Effects of Flash Evaporation Conditions on the Quality of UHT Milk by Changing the Dissolved Oxygen Content in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Flash Deoxidation
2.3. Determination of DO
2.4. Determination of Milk Fraction
2.5. Turbiscan Measurement
2.6. Particle Size Measurements
2.7. Measurements of Primary and Secondary Lipid Oxidation Products
2.8. Determination of Protein Carbonyl (PC)
2.9. Dityrosine Quantification
2.10. Determination of Lactulose
2.11. Determination of Furosine
2.12. Determination of 5-HMF
2.13. Determination of Melanoids
2.14. Determination of Color
2.15. Determination of Flavor
2.16. Statistical Analysis
3. Result and Discussion
3.1. Changes in Milk Fraction
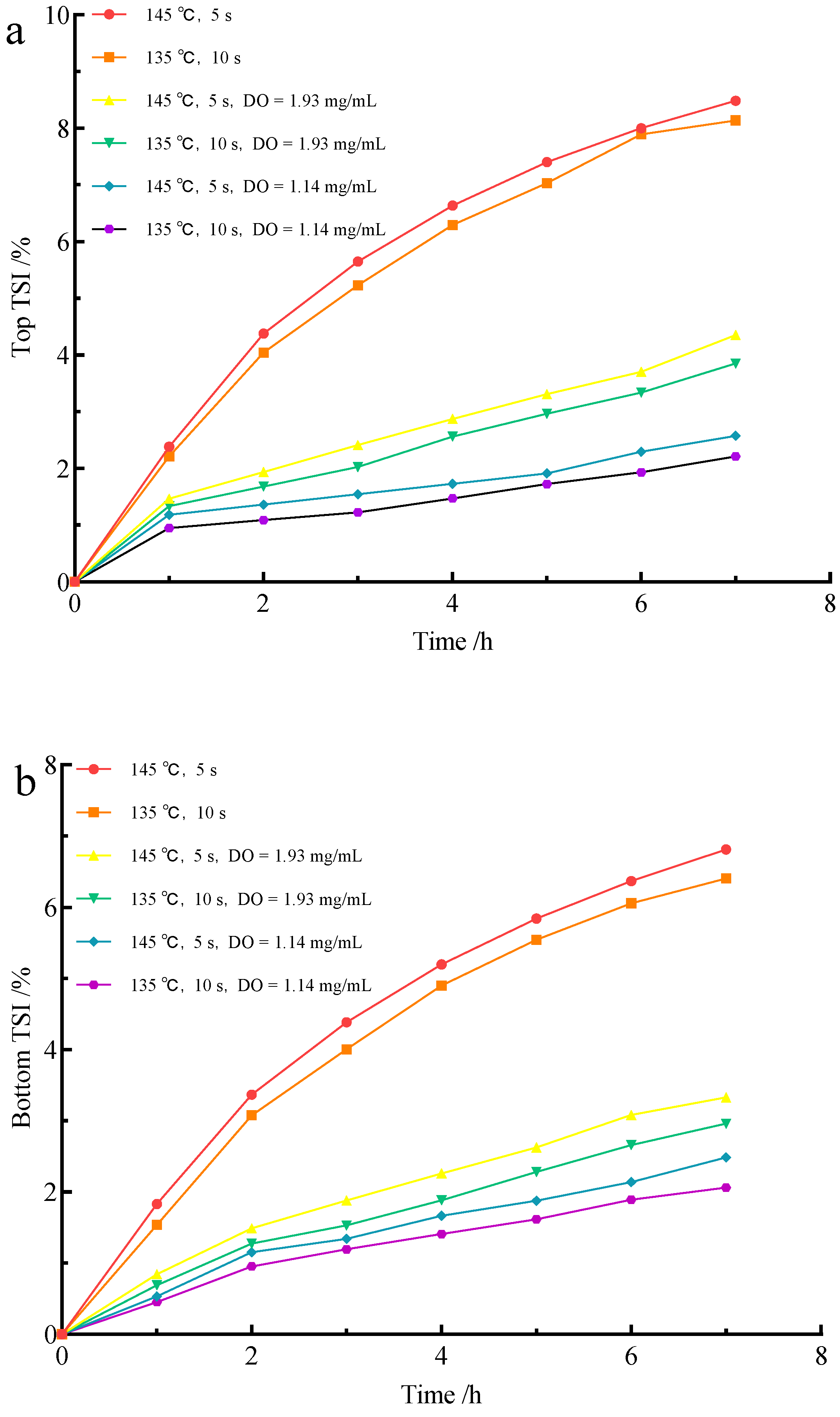
3.2. Effect of Flash Deoxidation on the Stability of UHT Milk
3.3. Effects of Flash Deoxygenation on the Oxidation of UHT Milk
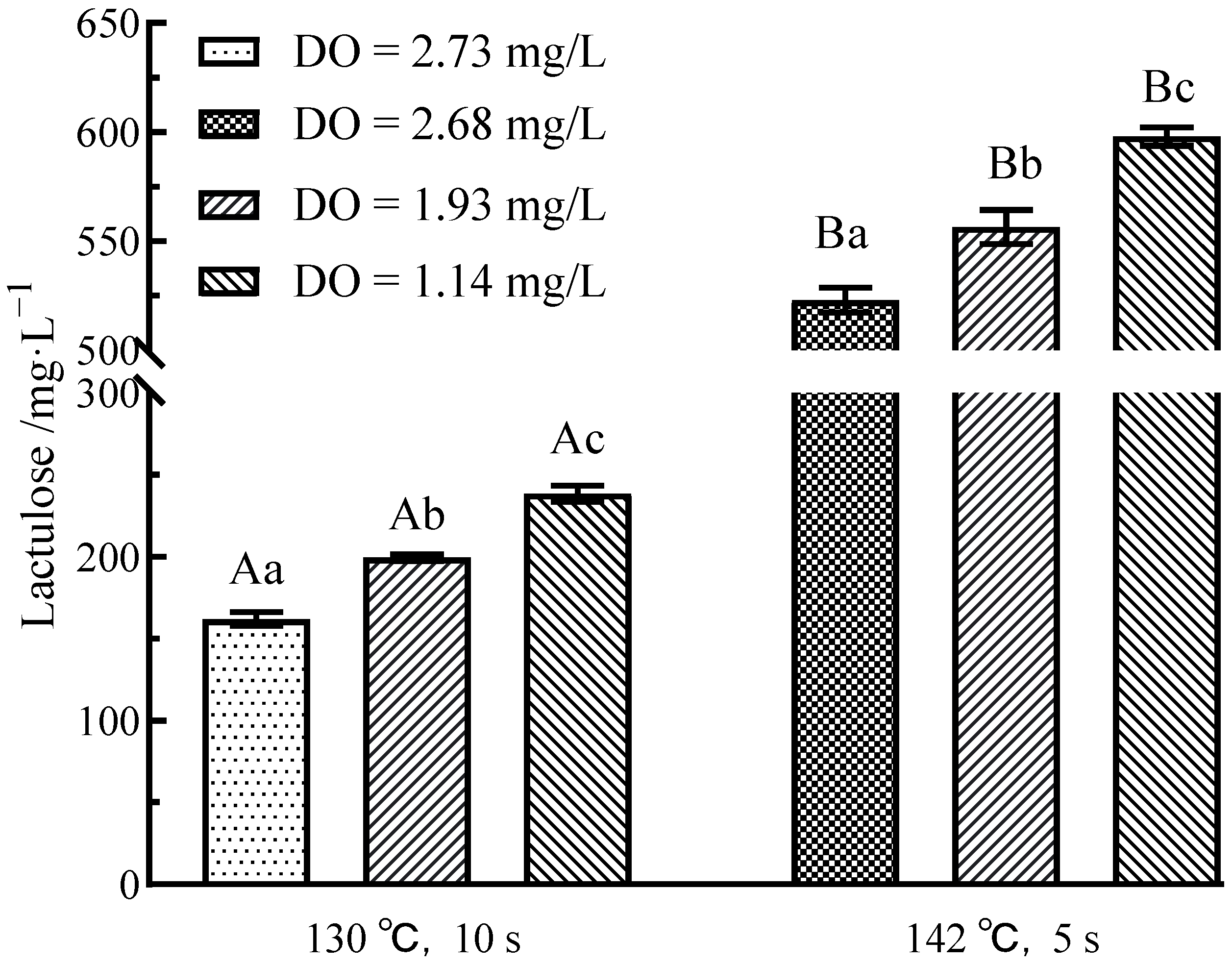
3.4. Effects of Flash Deoxygenation on Lactulose in UHT Milk
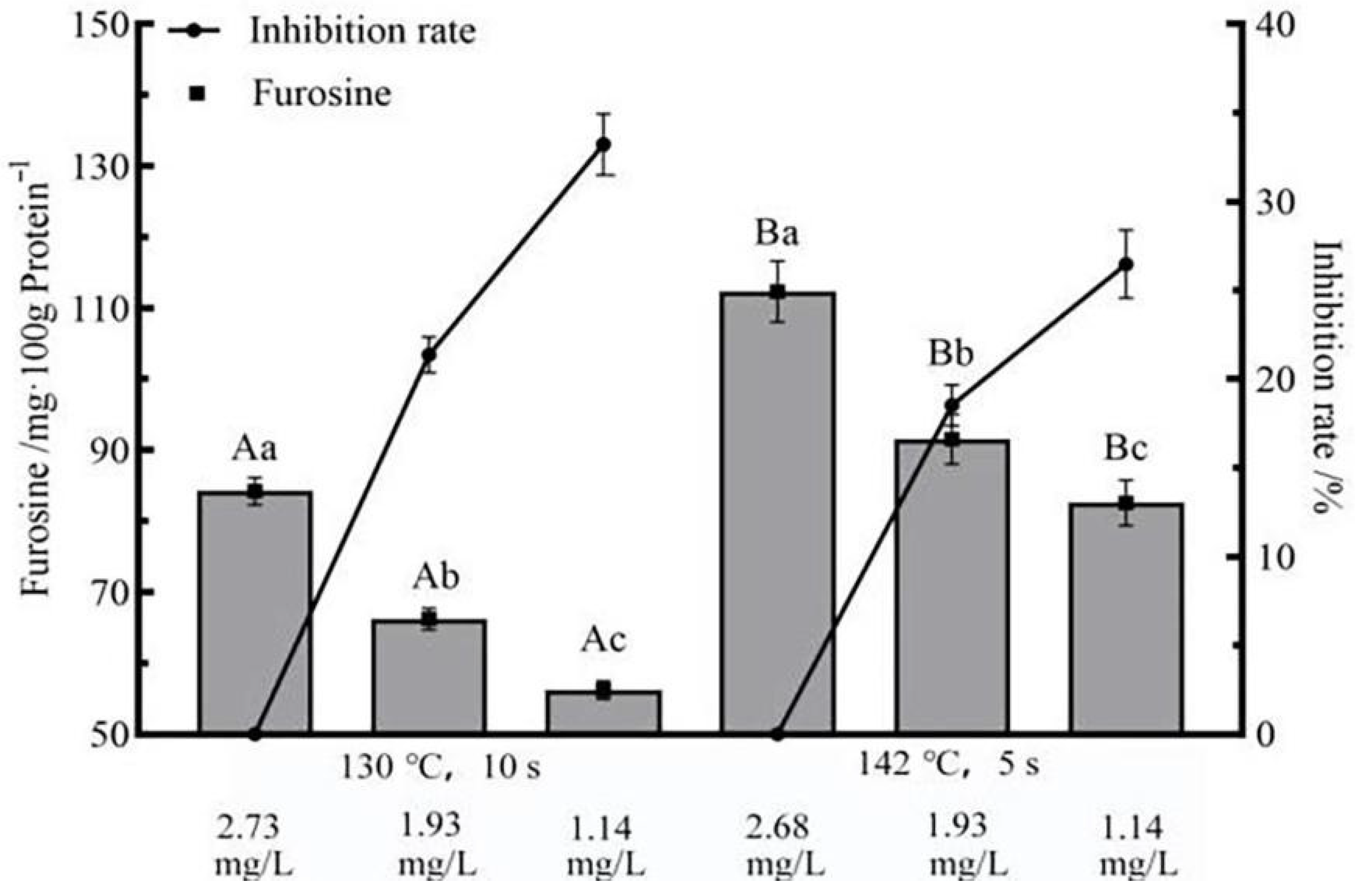
3.5. Effects of Flash Deoxygenation on Furosine in UHT Milk
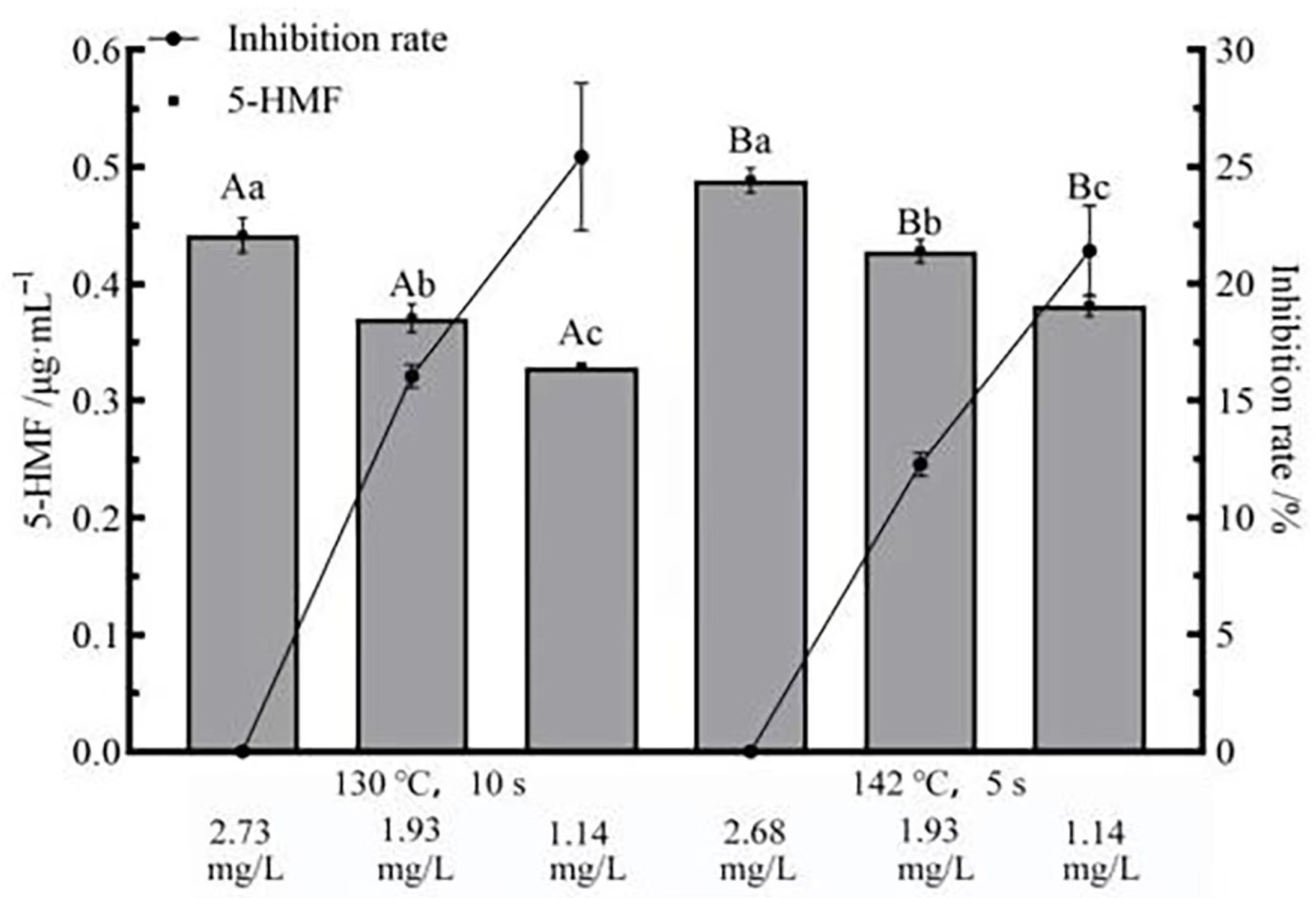
3.6. Effects of Flash Deoxygenation on 5-HMF in UHT Milk
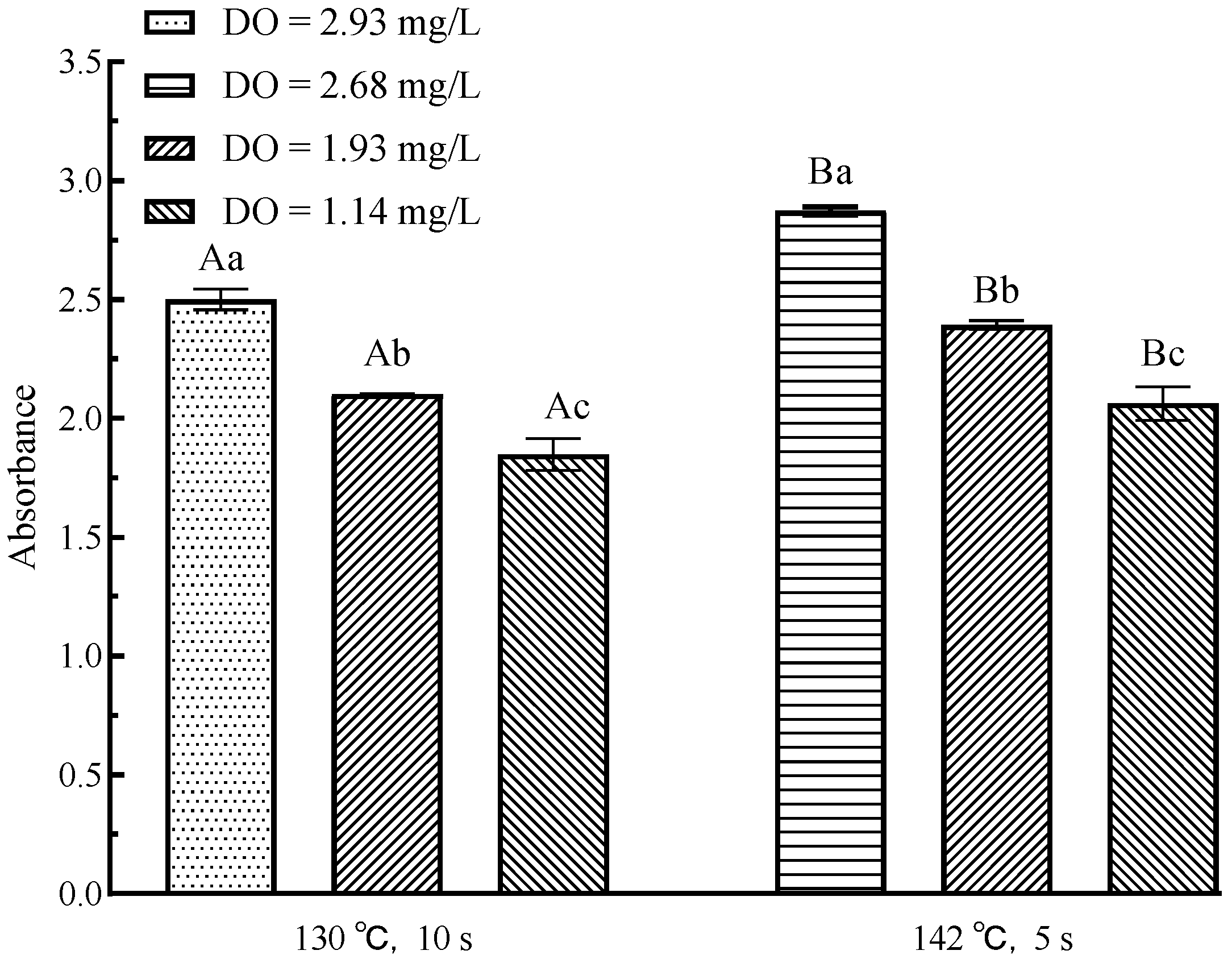
3.7. Effects of Flash Deoxygenation on Melanoids in UHT Milk
