Comparison of Wet Fractionation Methods for Processing Broccoli Agricultural Wastes and Evaluation of the Nutri-Chemical Values of Obtained Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Source
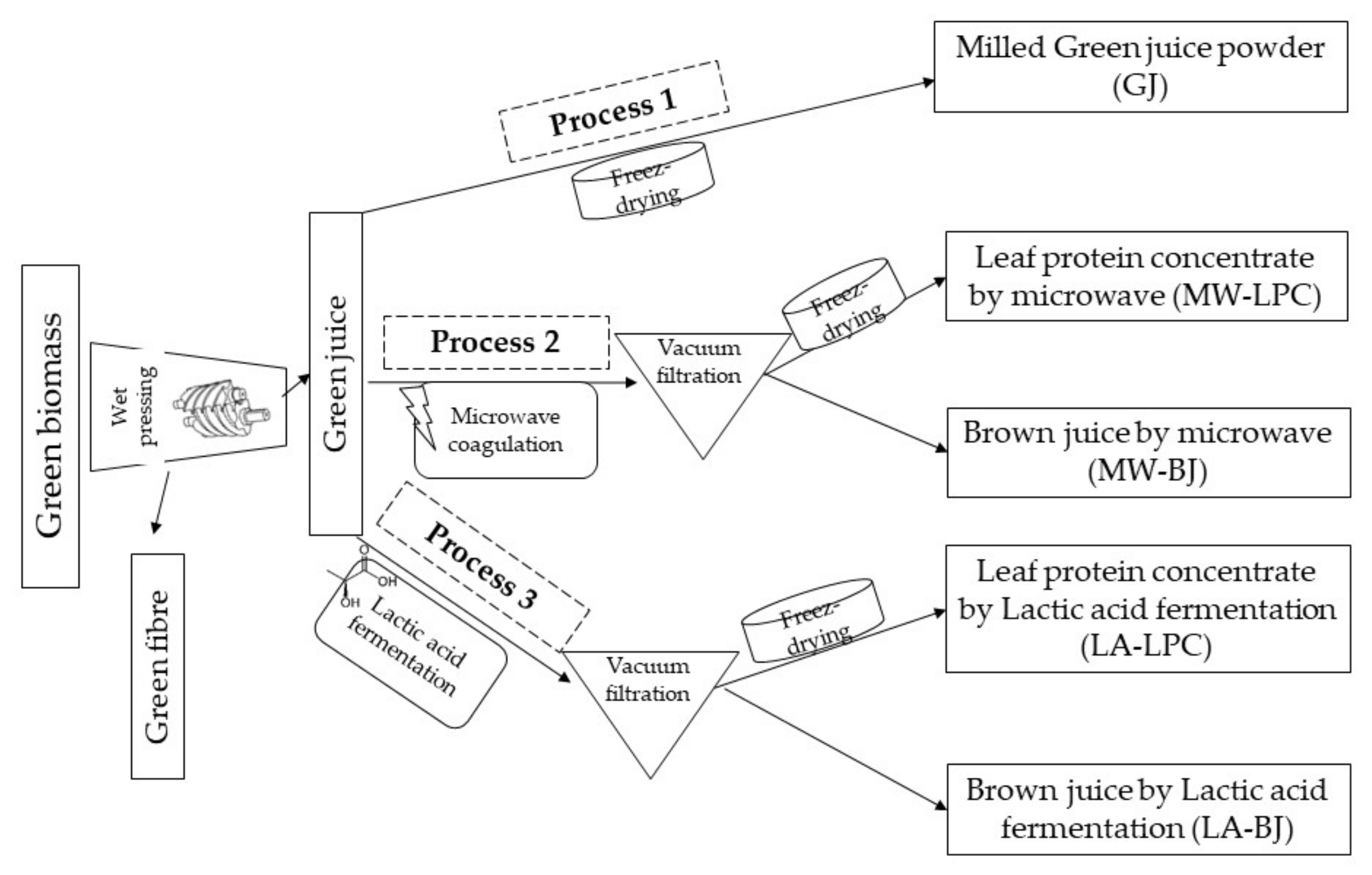
2.3. Processing of Green Biomass
2.4. Physicochemical Parameters of Obtained Products
2.5. Determination of Crude Protein Content
2.6. Quantification of Amino Acids Composition
2.7. Protein Expression Pattern by SDS-PAGE
2.8. Qualitative and Quantitative Phytochemical Analysis
2.9. Statistical Evaluation
3. Results
3.1. Yield and Physicochemical Traits of Processed Products of Broccoli Green Leaves
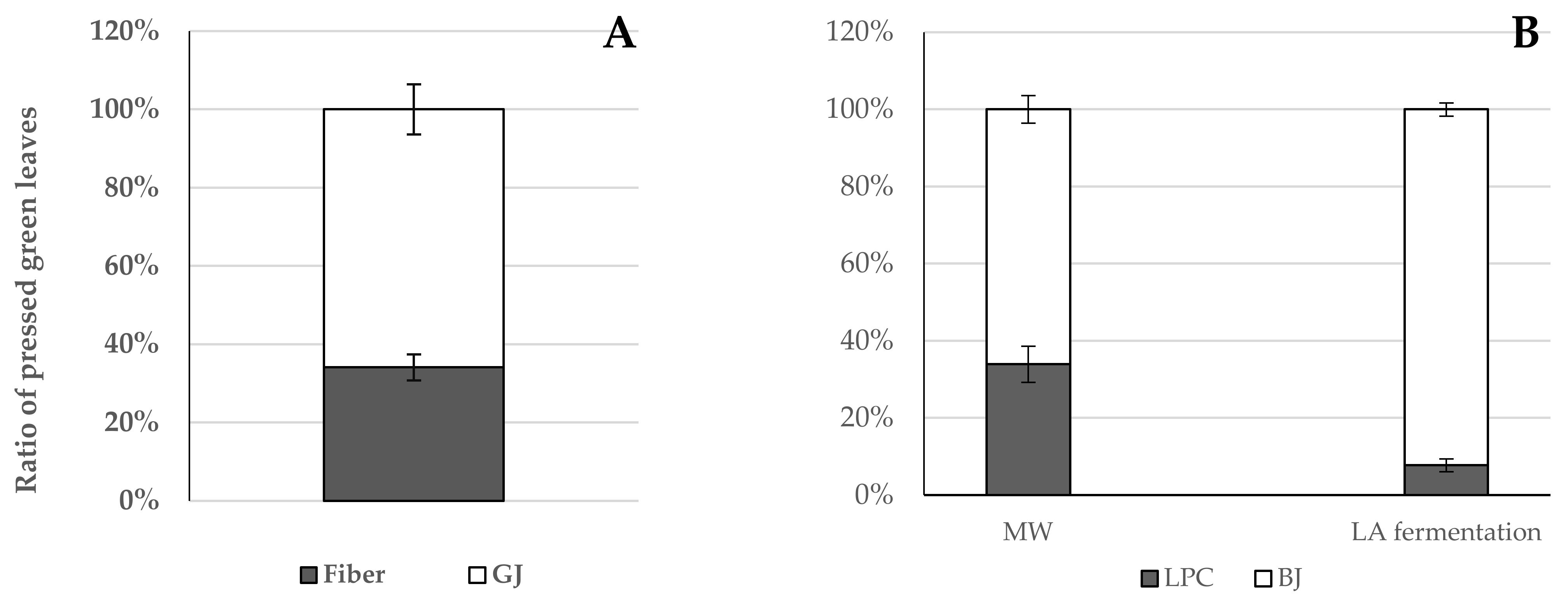
3.1.1. Fresh Yield
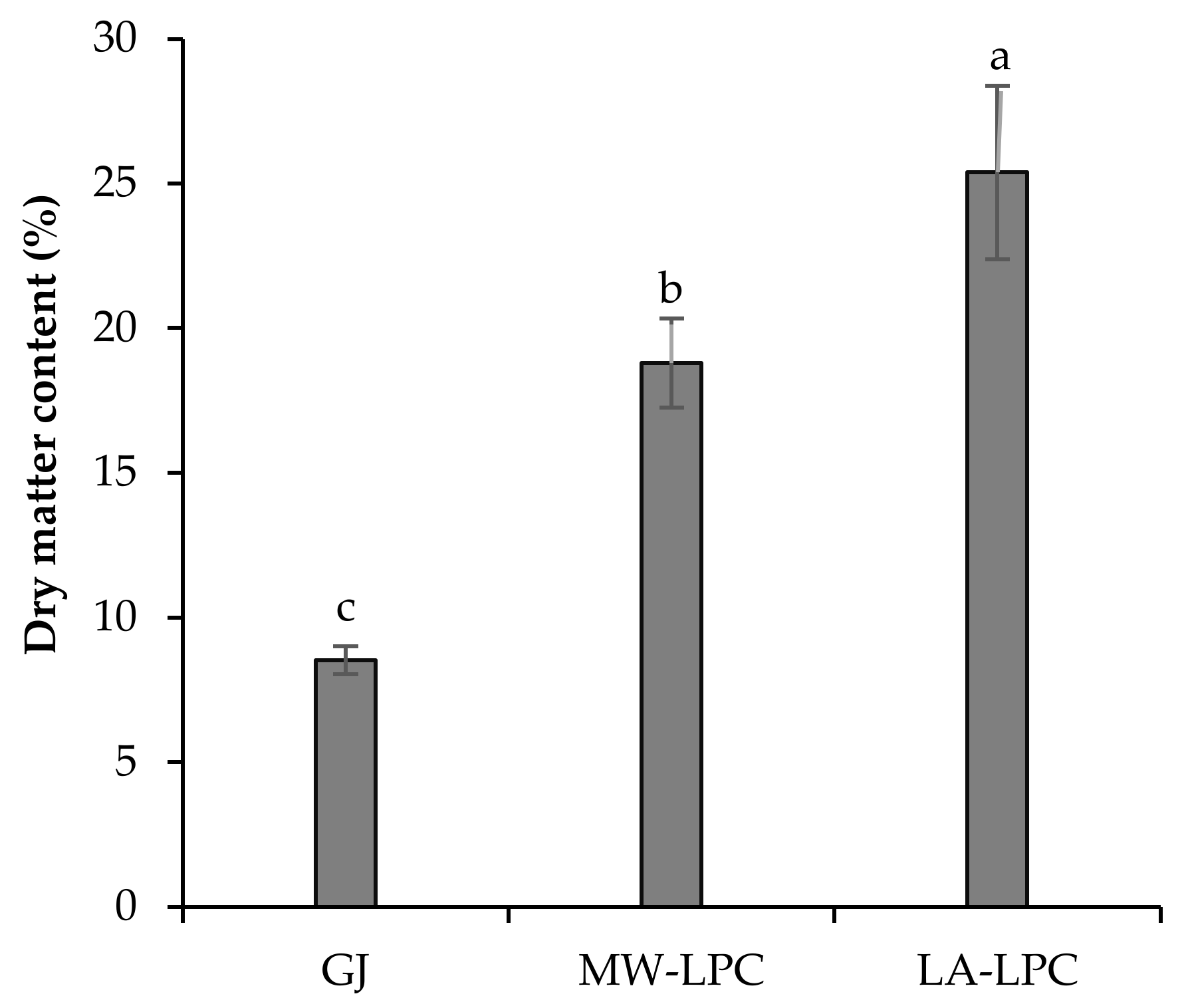
3.1.2. Dry Matter Content of Processed Products of Broccoli Green Leaves
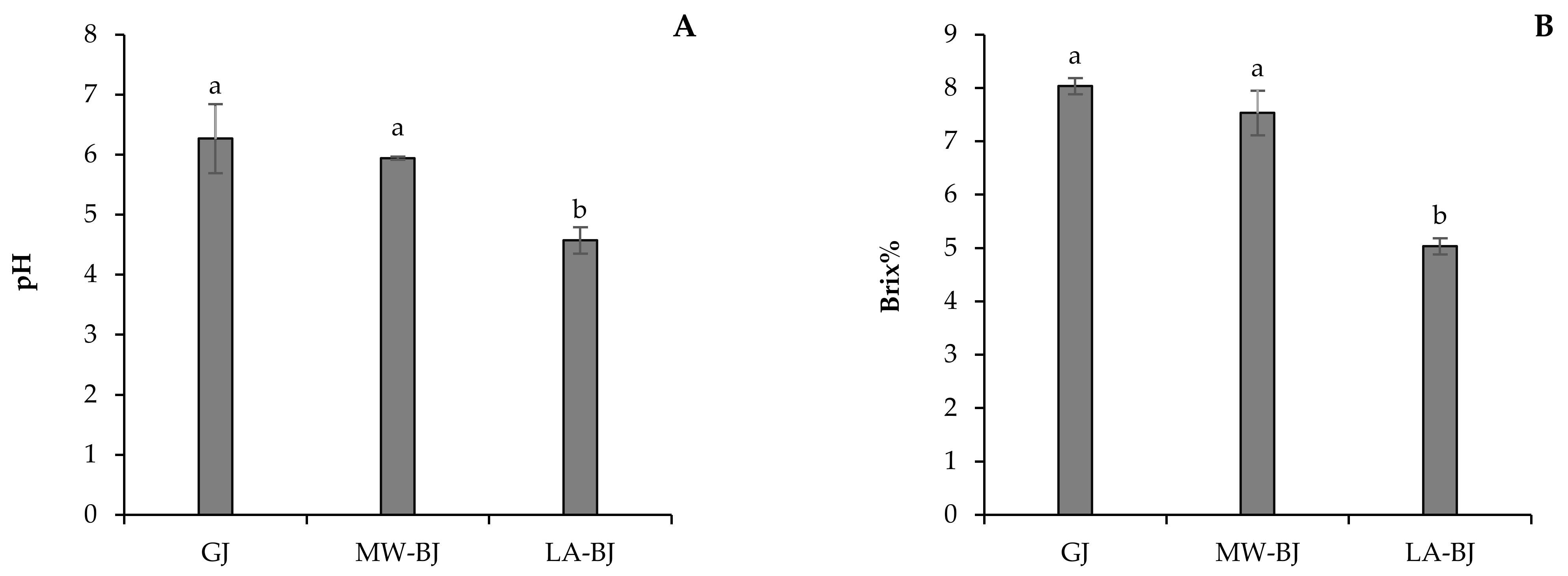
3.1.3. pH and Brix of GJ and BJ Fractions
3.2. Biochemical Assessment of Processed Broccoli Green Leaves
3.2.1. Crude Protein Content and Amino Acids Composition
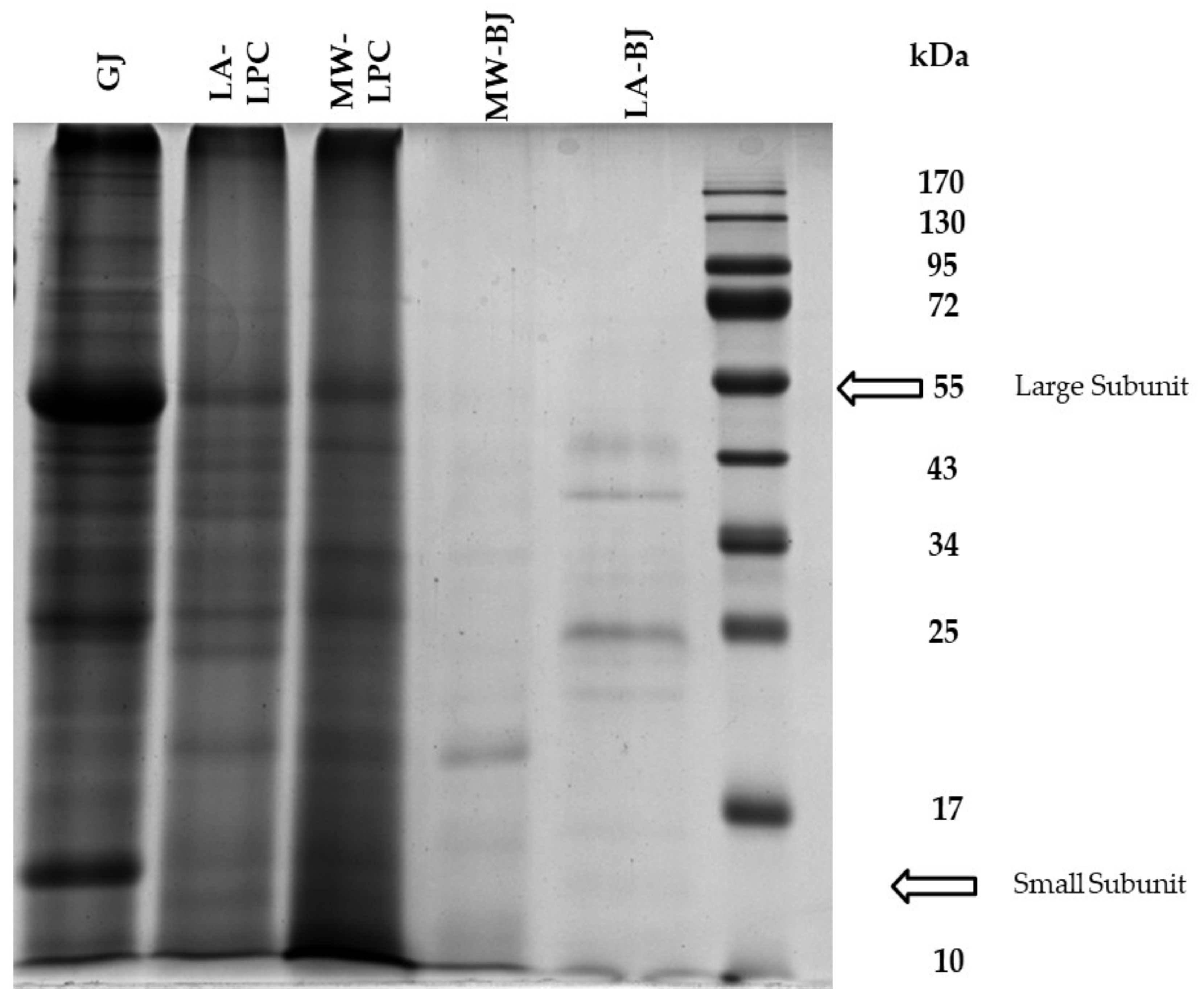
3.2.2. Protein Expression Pattern
3.2.3. Screening of Phytochemicals of GJ
3.2.4. Quantitative Analysis of the Phytochemicals of Fractions Obtained by Different Processes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nynäs, A.-L.; Newson, W.R.; Johansson, E. Protein Fractionation of Green Leaves as an Underutilized Food Source—Protein Yield and the Effect of Process Parameters. Foods 2021, 10, 2533. [Google Scholar] [CrossRef] [PubMed]
- Lübeck, M.; Lübeck, P.S. Application of lactic acid bacteria in green biorefineries. FEMS Microbiol. Lett. 2019, 366, fnz024. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W. Brassica: Characteristics and Properties. Encycl. Food Health 2016, 469–477. [Google Scholar]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products--a promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.-M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Röös, E.; Carlsson, G.; Ferawati, F.; Hefni, M.; Stephan, A.; Tidåker, P.; Witthöft, C. Less meat, more legumes: Prospects and challenges in the transition toward sustainable diets in Sweden. Renew. Agric. Food Syst. 2020, 35, 192–205. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.-C.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoids, polyphenols and micronutrient profiles of Brassica oleraceae and plum varieties and their contribution to measures of total antioxidant capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef]
