Attenuation of Chilling Injury and Improving Antioxidant Capacity of Persimmon Fruit by Arginine Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment of L-arginine
2.2. Assessment of Browning Index
2.3. Assessment of Malondialdehyde (MDA) and H2O2
2.4. Assessment of Firmness and Weight Loss
2.5. Assessment of Titratable Acidity and Total Soluble Solids
2.6. Assessment of Total Soluble Tannin
2.7. Assessment of Total Carotenoid
2.8. Assessment of Antioxidative Enzymes Activity
2.9. Assessment of Total Antioxidant Capacity
2.10. Statistical Analysis
3. Results and Discussion
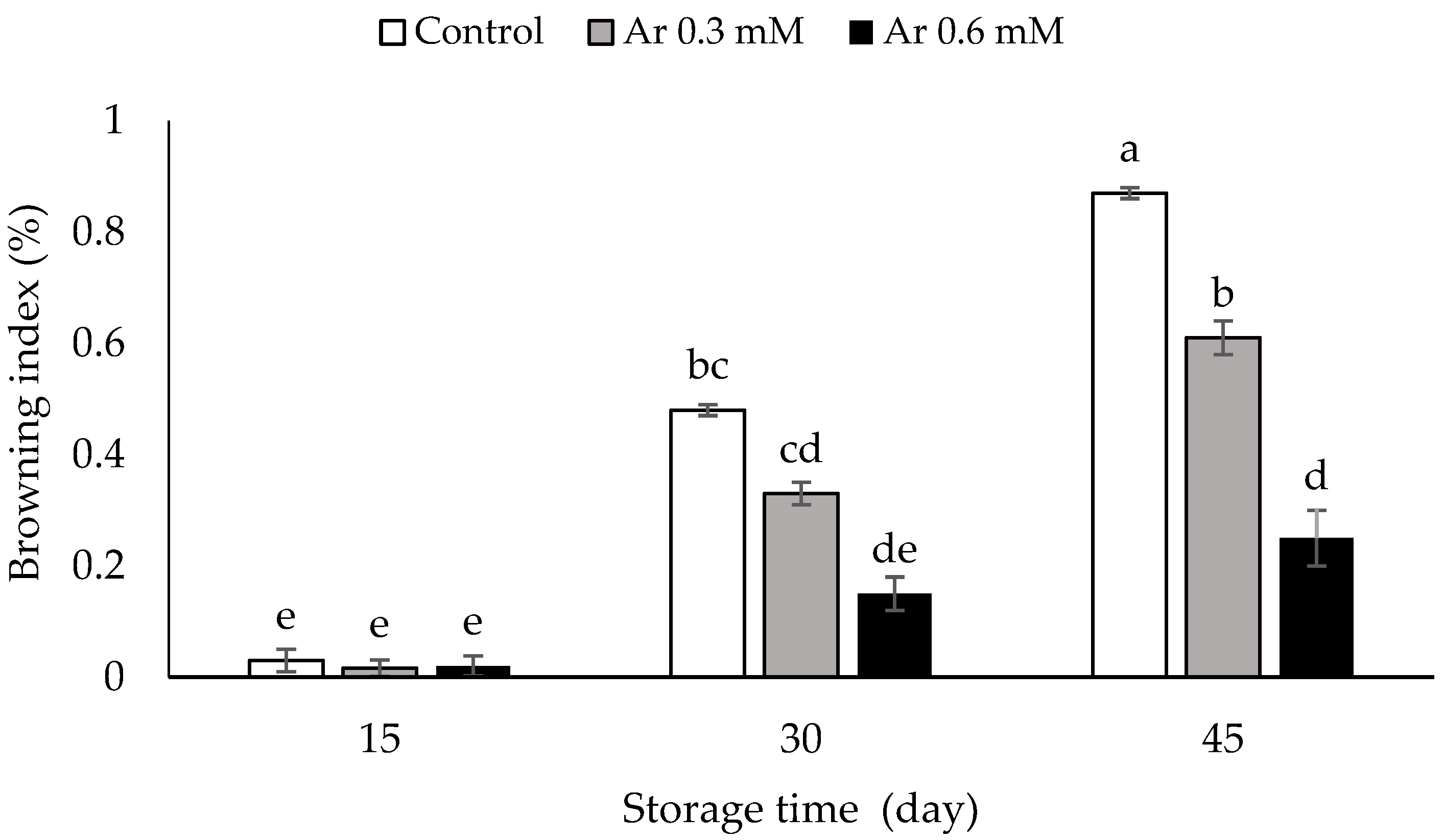
3.1. Browning Index
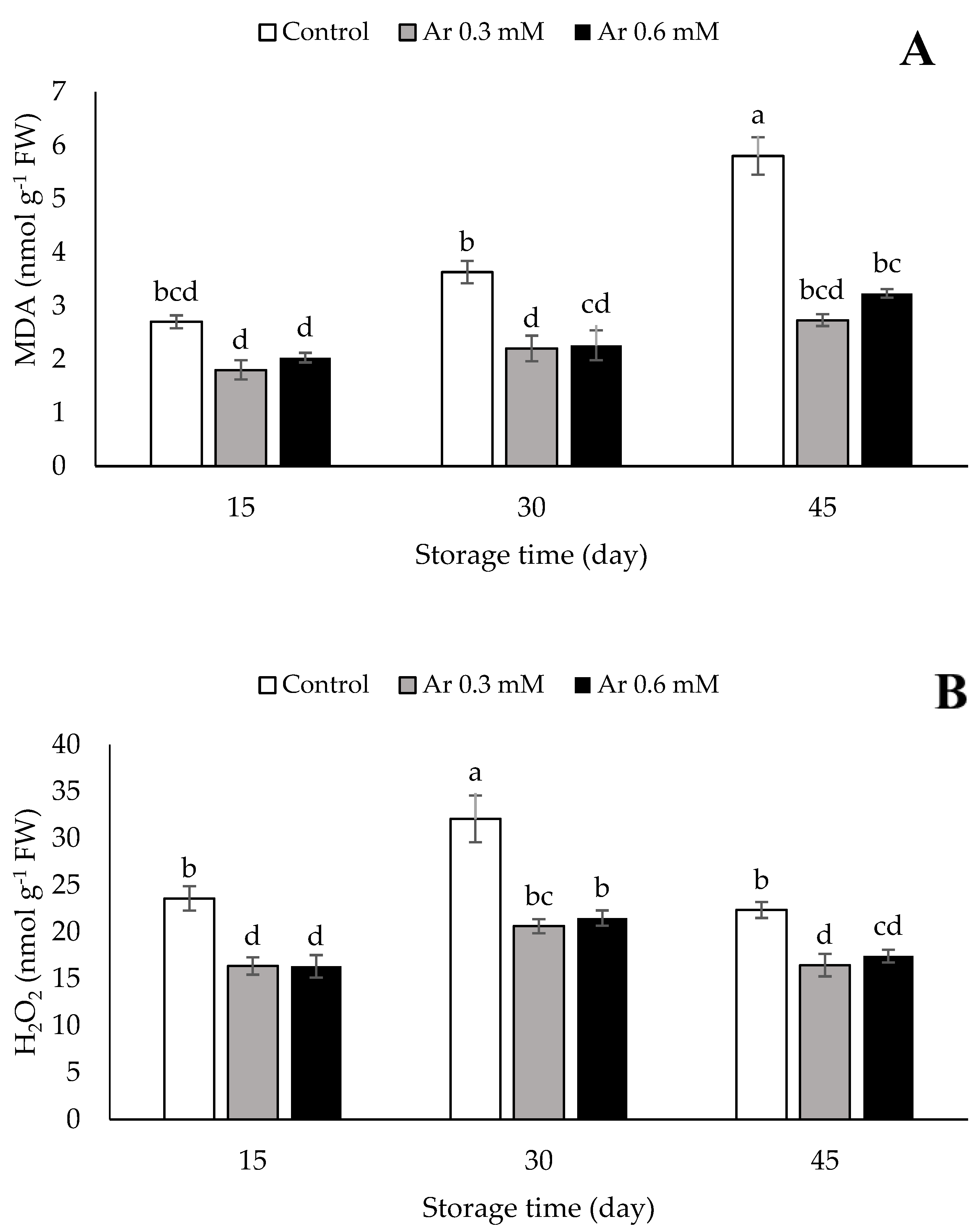
3.2. MDA and H2O2
3.3. Fruit Tissue Firmness
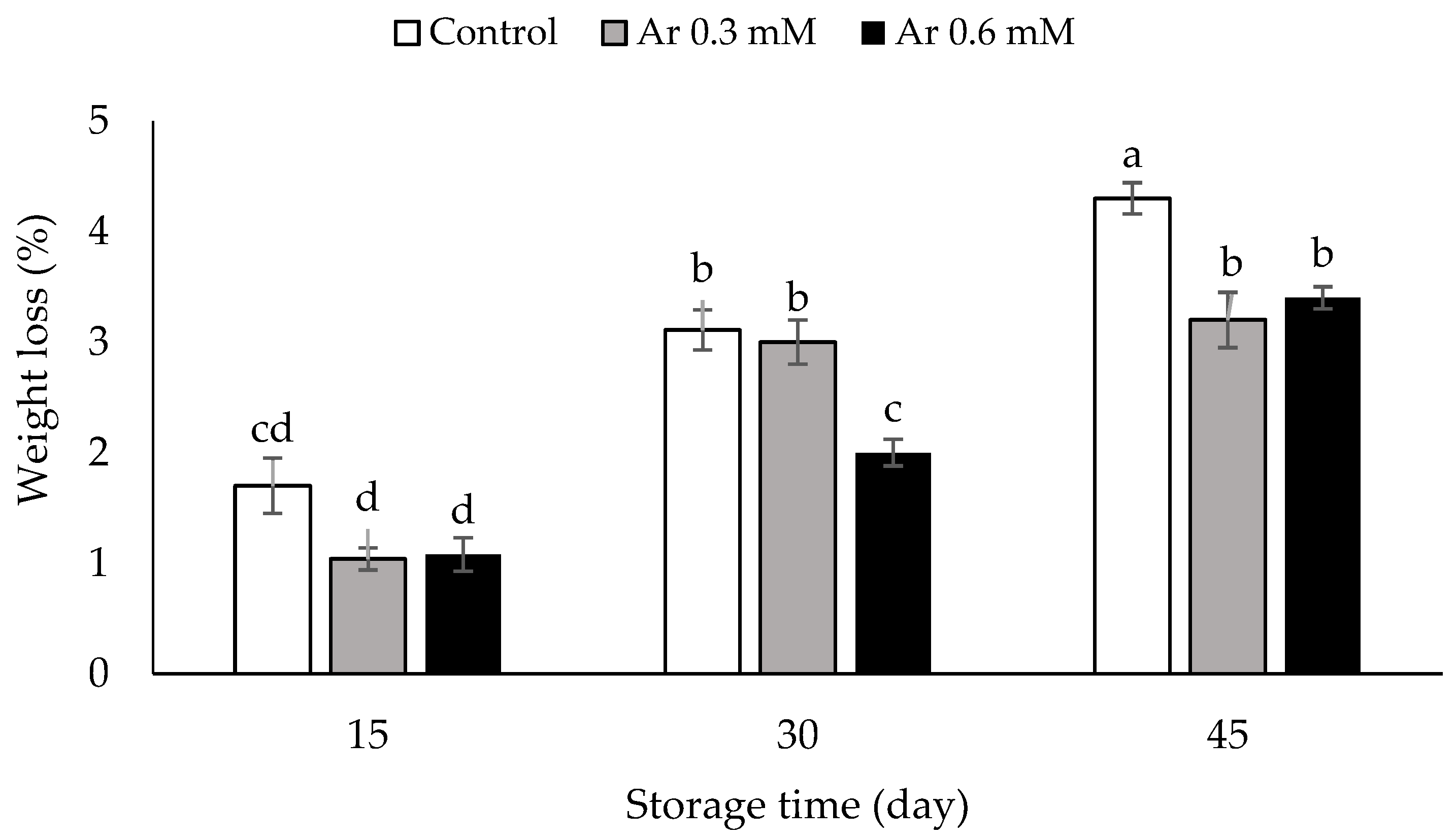
3.4. Weight Loss (%)
3.5. Total Soluble Solids (TSS) and Titratable Acid (TA)
3.6. Total Soluble Tannins
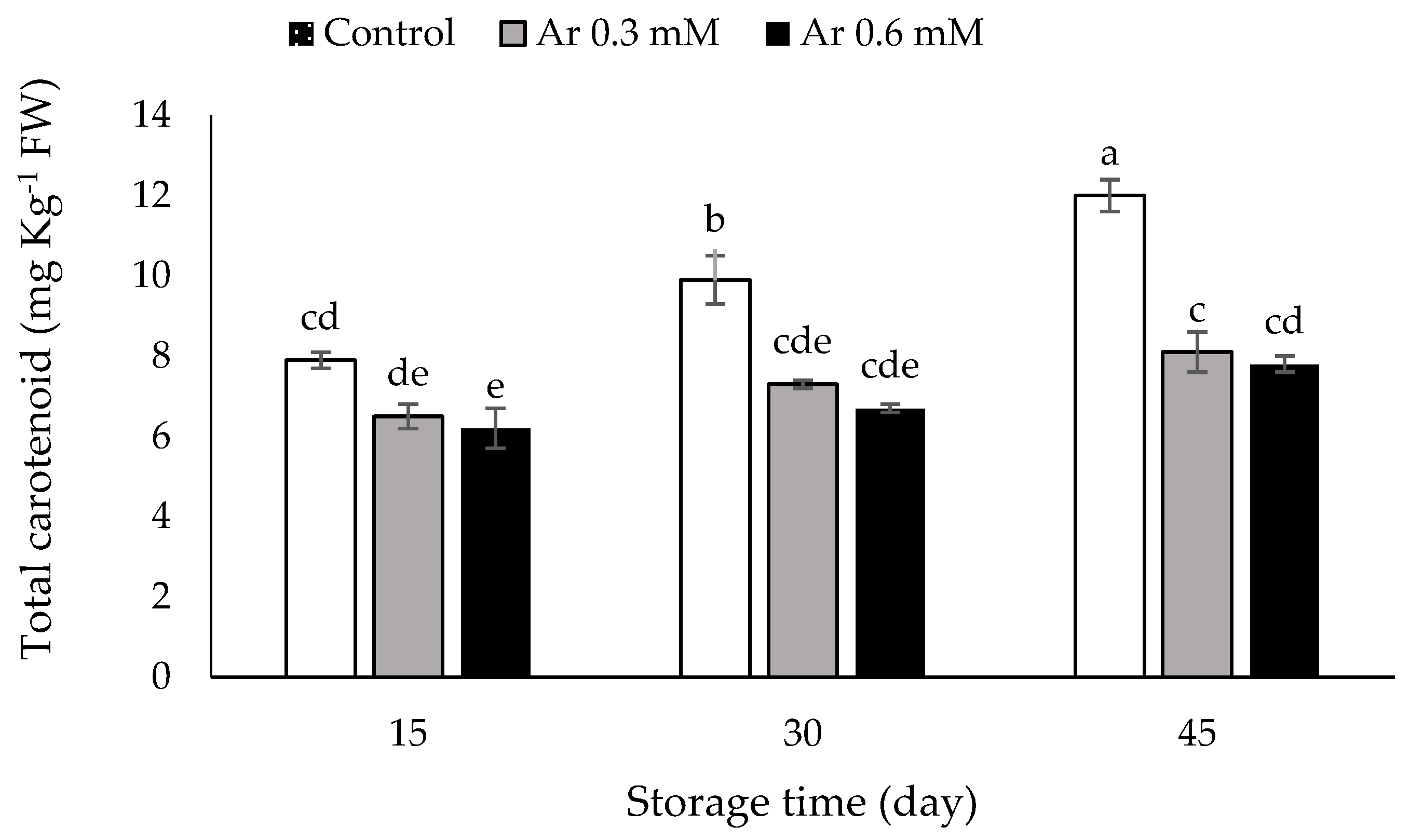
3.7. Total Carotenoids
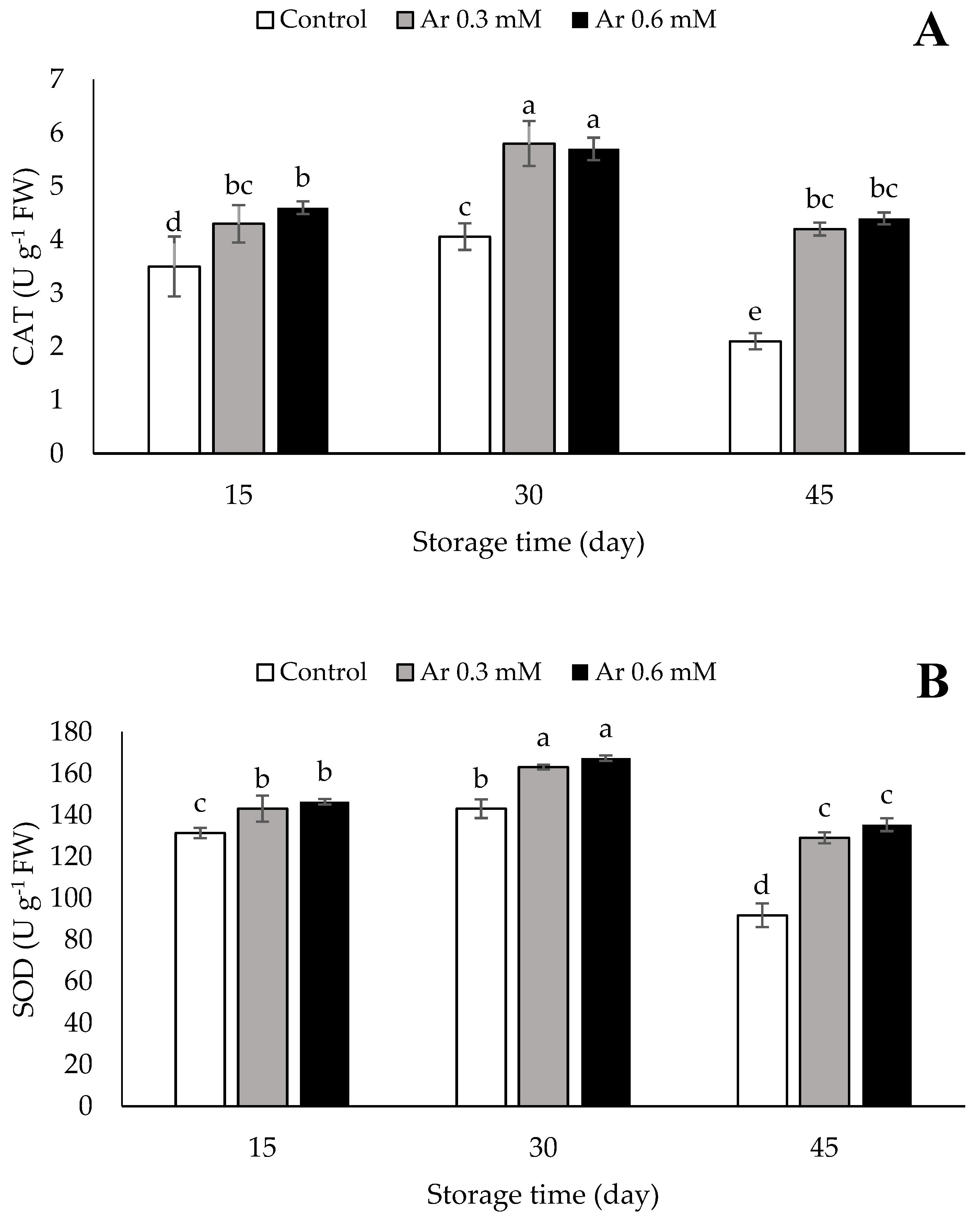
3.8. Catalase (CAT), Superoxide Dismutase (SOD), and Ascorbate Peroxidase (APX)
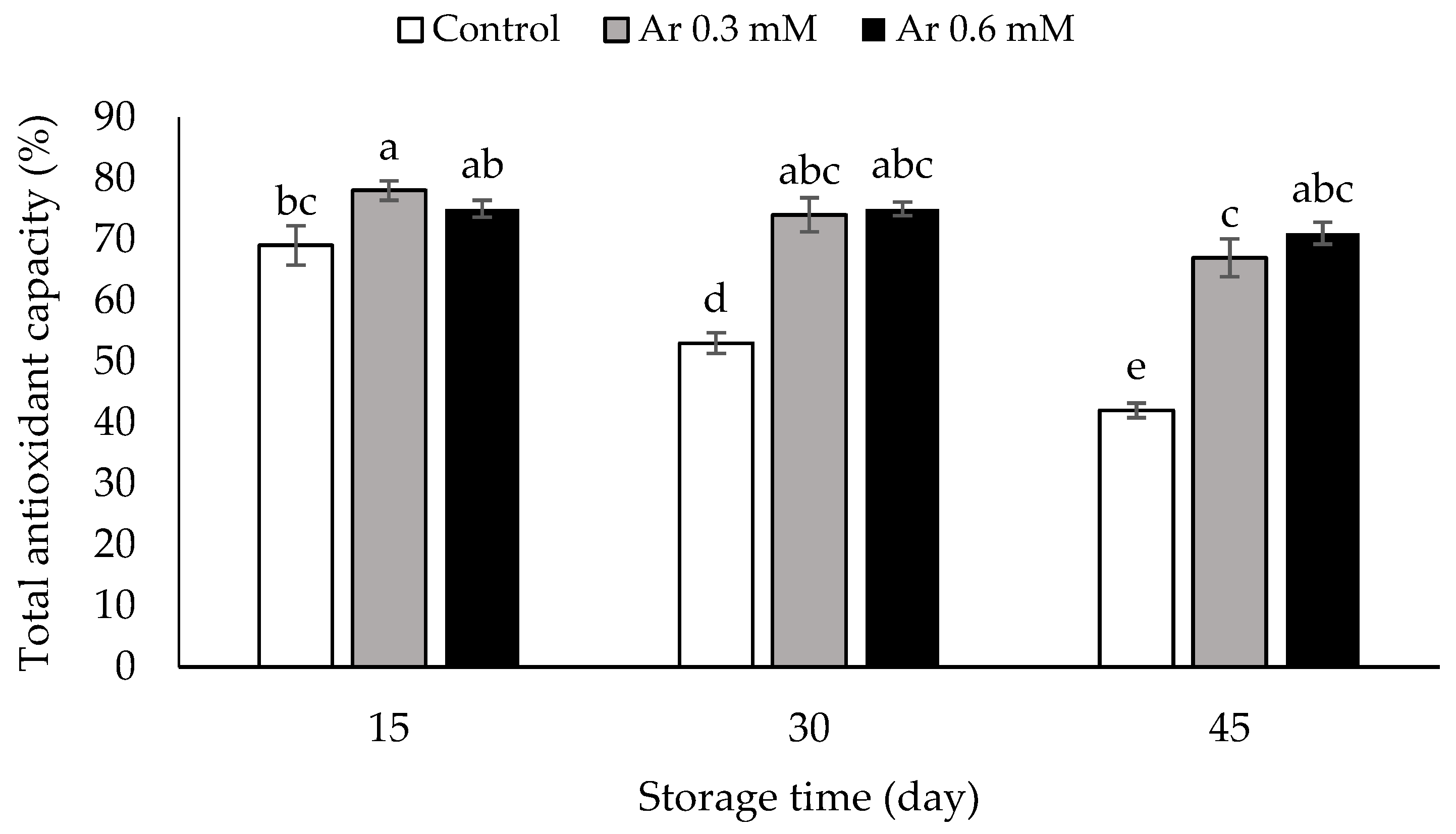
3.9. Total Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Munuera, I.; Hernando, I.; Larrea, V.; Besada, C.; Arnal, L.; Salvador, A. Microstructural study of chilling injury alleviation by 1-methylcyclopropene in persimmon. HortScience 2009, 44, 742–745. [Google Scholar] [CrossRef]