3.8. Effect of Flash Deoxygenation on the Color of UHT Milk
3.9. Effects of Flash Deoxygenation on the Content of Volatile Flavor Substances of UHT Milk
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Deeth, H. Improving UHT processing and UHT milk products. In Improving the Safety and Quality of Milk; Elsevier: Amsterdam, The Netherlands, 2010; pp. 302–329. [Google Scholar]
- Aït-Kaddour, A.; Hassoun, A.; Bord, C.; Schmidt-Filgueras, R.; Biancolillo, A.; Di Donato, F.; Temiz, H.T.; Cozzolino, D. Application of spectroscopic techniques to evaluate heat treatments in milk and dairy products: An overview of the last decade. Food Bioprocess Technol. 2021, 14, 781–803. [Google Scholar] [CrossRef]
- Ford, J. The influence of the dissolved oxygen in milk on the stability of some vitamins towards heating and during subsequent exposure to sunlight. J. Dairy Res. 1967, 34, 239–247. [Google Scholar] [CrossRef]
- García-Torres, R.; Ponagandla, N.; Rouseff, R.; Goodrich-Schneider, R.; Reyes-De-Corcuera, J. Effects of dissolved oxygen in fruit juices and methods of removal. Compr. Rev. Food Sci. Food Saf. 2009, 8, 409–423. [Google Scholar] [CrossRef]