- Prade, T.; Muneer, F.; Berndtsson, E.; Nynäs, A.-L.; Svensson, S.-E.; Newson, W.R.; Johansson, E. Protein fractionation of broccoli (Brassica oleracea, var. Italica) and kale (Brassica oleracea, var. Sabellica) residual leaves—A pre-feasibility assessment and evaluation of fraction phenol and fibre content. Food Bioprod. Process. 2021, 130, 229–243. [Google Scholar] [CrossRef]
- Rodríguez García, S.L.; Raghavan, V. Microwave-Assisted Extraction of Phenolic Compounds from Broccoli (Brassica oleracea) Stems, Leaves, and Florets: Optimization, Characterization, and Comparison with Maceration Extraction. Recent Prog. Nutr. 2022, 2, 011. [Google Scholar] [CrossRef]
- Mazarakis, N.; Snibson, K.; Licciardi, P.V.; Karagiannis, T.C. The potential use of l-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2020, 39, 664–675. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Gieteling, J.; de Jong, G.A.H.; Boom, R.M.; van der Goot, A.J. Recovery of protein from green leaves: Overview of crucial steps for utilisation. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Lübeck, M. Production of leaf protein concentrates in green biorefineries as alternative feed for monogastric animals. Anim. Feed Sci. Technol. 2020, 268, 114605. [Google Scholar] [CrossRef]
- Fári, M.G.; Domokos-Szabolcsy, É. Method for Producing Plant Protein Coagulum. WO-2019150144-A1, 31 January 2018. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Guba, A.; Bába, O.; Tőzsér, J.; Csősz, É.; Kalló, G. Fast and Sensitive Quantification of AccQ-Tag Derivatized Amino Acids and Biogenic Amines by UHPLC-UV Analysis from Complex Biological Samples. Metabolites 2022, 12, 272. [Google Scholar] [CrossRef]
- Kaszás, L.; Alshaal, T.; El-Ramady, H.; Kovács, Z.; Koroknai, J.; Elhawat, N.; Nagy, É.; Cziáky, Z.; Fári, M.; Domokos-Szabolcsy, É. Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.). Plants 2020, 9, 889. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; Ferreres, F. Characterisation of flavonols in broccoli (Brassica oleracea L. var. italica) by liquid chromatography-uV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. A 2004, 1054, 181–193. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Y.; Haytowitz, D.B.; Chen, P.; Pehrsson, P.R. Effects of domestic cooking on flavonoids in broccoli and calculation of retention factors. Heliyon 2019, 5, e01310. [Google Scholar] [CrossRef]
- De Fremery, D.; Miller, R.E.; Edwards, R.H.; Knuckles, B.E.; Bickoff, E.M.; Kohler, G.O. Centrifugal separation of white and green protein fractions from alfalfa juice following controlled heating. J. Agric. Food Chem. 1973, 21, 886–889. [Google Scholar] [CrossRef]
- Morrison, J.E.; Pirie, N.W. The large-scale production of protein from leaf extracts. J. Sci. Food Agric. 1961, 12, 1–5. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Alassali, A.; Cybulska, I.; Yousef, A.F.; Brown, J.J.; Andersen, M.; Ratkov, A.; Kiel, P. Microorganisms for Biorefining of Green Biomass. In Microorganisms in Biorefineries; Microbiology Monographs; Kamm, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 26, pp. 157–181. ISBN 978-3-662-45208-0. [Google Scholar]
- Novik, G.; Meerovskaya, O.; Savich, V. Waste Degradation and Utilization by Lactic Acid Bacteria: Use of Lactic Acid Bacteria in Production of Food Additives, Bioenergy and Biogas; IntechOpen: London, UK, 2017; ISBN 978-953-51-3490-9. [Google Scholar]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Molinuevo-Salces, B.; Kiel, P.; Steenfeldt, S.; Uellendahl, H.; Lübeck, M. Lactic acid fermentation for refining proteins from green crops and obtaining a high quality feed product for monogastric animals. J. Clean. Prod. 2017, 162, 875–881. [Google Scholar] [CrossRef]
- Weinberg, Z.G. Preservation of Forage Crops by Solid-state Lactic Acid Fermentation-Ensiling. In Current Developments in Solid-state Fermentation; Pandey, A., Soccol, C.R., Larroche, C., Eds.; Springer: New York, NY, USA, 2008; pp. 443–467. ISBN 978-0-387-75213-6. [Google Scholar]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, E2288. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Bákonyi, N.; Kisvarga, S.; Barna, D.; Tóth Ibolya, O.; El-Ramady, H.; Abdalla, N.; Kovács, S.; Rozbach, M.; Fehér, C.; Elhawat, N.; et al. Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia. Agronomy 2020, 10, 247. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