- Xue, J.; Huang, L.; Zhang, S.; Sun, H.; Gao, T. Study on the evaluation of carboxymethyl-chitosan concentration and temperature treatment on the quality of “Niuxin” persimmon during cold storage. JAST 2020, 145, 1–17. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Luo, Z.; Sun, J.; Li, L.; Yin, X.; Li, J.; Xu, Y. Effects of inside-out heat-shock via microwave on the fruit softening and quality of persimmon during postharvest storage. Food Chem. 2021, 349, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Niazi, Z.; Razavi, F.; Khademi, O.; Aghdam, M. Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 2021, 285, 110198. [Google Scholar] [CrossRef]
- Bagheri, M.; Esna-Ashari, M. Effects of postharvest methyl jasmonate treatment on persimmon quality during cold storage. Sci. Hortic. 2022, 294, 110–118. [Google Scholar] [CrossRef]
- Zhao, Q.; Jin, M.; Guo, L.; Pei, H.; Nan, Y.; Rao, J. Modified atmosphere packaging and 1-methylcyclopropene alleviate chilling injury of ‘Youhou’ sweet persimmon during cold storage. Food Packag. Shelf Life 2020, 24, 100479. [Google Scholar] [CrossRef]
- Denoya, G.; Pataro, G.; Ferrari, G. Effects of postharvest pulsed light treatments on the quality and antioxidant properties of persimmons during storage. Postharvest Biol. Technol. 2020, 160, 111055. [Google Scholar] [CrossRef]
- Babalar, M.; Pirzad, F.; Ali, M.; Sarcheshmeh, A.; Talaei, A.; Lessani, H. Arginine treatment attenuates chilling injury of pomegranate fruit during cold storage by enhancing antioxidant system activity. Postharvest Biol. Technol. 2018, 137, 31–37. [Google Scholar] [CrossRef]
