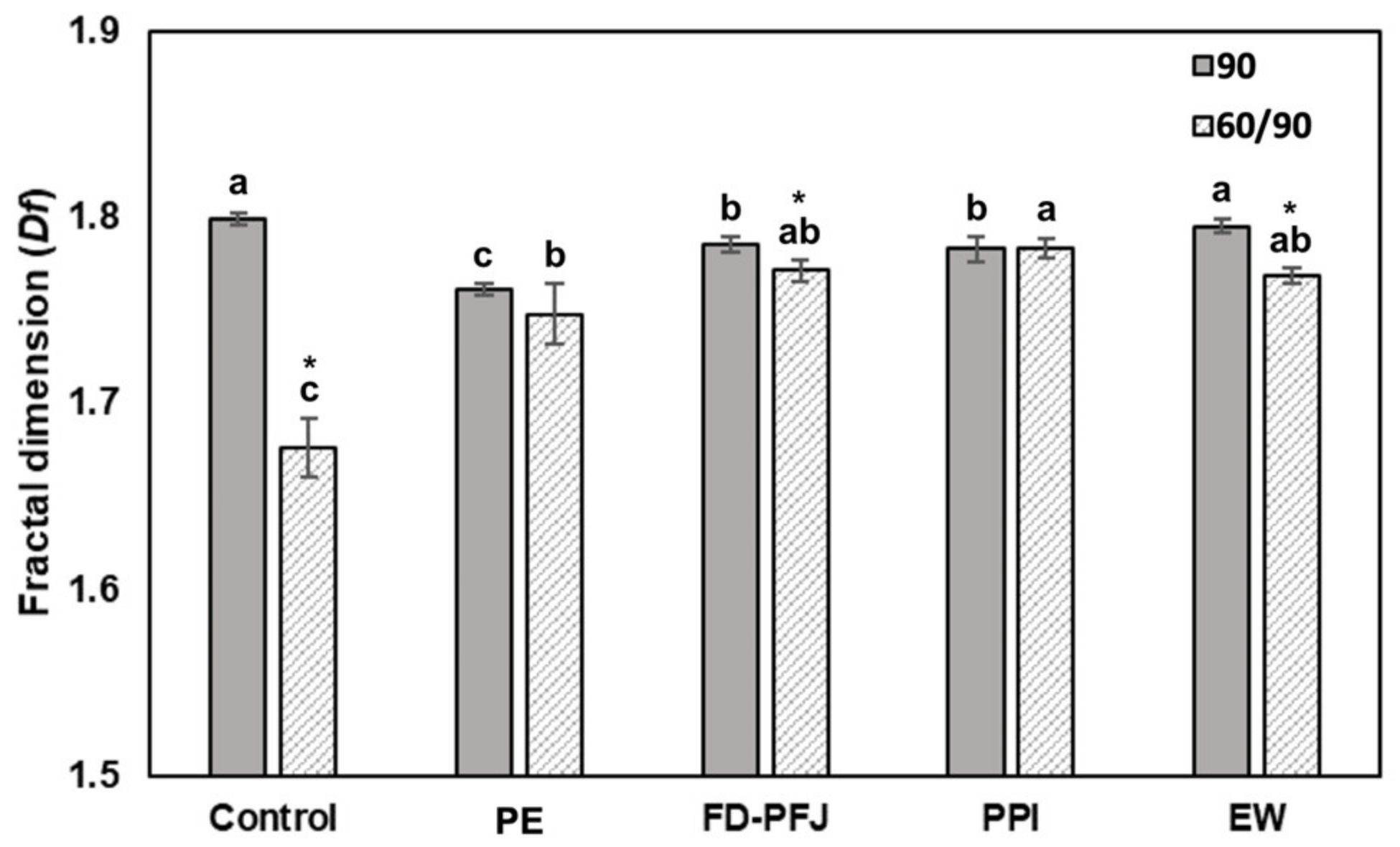
3.5. Microstructure and Its Patterns of Surimi Gel with Various Enzyme Inhibitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowler, M.R.; Park, J.W. Effect of salmon plasma protein on Pacific whiting surimi gelation under various ohmic heating conditions. LWT-Food Sci. Technol. 2015, 61, 309–315. [Google Scholar] [CrossRef]
- Klesk, K.; Yongsawatdigul, J.; Park, J.W.; Viratchakul, S.; Virulhakul, P. Gel forming ability of tropical tilapia surimi as compared with Alaska pollock and Pacific whiting surimi. J. Aquat. Food Prod. Technol. 2000, 9, 91–104. [Google Scholar] [CrossRef]
- An, H.; Weerasinghe, V.; Seymour, T.A.; Morrissey, M.T. Cathepsin degradation of Pacific whiting surimi proteins. J. Food Sci. 1994, 59, 1013–1017. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, J.W. Acid-aided protein recovery from enzyme-rich Pacific whiting. J. Food Sci. 2002, 67, 2962–2967. [Google Scholar] [CrossRef]
- Fowler, M.R.; Park, J.W. Salmon blood plasma: Effective inhibitor of protease-laden Pacific whiting surimi and salmon mince. Food Chem. 2015, 176, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.T.; Wu, J.W.; Lin, D.; An, H. Protease inhibitor effects on torsion measurements and autolysis of Pacific whiting surimi. J. Food Sci. 1993, 58, 1050–1054. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Benjakul, S.; Visessanguan, W.; Lanier, T.C. Chicken plasma protein: Proteinase inhibitory activity and its effect on surimi gel properties. Food Res. Int. 2004, 37, 156–165. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Benjakul, S. Whey protein concentrate: Autolysis inhibition and effects on the gel properties of surimi prepared from tropical fish. Food Chem. 2008, 106, 1077–1084. [Google Scholar] [CrossRef]
- Visessanguan, W.; Benjakul, S.; An, H. Porcine plasma proteins as a surimi protease inhibitor: Effects on actomyosin gelation. J. Food Sci. 2000, 65, 607–611. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Liu, X.; Qiao, M.; Yi, S.; Li, X.; Li, J. Effects of chickpea and peanut protein isolates on the gelling properties of hairtail (Trichiurus haumela) myosin. LWT-Food Sci. Technol. 2022, 163, 113562. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Serine protease inhibitors from squid ovary: Extraction and its effect on proteolysis and gel properties of surimi. J. Food Sci. Technol. 2017, 54, 267–275. [Google Scholar] [CrossRef]