| GJ | MW-LPC | LA-LPC | MW-BJ | LA-BJ | |
|---|---|---|---|---|---|
| Crude Protein Content (m/m%) | |||||
| 27.43 ± 0.12 c | 34.30 ± 1.77 b | 39.18 ± 0.17 a | 1.96 ± 0.01 a | 1.30 ± 0.03 b | |
| Amino acids composition | |||||
| g 100 g−1 DW | g 100 g−1 FW | ||||
| His | 0.286 ± 0.12 | 0.503 ± 0.01 | 0.253 ± 0.01 | 0.001 ± 0.00 | 0.002 ± 0.00 |
| Asn | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 |
| Ser | 1.285 ± 0.49 | 1.217 ± 0.01 | 1.673 ± 0.48 | 0.002 ± 0.00 | 0.007 ± 0.00 |
| Gln | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 |
| Arg | 1.194 ± 0.23 | 1.342 ± 0.00 | 2.371 ± 0.07 | 0.002 ± 0.01 | 0.005 ± 0.00 |
| Gly | 0.986 ± 0.17 | 1.135 ± 0.00 | 1.248 ± 0.02 | 0.001 ± 0.01 | 0.004 ± 0.00 |
| Asp | 2.013 ± 0.24 | 2.443 ± 0.08 | 2.134 ± 0.03 | 0.005 ± 0.01 | 0.010 ± 0.00 |
| Glu | 2.644 ± 0.06 | 2.648 ± 0.03 | 2.063 ± 0.19 | 0.008 ± 0.04 | 0.013 ± 0.00 |
| Thr | 0.873 ± 0.05 | 1.082 ± 0.01 | 0.751 ± 0.27 | 0.002 ± 0.00 | 0.004 ± 0.00 |
| Ala | 1.080 ± 0.02 | 1.365 ± 0.02 | 1.189 ± 0.07 | 0.002 ± 0.01 | 0.005 ± 0.00 |
| Pro | 0.913 ± 0.06 | 1.198 ± 0.01 | 1.533 ± 0.02 | 0.002 ± 0.00 | 0.004 ± 0.00 |
| Cys | 0.000 ± 0.00 | 0.128 ± 0.00 | 0.067 ± 0.05 | 0.000 ± 0.00 | 0.000 ± 0.00 |
| Lys | 1.115 ± 0.05 | 1.403 ± 0.03 | 1.092 ± 0.08 | 0.002 ± 0.00 | 0.005 ± 0.00 |
| Tyr | 0.743 ± 0.01 | 1.047 ± 0.01 | 0.668 ± 0.02 | 0.001 ± 0.00 | 0.003 ± 0.00 |
| Met | 0.309 ± 0.04 | 0.455 ± 0.00 | 0.321 ± 0.02 | 0.000 ± 0.00 | 0.001 ± 0.00 |
| Val | 0.980 ± 0.09 | 1.407 ± 0.01 | 1.056 ± 0.04 | 0.002 ± 0.00 | 0.004 ± 0.00 |
| Ile | 0.701 ± 0.11 | 1.321 ± 0.36 | 0.787 ± 0.03 | 0.001 ± 0.00 | 0.003 ± 0.00 |
| Leu | 1.568 ± 0.07 | 1.892 ± 0.34 | 1.812 ± 0.06 | 0.002 ± 0.00 | 0.007 ± 0.00 |
| Phe | 1.039 ± 0.02 | 1.411 ± 0.01 | 1.271 ± 0.01 | 0.001 ± 0.00 | 0.004 ± 0.00 |
| Trp | 0.127 ± 0.18 | 0.255 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.01 | 0.000 ± 0.00 |
| Identified Compounds | Formula | [M + H] + (m/z) | [M − H]−(m/z) |
|---|---|---|---|
| Vitamins/vitamin like substances | |||
| Nicotinic acid | C6H5NO2 | 124.040 | |
| Nicotinamide | C6H6N2O | 123.056 | |
| Cabagin-U (Vitamin U) | C6H14NO2S | 164.075 | |