- Eslami, M.; Nasibi, F.; Kalantari, K.; Khezri, M.; Oloumi, H. Effect of exogenous application of-arginine and sodium nitroprusside on fruit abscission and physiological disorders of pistachio (Pistacia vera L.) Scions. Int. J. Hortic. Sci. Technol. 2019, 6, 51–62. [Google Scholar]
- Shu, P.; Min, D.; Ai, W.; Li, J.; Zhou, J.; Li, Z.; Zhang, X.; Shi, Z.; Sun, Y.; Jiang, Y.; et al. L-arginine treatment attenuates postharvest decay and maintains quality of strawberry fruit by promoting nitric oxide synthase pathway. Postharvest Biol. Technol. 2020, 168, 121–153. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Postharvest Biol. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Hasan, M.H.; Naveed, R.; Rehman, U.; Malik, A.U.; Haider, M.W.; Ahmed, Z.; Khan, A.; Anwar, R. Pre-storage Application of L-arginine Alleviates Chilling Injury and Maintains Postharvest Quality of Cucumber (Cucumis sativus). Int. J. Hortic. Sci. Technol. 2019, 2, 102–108. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Tang, X.; Wang, G.; Wu, S.; Li, X.; Huang, X.; Qu, T.; Chen, J.; Tang, X. Effect of L-arginine on maintaining storage quality of the white button mushroom (Agaricus bisporus). Food Bioprocess Technol. 2019, 18, 1942–2043. [Google Scholar] [CrossRef]
- Zhang, X.; Min, D.; Li, F.; Ji, N.; Meng, D.; Li, L. Synergistic effects of l-arginine and methyl salicylate on alleviating postharvest disease caused by botrysis cinerea in tomato fruit. J. Agric. Food Chem. 2017, 24, 4890–4896. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Li, Y. Use of arginine to inhibit browning on fresh cut apple and lettuce. Postharvest Biol. Technol. 2016, 113, 66–68. [Google Scholar] [CrossRef]