- Schröder, M.J.A. Effect of oxygen on the keeping quality of milk: I. Oxidized flavour development and oxygen uptake in milk in relation to oxygen availability. J. Dairy Res. 1982, 49, 407–424. [Google Scholar] [CrossRef]
- Eitenmiller, R.R.; Landen Jr., W.; Ye, L. Vitamin Analysis for the Health and Food Sciences; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Giroux, H.J.; Acteau, G.V.; Sabik, H.; Britten, M. Influence of dissolved gases and heat treatments on the oxidative degradation of polyunsaturated fatty acids enriched dairy beverage. J. Agric. Food Chem. 2008, 56, 5710–5716. [Google Scholar] [CrossRef]
- Rada-Mendoza, M.; Villamiel, M.; Olano, A. Dissolved air effects on lactose isomerisation and furosine formation during heat treatment of milk. Le Lait 2002, 82, 629–634. [Google Scholar] [CrossRef]
- Shaw, P.; Nagy, S.; Rouseff, R. shelf life of citrus products. Dev. Food Sci. 1993, 33, 755–778. [Google Scholar]
- Perkins, M.L.; Zerdin, K.; Rooney, M.L.; D’Arcy, B.R.; Deeth, H.C. Active packaging of UHT milk to prevent the development of stale flavour during storage. Packag. Technol. Sci. Int. J. 2007, 20, 137–146. [Google Scholar] [CrossRef]
- Jordan, M.; Goodner, K.; Laencina, J. Deaeration and pasteurization effects on the orange juice aromatic fraction. LWT-Food Sci. Technol. 2003, 36, 391–396. [Google Scholar] [CrossRef]