| Pantothenic acid | C9H17NO5 | 220.119 | |
| Riboflavin | C17H20N4O6 | 377.146 | |
| Phylloquinone | C31H46O2 | 451.358 | |
| Biotin | C10H16N2O3S | 245.095 | |
| Flavonoids | |||
| Chalcones | |||
| Isoliquiritigenin (2′,4,4′-trihydroxychalcone) | C15H12O4 | 255.065 | |
| Flavonols | |||
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | C15H10O7 | 301.035 | |
| Quercetin-O-hexoside-O-hexosylhexoside isomer 1 | C33H40O22 | 787.193 | |
| Quercetin-3-O-[caffeoyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-glucoside | C42H46O25 | 949.225 | |
| Quercetin-O-(sinapoyl)hexosylhexoside-O-hexoside | C44H50O26 | 993.251 | |
| Quercetin-3-O-[feruloyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-glucoside | C43H48O25 | 963.241 | |
| Quercetin-O-hexoside-O-hexosylhexoside isomer 2 | C33H40O22 | 787.193 | |
| Quercetin-O-hexosylhexoside isomer 1 | C27H30O17 | 625.140 | |
| Quercetin-di-O-hexoside | C27H30O17 | 625.140 | |
| Quercetin-O-hexosylhexoside isomer 2 | C27H30O17 | 625.140 | |
| Quercetin-3-O-glucoside (Isoquercitrin) | C21H20O12 | 463.088 | |
| Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 285.040 | |
| Kaempferol-O-hexoside-O-hexosylhexoside | C33H40O21 | 771.198 | |
| Kaempferol-7-O-glucoside-3-O-sophoroside | C33H40O21 | 771.198 | |
| Kaempferol-O-(caffeoyl)hexosylhexoside-O-hexoside | C42H46O24 | 933.251 | |
| Kaempferol-3-O-[caffeoyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-glucoside | C42H46O24 | 933.251 | |
| Kaempferol-O-(caffeoyl)hexosylhexoside-O-hexosylhexoside | C48H56O29 | 1095.283 | |
| Kaempferol-3-O-[caffeoyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-[glucosyl-(1→4)-glucoside] | C48H56O29 | 1095.283 | |
| Kaempferol-3-O-[sinapoyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-glucoside | C44H50O25 | 977.256 | |
| Kaempferol-3-O-[sinapoyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-[glucosyl-(1→4-)glucoside] | C50H60O30 | 1139.309 | |
| Kaempferol-3-O-[feruloyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-glucoside | C43H48O24 | 947.246 | |
| Kaempferol-3-O-[feruloyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-[glucosyl-(1→4)-glucoside] | C49H58O29 | 1109,299 | |
| Kaempferol-O-[p-coumaroyl-(→2)-glucosyl-(1→2)-glucoside]-7-O-glucoside | C42H46O23 | 917.235 | |