- Xiangyang, W.; Gu, S.; Chen, B.; Xing, J. Effect of postharvest L-arginine or cholesterol treatment on the quality of green asparagus (Asparagus officinalis L.) spears during low temperature storage. Sci. Hort. 2017, 225, 788–794. [Google Scholar]
- Chang, L.; Yu, Y.; Zhang, L.; Wang, X.; Zhan, S. Arginine induces the resistance of postharvest jujube fruit against Alternaria rot. Food Qual. Saf. 2022, 29, 11–32. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Iranzo, B.; Pertegaz, J.C.; Balsalobre, A.P. Characterization and measurement of astringency and tannin content in Rojo Brillanteg persimmon quality and systems. Acta Hortic. 1984, 601, 227–231. [Google Scholar]
- Taira, S. Astringency in persimmon. Modern methods of plant analysis. In Fruit Analysis; Springer: Berlin/Heidelberg, Germany, 1996; pp. 97–110. [Google Scholar]
- Wang, Z.F.; Ying, T.J.; Bao, B.L.; Huang, X.D. Characteristics of fruit ripening in tomato mutant. J. Zheijang Univ. Sci. Biomed. Biotechnol. 2005, 6, 502–507. [Google Scholar]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin (II)-quercetin complex synthesis, spectral characterization and antioxidant activity. Food Chem. 2012, 131, 422–427. [Google Scholar] [CrossRef]
- Arnal, L.; Del Rio, M.A. Quality of persimmon fruit (Diospyros kaki L.) cv. ‘RojoBrillante’ during storage at different temperatures. Span. J. Agric. Res. 2004, 2, 243–247. [Google Scholar] [CrossRef]