- Mahmud, H.; Kumar, A.; Narbaitz, R.M.; Matsuura, T. Membrane air stripping: A process for removal of organics from aqueous solutions. Sep. Sci. Technol. 1998, 33, 2241–2255. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Firestone, D. Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Fenaille, F.; Parisod, V.; Visani, P.; Populaire, S.; Tabet, J.-C.; Guy, P.A. Modifications of milk constituents during processing: A preliminary benchmarking study. Int. Dairy J. 2006, 16, 728–739. [Google Scholar] [CrossRef]
- Scheidegger, D.; Pecora, R.; Radici, P.; Kivatinitz, S. Protein oxidative changes in whole and skim milk after ultraviolet or fluorescent light exposure. J. Dairy Sci. 2010, 93, 5101–5109. [Google Scholar] [CrossRef]
- Sunds, A.V.; Rauh, V.M.; Sørensen, J.; Larsen, L.B. Maillard reaction progress in UHT milk during storage at different temperature levels and cycles. Int. Dairy J. 2018, 77, 56–64. [Google Scholar] [CrossRef]
- Kiesner, C.; Eggers, R. Concept of a sterile concentration process for milk by multistage flash evaporation. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 1994, 17, 374–381. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Urban, T.; Nosal-Wiercińska, A.; Zarko, V.I.; Gun’ko, V.M. Comparison of stability properties of poly (acrylic acid) adsorbed on the surface of silica, alumina and mixed silica-alumina nanoparticles—Application of turbidimetry method. Cent. Eur. J. Chem. 2014, 12, 476–479. [Google Scholar] [CrossRef]
- Brooker, B. The adsorption of crystalline fat to the air-water interface of whipped cream. Food Struct. 1990, 9, 5. [Google Scholar]
- Sun, D.-W. Thermal Food processing: New Technologies and Quality Issues; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Jeurnink, T.J. Fouling of heat exchangers by fresh and reconstituted milk and the influence of air bubbles. Milchwissenschaft 1995, 50, 189–192. [Google Scholar]
- Deeth, H.; Lewis, M. Protein stability in sterilised milk and milk products. In Advanced Dairy Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 247–286. [Google Scholar]
- Hui, Y.H. Handbook of Food Science, Technology, and Engineering; CRC Press: Boca Raton, FL, USA, 2006; Volume 149. [Google Scholar]
- Chen, B.; Grandison, A.; Lewis, M.J. Comparison of heat stability of goat milk subjected to ultra-high temperature and in-container sterilization. J. Dairy Sci. 2012, 95, 1057–1063. [Google Scholar] [CrossRef]