- Strætkvern, K.O.; Schwarz, J.G. Recovery of native potato protein comparing expanded bed adsorption and ultrafiltration. Food Bioprocess Technol. 2012, 5, 1939–1949. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J. Chemical composition and nutritional value of protein concentrates isolated from potato (Solanum tuberosum L.) fruit juice by precipitation with ethanol or ferric chloride. J. Agric. Food Chem. 2009, 57, 9028–9034. [Google Scholar] [CrossRef]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef]
- Løkra, S.; Strætkvern, K.O. Industrial proteins from potato juice. A review. Food 2009, 3, 88–95. [Google Scholar]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT-Food Sci. Technol. 2012, 46, 97–103. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Park, J.W.; Kolbe, E.; Dagga, Y.A.; Morrissey, M.T. Ohmic heating maximizes gel functionality of Pacific whiting surimi. J. Food Sci. 1995, 60, 10–14. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, Y.; Yu, J.; Hu, M.; Xue, Y.; Xue, C. Alaska pollock surimi addition affects Pacific white shrimp (Litopenaeus vannamei) surimi gel properties. Rheol. Acta 2021, 60, 741–749. [Google Scholar] [CrossRef]
- Zhu, J.; Fan, D.; Zhao, J.; Zhang, H.; Huang, J.; Zhou, W.; Zhang, W.; Chen, W. Enhancement of the gelation properties of surimi from yellowtail Seabream (Parargyrops edita, Sparidae) with Chinese Oak silkworm pupa, Antheraea pernyi. J. Food Sci. 2016, 81, E396–E403. [Google Scholar] [CrossRef]
- Vikelouda, M.; Kiosseoglou, V. The use of carboxymethylcellulose to recover potato proteins and control their functional properties. Food Hydrocoll. 2004, 18, 21–27. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chai, P.P.; Park, J.W. Physical properties of fish proteins cooked with starches or protein additives under ohmic heating. J. Food Qual. 2007, 30, 783–796. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Moon, J.H.; Yoon, W.B.; Park, J.W. Assessing the textural properties of Pacific whiting and Alaska pollock surimi gels prepared with carrot under various heating rates. Food Biosci. 2017, 20, 12–18. [Google Scholar] [CrossRef]
- Lineweaver, H.; Murray, C.W. Identification of the trypsin inhibitor of egg white with ovomucoid. J. Biol. Chem. 1947, 171, 565–581. [Google Scholar] [CrossRef]