- Rasouli, M.; Khademi, O. Influence of storage temperature, hot water, and 1-MCP treatments on the postharvest quality of ‘karaj ’ persimmon. Curr. Opin. Plant Biol. 2018, 3, 320–337. [Google Scholar] [CrossRef]
- MacRae, E.A. Development of chilling injury in New Zealand grown ‘Fuyu’ persimmon during storage. N. Z. J. Agric. Res. 1987, 15, 333–344. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, K.; Shi, X.; Xia, Y.; Zhouand, J.; Yu, Q. Hydrogen peroxide is involved in the coldacclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biol. 2012, 60, 141–149. [Google Scholar]
- Hu, Y.; Jiang, F.; Wang, F.; Yu, D. Jasmonate regulates the inducer of CBF expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.W.; Gong, D.Q.; Liang, G.B.; Han, R.H.; Xie, J.H.; Li, W.C. Enhanced preservation effects of sugar apple fruit by salicylic acid treatment during storage. J. Sci. Food Agric. 2008, 88, 2693–2699. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implication of arginine metabolism in plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plants. Plant Mol. Biol. 2001, 47, 311–339. [Google Scholar] [CrossRef]
- Ali, Z.M.; Chin, L.H.; Lazan, H. A comparative study on wall degrading enzymes, pectin modifcations and softening during ripening of selected tropical fruits. Plant Sci. 2004, 167, 317–327. [Google Scholar] [CrossRef]
- Lv, J.; Pang, Q.; Chen, X.; Li, T.; Fang, J.; Lin, S.; Jia, H. Transcriptome analysis of strawberry fruit in response to exogenous arginine. Planta 2020, 252, 82–98. [Google Scholar] [CrossRef]