| Kaempferol-3,7-di-O-glucoside (Paeonoside) | C27H30O16 | 609,146 | |
| Kaempferol-O-(sinapoyl)hexosylhexoside-O-(sinapoyl)hexoside | C55H60O29 | 1183.314 | |
| Kaempferol-di-O-hexoside | C27H30O16 | 609.146 | |
| Kaempferol-O-(caffeoyl)hexosylhexoside | C36H36O19 | 771.177 | |
| Kaempferol-O-(sinapoyl)hexosylhexoside | C38H40O20 | 815.203 | |
| Kaempferol-7-O-sophoroside | C27H30O16 | 609.146 | |
| Kaempferol-O-(feruloyl)hexosylhexoside | C37H38O19 | 785.193 | |
| Kaempferol-O-(4-coumaroyl)hexosylhexoside | C36H36O18 | 755.182 | |
| Kaempferol-O-(disinapoyl)hexosylhexosylhexoside-O-hexoside | C61H70O34 | 1345.367 | |
| Kaempferol-O-hexosylhexoside | C27H30O16 | 609.146 | |
| Kaempferol-3-O-glucoside (Astragalin) | C21H20O11 | 447.093 | |
| Isorhamnetin-O-hexosylhexoside | C28H32O17 | 639.156 | |
| Isorhamnetin-3-O-glucoside | C22H22O12 | 477.103 | |
| Isorhamnetin-7-O-glucoside-3-O-sophoroside (Brassicoside) | C34H42O22 | 801.209 | |
| Flavanones | |||
| 4′.7-Dihydroxyflavanone (Liquiritigenin) | C15H12O4 | 255.066 | |
| 4′,5,7-Trihydroxyflavanone (Naringenin) | C15H12O5 | 271.061 | |
| Flavons | |||
| Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 269.045 | |
| Apigenin-7-O-glucuronide | C21H28O11 | 445.077 | |
| Luteolin (3′.4′.5.7-Tetrahydroxyflavone) | C15H10O6 | 285.039 | |
| Phenolic acids | |||
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | C16H18O9 | 355.103 | |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | C16H18O9 | 355.103 | |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | C16H18O9 | 355.103 | |
| Caffeic acid | C9H8O4 | 179.034 | |
| 4-Coumaric acid | C9H8O3 | 163.040 | |
| Sinapic acid | C11H12O5 | 225.076 | |
| Di-O-sinapoylgentiobiose | C34H42O19 | 753.224 | |
| Tri-O-sinapoylgentiobiose | C45H52O23 | 959.282 | |
| Feruloyl-sinapoyldihexoside | C33H40O18 | 723.214 | |
| Di-O-sinapoylglucose | C28H32O14 | 591.171 | |
| Feruloyl-disinapoyldihexoside | C44H50O22 | 929.272 | |
| Syringaldehyde (3,5-Dimethoxy-4-hydroxybenzaldehyde) | C9H10O4 | 183.066 | |
| Glucosinolates | |||
| Glucobrassicin (3-Indolylmethyl glucosinolate) | C16H20N2O9S2 | 447.053 | |
| 3-Methylsulphinylpropyl isothiocyanate | C5H9NOS2 | 164.020 | |
| 4-Methoxy-3-indolylmethyl glucosinolate | C17H22N2O10S2 | 477.064 | |