- Thawornchinsombut, S.; Park, J.W. Role of pH in solubility and conformational changes of Pacific whiting muscle proteins. J. Food Biochem. 2004, 28, 135–154. [Google Scholar] [CrossRef]
- Hunt, A.; Park, J.W.; Handa, A. Effect of various types of egg white on characteristics and gelation of fish myofibrillar proteins. J. Food Sci. 2009, 74, C683–C692. [Google Scholar] [CrossRef]
- Zhang, D.; Mu, T.; Sun, H. Calorimetric, rheological, and structural properties of potato protein and potato starch composites and gels. Starch-Stärke 2017, 69, 1600329. [Google Scholar] [CrossRef]
- Katzav, H.; Chirug, L.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Comparison of thermal and high-pressure gelation of potato protein isolates. Foods 2020, 9, 1041. [Google Scholar] [CrossRef]
- Witczak, T.; Juszczak, L.; Ziobro, R.; Korus, J. Rheology of gluten-free dough and physical characteristics of bread with potato protein. J. Food Process Eng. 2017, 40, e12491. [Google Scholar] [CrossRef]
- Clemente, M.; Corigliano, M.G.; Pariani, S.A.; Sánchez-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant serine protease inhibitors: Biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci. 2019, 20, 1345. [Google Scholar] [CrossRef] [PubMed]
- Oujifard, A.; Benjakul, S.; Ahmad, M.; Seyfabadi, J. Effect of bambara groundnut protein isolate on autolysis and gel properties of surimi from threadfin bream (Nemipterus bleekeri). LWT-Food Sci. Technol. 2012, 47, 261–266. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella). LWT-Food Sci. Technol. 2013, 50, 511–518. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Kishimura, H.; Osako, K.; Simpson, B.K. Trypsin inhibitor from yellowfin tuna (Thunnus albacores) roe: Effects on gel properties of surimi from bigeye snapper (Priacanthus macracanthus). LWT-Food Sci. Technol. 2016, 65, 122–127. [Google Scholar] [CrossRef]
- Singh, A.; Prabowo, F.F.; Benjakul, S.; Pranoto, Y.; Chantakun, K. Combined effect of microbial transglutaminase and ethanolic coconut husk extract on the gel properties and in-vitro digestibility of spotted golden goatfish (Parupeneus heptacanthus) surimi gel. Food Hydrocoll. 2020, 109, 106107. [Google Scholar] [CrossRef]