- Han, Y.; Ban, Q.; Hou, Y.; Meng, K.; Suo, J.; Rao, J. Isolation and Characterization of Two Persimmon Xyloglucan Endotransglycosylase/Hydrolase (XTH) Genes That Have Divergent Functions in Cell Wall Modification and Fruit Postharvest Softening. Front. Plant Sci. 2016, 7, 624. [Google Scholar] [CrossRef]
- Pan, C.; Yao, S.; Xiong, W.; Luo, S.; Wang, Y.; Wang, A.; Xiao, D.; Zhan, J.; He, L. Nitric oxide inhibits al-induced programmed cell death in root tips of peanut (Arachis hypogaea L.) by affecting physiological properties of antioxidants systems and cell wall. Front. Physiol. 2017, 8, 1037. [Google Scholar] [CrossRef]
- Ali, M.H.; Khan, A.S.; Jaskani, M.J.; Anwar, R.; Ali, S.; Malik, A.U.; Hasan, M.U.; Rehman, R.N.U.; Ayyub, S. Pre-storage application of L-arginine mitigates chilling injury and maintains quality of Sandhuri guava fruit. J. Food Sci. Tecnol. 2022, 46, 16405. [Google Scholar] [CrossRef]
- Naeem, M. Effect of various temperatures on the postharvest quality and storage life of persimmon fruit. Postharvest Biol. Technol. 2019, 7, 9–24. [Google Scholar]
- Ghasemnekhad, M.; Zreh, S.; Rassa, M.; Hassan, R.; Sajedi, R. Effect of Chitosan coating on maintenance of aril quality. microbial population and PPO activity of pomegranate (Punica granatum L. Var. Tarom) at cold storage temperature. J. Food Sci. Technol. 2012, 93, 368–378. [Google Scholar]
- Taira, S.; Ono, M.; Matsumoto, N. Reduction of persimmon astringency by complex formation between pectin and tannin. Postharvest Biol. Technol. 1997, 12, 265–271. [Google Scholar] [CrossRef]