| Sulforaphane | C6H11NOS2 | 178.036 | |
| Neoglucobrassicin (1-Methoxy-3-indolylmethyl glucosinolate) | C17H22N2O10S2 | 477.064 | |
| Coumarins | |||
| Scopoletin (7-Hydroxy-6-methoxycoumarin) | C10H8O4 | 193.050 | |
| Other phytocompounds | |||
| γ-Aminobutyric acid | C4H9NO2 | 104.071 | |
| Indole-4-carbaldehyde | C9H7NO | 146.061 | |
| Abscisic acid | C15H20O4 | 263.128 | |
| Kynurenic acid | C10H7NO3 | 190.050 | |
| Vitamins | GJ | MW-LPC | LA-LPC | MW-BJ | LA-BJ |
|---|---|---|---|---|---|
| μg g−1 DW | ng mL−1 | ||||
| Nicotinamide | 9.71 ± 0.17 a | 6.95 ±0.17 b | 4.03 ± 0.20 c | 839.73 ± 101.00 a | 386.36 ± 16.77 b |
| Nicotinic acid | 19.18 ± 0.27 a | 11.97 ± 0.14 b | 11.33 ± 0.43 b | 2093.35 ± 480.15 a | 1620.00 ± 10 a |
| Biotin | 1.54 ± 0.06 a | nd ‡ | nd | nd | nd |
| Riboflavin | 5.01 ± 0.11 b | 3.85 ± 0.08 c | 5.35 ± 0.03 a | 644.37 ± 19.85 b | 1069.55 ± 5.46 a |
| Flavonoids | GJ | MW-LPC | LA-LPC | MW-BJ | LA-BJ |
|---|---|---|---|---|---|
| μg g−1 DW | ng mL−1 | ||||
| Isoliquiritigenin (2′,4,4′-trihydroxychalcone) | 0.22 ± 0.03 a | 0.24 ± 0.04 a | 0.21 ± 0.03 a | 1.57 ± 0.09 b | 2.10 ± 0.07 a |
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | 1.90 ± 0.24 c | 20.26 ± 0,53 b | 48.75 ± 1.30 a | 209.66 ± 116.18a | 7390.00 ± 275.13 b |
| Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | 2.63 ± 0.12 c | 20.91 ± 0.30 b | 895.26 ± 17.13a | 340.00 ± 45.83 b | 13046.67 ± 1956.76 a |
| 4′.7-Dihydroxyflavanone (Liquiritigenin) | 0.14 ± 0.02 a | 0.14 ± 0.01 a | 0.13 ± 0.02 a | 1.19 ± 0.06 b | 1.37 ± 0.04 a |
| Naringenin (4′,5,7-Trihydroxyflavanone) | 1.08 ± 0.06 b | 1.08 ± 0.05 b | 3.13 ± 0.14 a | nd | 38.18 ± 1.85 a |
| Genkwanin | 0.12 ± 0.01 a | nd ‡ | 0.14 ± 0.02 a | nd | 14.73 ± 0.32 a |
| Luteolin | 0.25 ± 0.03 b | 0.26 ± 0.03 b | 2.04 ± 0.11 a | 23.79 ± 9.47 b | 80.34 ± 3.32 a |
| Apigenin | 0.15 ± 0.02 ab | 0.11 ± 0.02 b | 0.20 ± 0.02 a | 1.85 ± 0.16 a | 2.04 ± 0.10 a |
| Apigenin-7-O-glucuronide | 0.17 ± 0.02 ab | 0.14 ± 0.02 b | 0.22 ± 0.02 a | 1.09 ± 0.04 b | 1.17 ± 0.04 a |
| GJ | MW-LPC | LA-LPC | MW-BJ | LA-BJ | |
|---|---|---|---|---|---|
| Phenolic Acids/Glucosinolates | μg g−1 DW | ng mL−1 | |||