- García-Armenta, E.; Gutiérrez-López, G.F. Fractal microstructure of foods. Food Eng. Rev. 2022, 14, 1–19. [Google Scholar] [CrossRef]

| Ingredient (g) | Inhibitor (%) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 3 | |
| Pacific whiting surimi | 623.1 | 607.5 | 591.9 | 560.7 | 529.6 |
| Ice | 160.9 | 172.5 | 184.1 | 207.3 | 230.4 |
| Salt | 16 | 16 | 16 | 16 | 16 |
| Inhibitor | 0 | 4 | 8 | 16 | 24 |
| Total | 800 | 800 | 800 | 800 | 800 |
| Concentration (%) | Breaking Force (g) | Penetration Distance (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| PE | FD-PFJ | PPI | EW | PE | FD-PFJ | PPI | EW | |
| 0 | 262.8 ± 9.8 a | 262.8 ± 9.8 c | 262.8 ± 9.8 e | 262.8 ± 9.8 d | 11.3 ± 0.4 a | 11.3 ± 0.4 b | 11.3 ± 0.4 c | 11.3 ± 0.4 c |
| 0.5 | 256.9 ± 11.2 aD | 338.0 ± 18.8 bA | 319.4 ± 15.2 dB | 279.5 ± 14.8 cC | 10.8 ± 0.3 bB | 12.0 ± 0.6 aA | 11.9 ± 0.5 bA | 10.8 ± 0.3 dB |
| 1 | 268.4 ± 15.7 aC | 379.9 ± 17.6 aA | 382.3 ± 18.2 cA | 309.2 ± 15.5 bB | 10.9 ± 0.4 bD | 12.4 ± 0.3 aB | 13.1 ± 0.5 aA | 11.5 ± 0.7 bcC |
| 2 | 260.7 ± 8.4 aC | 322.4 ± 14.8 bB | 445.2 ± 16.8 bA | 314.5 ± 10.9 bB | 10.6 ± 0.3 bD | 11.3 ± 0.5 bC | 13.5 ± 0.4 aA | 12.0 ± 0.5 aB |
| 3 | 262.2 ± 11.3 aC | 199.5 ± 14.0 dD | 469.4 ± 9.3 aA | 336.7 ± 14.9 aB | 10.6 ± 0.3 bC | 6.7 ± 0.3 cD | 13.1 ± 0.2 aA | 11.8 ± 0.3 abB |
| Concentration (%) | Breaking Force (g) | Penetration Distance (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| PE | FD-PFJ | PPI | EW | PE | FD-PFJ | PPI | EW | |
| 0 | 43.0 ± 3.0 c | 43.0 ± 3.0 d | 43.0 ± 3.0 e | 43.0 ± 3.0 e | 1.4 ± 0.3 c | 1.4 ± 0.3 e | 1.4 ± 0.3 d | 1.4 ± 0.3 d |
| 0.5 | 43.8 ± 1.4 cD | 53.7 ± 4.5 cC | 126.7 ± 2.4 dA | 108.6 ± 10.7 dB | 1.2 ± 0.2 cC | 2.1 ± 0.3 dB | 5.9 ± 0.3 cA | 5.4 ± 0.6 cA |
| 1 | 46.0 ± 2.4 cD | 91.0 ± 4.9 bC | 211.6 ± 9.0 cA | 194.3 ± 11.0 cB | 1.5 ± 0.2 cC | 3.8 ± 0.3 cB | 8.9 ± 0.4 aA | 8.7 ± 0.5 bA |
| 2 | 62.3 ± 3.1 bD | 135.5 ± 15.8 aC | 248.2 ± 13.1 bB | 284.8 ± 9.9 bA | 2.4 ± 0.1 bD | 6.0 ± 0.6 aC | 9.8 ± 0.6 bB | 11.1 ± 0.5 aA |
| 3 | 80.8 ± 3.6 aD | 85.6 ± 2.5 aC | 266.0 ± 9.4 aB | 330.5 ± 8.0 aA | 3.3 ± 0.3 aC | 3.2 ± 0.2 bC | 9.1 ± 0.3 aB | 11.4 ± 0.2 aA |
| Concentration (%) | 90 | 60/90 | ||||||
|---|---|---|---|---|---|---|---|---|
| PE | FD-PFJ | PPI | EW | PE | FD-PFJ | PPI | EW | |
| 0 | 49.7 ± 1.5 b | 49.7 ± 1.5 a | 49.7 ± 1.5 b | 49.7 ± 1.5 bc | 26.8 ± 1.6 b | 26.8 ± 1.6 c | 26.8 ± 1.6 d | 26.8 ± 1.6 c |