- Khademi, O.; Mostofi, Y.; Zamani, Z.; Fatahi, R. The effect of deastringency treatments on increasing the marketability of persimmon fruit. Acta Hortic. 2009, 877, 687–691. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Alquezar, B.; Rodrigo, M.J.L.; Zacarias, L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara. Phytochemistry 2008, 69, 1997–2007. [Google Scholar] [CrossRef]
- Schon, K.I. The carotenoids of Diospyros fruits. II. The carotenoids of arbutus fruits Arbutus unedo. Biochem. J. 1935, 29, 1779. [Google Scholar]
- Zhang, Y.Z.; Zhang, M.L.; Yang, H.Q. Postharvest chitosan-gsalicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef]
- Zhao-Liang, L.; Young-Bing, Y.; Cheng-Lian, L.; Zong-Xun, C.; Tsung-Hsum, T. Regulation of antioxidant enzymes by salycilic acid in cucumber leaves. Acta Bot. Sin. 1998, 40, 356–361. [Google Scholar]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Pignocchi, C.; Foyer, C.H. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr. Opin. Plant Biol. 2003, 6, 379–389. [Google Scholar] [CrossRef]
- Duan, X.W.; Liu, T.; Zhang, D.; Su, X.G.; Lin, H.T.; Jiang, Y.M. Effect of pureoxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Li, F.; Zhang, Y.; Meng, D.; Sheng, J. Up-regulating arginase contributes to amelioration of chilling stress and the antioxidant system in cherry tomato fruits. J. Sci. Food Agri. 2010, 90, 2195–2202. [Google Scholar] [CrossRef]