| Chlorogenic acid (3-O-Caffeoylquinic acid) | 26.36 ± 2.02 a | 26.52 ± 0.47 a | n.d | 3723.33 ± 183.39 b | 32.36 ± 4.68 a |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | 644.71 ± 16.12 a | 530.81 ± 22.51 b | n.d | 94880.00 ± 4762.02 a | 4.94 ± 1.20 b |
| Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | 57.15 ± 2.20 b | 76.18 ± 2.08 a | n.d | 16733.33 ± 1095.10 a | 12.13 ± 0.56 b |
| Syringaldehyde | 0.39 ± 0.03 b | 1.27 ± 0.03 a | 0.17 ± 0.03 c | 301.12 ± 81.34 a | 34.46 ± 3.59 b |
| p-Coumaric acid | 2.73 ± 0.20 b | 8.26 ± 0.15 a | 0.23 ± 0.05 c | 2623.33 ± 1114.64 b | 22.92 ± 0.75 a |
| Ferulic acid | 4.71 ± 0.21 b | 11.51 ± 0.48 a | 3.55 ± 0.25 c | 6923.33 ± 3122.38 b | 530.29 ± 32.98 a |
| Sinapic acid | 352.20 ± 10.60 a | 208.51 ± 8.19 b | 109.09 ± 11.91 c | 20190.00 ± 1047.62 a | 8376.67 ± 370.04 b |
| Sulforaphane | 17.65 ± 0.12 a | 12.57 ± 0.25 b | n.d | 2083.33 ± 456.33 a | n.d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domokos-Szabolcsy, É.; Elhawat, N.; Domingos, G.J.; Kovács, Z.; Koroknai, J.; Bodó, E.; Fári, M.G.; Alshaal, T.; Bákonyi, N. Comparison of Wet Fractionation Methods for Processing Broccoli Agricultural Wastes and Evaluation of the Nutri-Chemical Values of Obtained Products. Foods 2022, 11, 2418. https://doi.org/10.3390/foods11162418
Domokos-Szabolcsy É, Elhawat N, Domingos GJ, Kovács Z, Koroknai J, Bodó E, Fári MG, Alshaal T, Bákonyi N. Comparison of Wet Fractionation Methods for Processing Broccoli Agricultural Wastes and Evaluation of the Nutri-Chemical Values of Obtained Products. Foods. 2022; 11(16):2418. https://doi.org/10.3390/foods11162418
Chicago/Turabian StyleDomokos-Szabolcsy, Éva, Nevien Elhawat, Geraldo Jorge Domingos, Zoltán Kovács, Judit Koroknai, Enikő Bodó, Miklós Gábor Fári, Tarek Alshaal, and Nóra Bákonyi. 2022. "Comparison of Wet Fractionation Methods for Processing Broccoli Agricultural Wastes and Evaluation of the Nutri-Chemical Values of Obtained Products" Foods 11, no. 16: 2418. https://doi.org/10.3390/foods11162418
APA StyleDomokos-Szabolcsy, É., Elhawat, N., Domingos, G. J., Kovács, Z., Koroknai, J., Bodó, E., Fári, M. G., Alshaal, T., & Bákonyi, N. (2022). Comparison of Wet Fractionation Methods for Processing Broccoli Agricultural Wastes and Evaluation of the Nutri-Chemical Values of Obtained Products. Foods, 11(16), 2418. https://doi.org/10.3390/foods11162418