| 0.5 | 66.9 ± 1.0 aA | 50.5 ± 2.1 aB | 48.3 ± 0.4 bB | 50.9 ± 1.3 bcB | 51.2 ± 2.8 aAB | 34.5 ± 2.0 bC | 46.4 ± 1.5 bcB | 53.4 ± 1.9 aA |
| 1 | 66.7 ± 2.0 aA | 49.5 ± 1.1 aB | 51.0 ± 0.4 bB | 53.3 ± 3.3 aB | 52.0 ± 4.0 aA | 40.9 ± 3.3 aB | 48.5 ± 0.6 bAB | 52.6 ± 2.3 aA |
| 2 | 65.2 ± 1.9 aA | 41.8 ± 1.4 bC | 64.4 ± 1.8 aA | 51.9 ± 1.0 abB | 54.4 ± 2.0 aB | 32.7 ± 1.3 bC | 62.3 ± 1.3 aA | 51.8 ± 2.4 aB |
| 3 | 66.2 ± 1.5 aA | 32.6 ± 0.5 cC | 47.4 ± 0.8 bB | 47.7 ± 2.3 cB | 53.0 ± 3.2 aA | 27.4 ± 3.1 cC | 42.6 ± 0.8 cB | 50.0 ± 1.8 bA |
| Concentration (%) | 90 | 60/90 | ||||||
|---|---|---|---|---|---|---|---|---|
| PE | FD-PFJ | PPI | EW | PE | FD-PFJ | PPI | EW | |
| 0 | 77.5 ± 0.6 a | 77.5 ± 0.6 d | 77.5 ± 0.6 b | 77.5 ± 0.6 c | 80.5 ± 0.3 a | 80.5 ± 0.3 bc | 80.5 ± 0.3 a | 80.5 ± 0.3 a |
| 0.5 | 76.3 ± 0.3 bC | 77.8 ± 0.6 dA | 77.3 ± 0.3 bB | 78.1 ± 0.6 bcA | 79.7 ± 0.3 bB | 80.2 ± 0.7 bcA | 79.0 ± 0.5 cC | 79.1 ± 0.4 cBC |
| 1 | 76.0 ± 0.4 cC | 78.5 ± 0.5 cA | 77.5 ± 0.2 bB | 77.7 ± 0.7 abB | 78.6 ± 0.5 cC | 80.1 ± 0.5 cA | 79.1 ± 0.3 cB | 78.9 ± 0.4 cBC |
| 2 | 74.5 ± 0.4 dD | 79.4 ± 0.7 bA | 77.2 ± 0.3 bC | 78.1 ± 0.4 abB | 77.1 ± 0.4 dD | 80.7 ± 0.6 bA | 79.3 ± 0.2 bcB | 79.1 ± 0.4 cC |
| 3 | 73.1 ± 0.3 eC | 80.0 ± 0.7 aA | 78.3 ± 0.2 aB | 78.3 ± 0.6 aB | 75.7 ± 0.4 eC | 81.8 ± 0.5 aA | 79.5 ± 0.1 bB | 79.7 ± 0.2 bB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, W.B.; Park, J.W.; Jung, H. Effects of Potato Protein Isolated Using Ethanol on the Gelation and Anti-Proteolytic Properties in Pacific Whiting Surimi. Foods 2022, 11, 3114. https://doi.org/10.3390/foods11193114
Yoon WB, Park JW, Jung H. Effects of Potato Protein Isolated Using Ethanol on the Gelation and Anti-Proteolytic Properties in Pacific Whiting Surimi. Foods. 2022; 11(19):3114. https://doi.org/10.3390/foods11193114
Chicago/Turabian StyleYoon, Won Byong, Jae Won Park, and Hwabin Jung. 2022. "Effects of Potato Protein Isolated Using Ethanol on the Gelation and Anti-Proteolytic Properties in Pacific Whiting Surimi" Foods 11, no. 19: 3114. https://doi.org/10.3390/foods11193114
APA StyleYoon, W. B., Park, J. W., & Jung, H. (2022). Effects of Potato Protein Isolated Using Ethanol on the Gelation and Anti-Proteolytic Properties in Pacific Whiting Surimi. Foods, 11(19), 3114. https://doi.org/10.3390/foods11193114