- Nasr, F.; Pateiro, M.; Rabiei, V.; Razavi, F.; Formaneck, S.; Gohari, G.; Lorenzo, J. Chitosan-Phenylalanine Nanoparticles (Cs-Phe Nps) Extend the Postharvest Life of Persimmon (Diospyros kaki) Fruits under Chilling Stress. Coatings 2021, 11, 819–827. [Google Scholar] [CrossRef]
- Dalvi, L.T.; Moreira, D.; Alonso, A.; de Avellar, I.G.; Hermes-Lima, M. Antioxidant activity and mechanism of commercial Rama Forte persimmon fruits (Diospyros kaki). Peer J. 2018, 6, 5223. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, F.; Razavi, F.; Rabiei, V.; Gohari, G.; Ali, S.; Hano, C. Attenuation of Chilling Injury and Improving Antioxidant Capacity of Persimmon Fruit by Arginine Application. Foods 2022, 11, 2419. https://doi.org/10.3390/foods11162419
Nasr F, Razavi F, Rabiei V, Gohari G, Ali S, Hano C. Attenuation of Chilling Injury and Improving Antioxidant Capacity of Persimmon Fruit by Arginine Application. Foods. 2022; 11(16):2419. https://doi.org/10.3390/foods11162419
Chicago/Turabian StyleNasr, Fahimeh, Farhang Razavi, Vali Rabiei, Gholamreza Gohari, Sajid Ali, and Christophe Hano. 2022. "Attenuation of Chilling Injury and Improving Antioxidant Capacity of Persimmon Fruit by Arginine Application" Foods 11, no. 16: 2419. https://doi.org/10.3390/foods11162419
APA StyleNasr, F., Razavi, F., Rabiei, V., Gohari, G., Ali, S., & Hano, C. (2022). Attenuation of Chilling Injury and Improving Antioxidant Capacity of Persimmon Fruit by Arginine Application. Foods, 11(16), 2419. https://doi.org/10.3390/foods11162419