- Jukkola, A.; Rojas, O.J. Milk fat globules and associated membranes: Colloidal properties and processing effects. Adv. Colloid Interface Sci. 2017, 245, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Akoh, C.C. Characterisation and optimisation of physical and oxidative stability of structured lipid-based infant formula emulsion: Effects of emulsifiers and biopolymer thickeners. Food Chem. 2013, 141, 2486–2494. [Google Scholar] [CrossRef]
- Deeth, H. Stability and Spoilage of Lipids in Milk and Dairy Products. In Advanced Dairy Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2, pp. 345–373. [Google Scholar]
- Fenaille, F.; Visani, P.; Fumeaux, R.; Milo, C.; Guy, P.A. Comparison of mass spectrometry-based electronic nose and solid phase microextraction gas chromatography mass spectrometry technique to assess infant formula oxidation. J. Agric. Food Chem. 2003, 51, 2790–2796. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Comparative effects of curcumin when delivered in a nanoemulsion or nanoparticle form for food applications: Study on stability and lipid oxidation inhibition. LWT 2019, 113, 108319. [Google Scholar] [CrossRef]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R. Modification of proteins by reactive lipid oxidation products and biochemical effects of lipoxidation. Essays Biochem. 2020, 64, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Balbis, E.; Sukkar, S.G.; Furfaro, A.; Sacchi-Nemours, A.M.; Ferrari, C.; Patriarca, S.; Cottalasso, D. Oxidative stress in the animal model: The possible protective role of milk serum protein. Mediterr. J. Nutr. Metab. 2010, 3, 173–178. [Google Scholar] [CrossRef]
- Schuster-Wolff-Bühring, R.; Fischer, L.; Hinrichs, J. Production and physiological action of the disaccharide lactulose. Int. Dairy J. 2010, 20, 731–741. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Montilla, A.; Megías-Pérez, R.; Olano, A.; Moreno, F.J.; Villamiel, M. Impact of high-intensity ultrasound on the formation of lactulose and Maillard reaction glycoconjugates. Food Chem. 2014, 157, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yi, S.; Lu, J.; Pang, X.; Xu, X.; Lv, J.; Zhang, S. Effect of different heat treatments on the Maillard reaction products, volatile compounds and glycation level of milk. Int. Dairy J. 2021, 123, 105182. [Google Scholar] [CrossRef]
- Aktağ, I.G.; Hamzalıoğlu, A.; Gökmen, V. Lactose hydrolysis and protein fortification pose an increased risk for the formation of Maillard reaction products in UHT treated milk products. J. Food Compos. Anal. 2019, 84, 103308. [Google Scholar] [CrossRef]
- Li, Y.; Quan, W.; Jia, X.; He, Z.; Wang, Z.; Zeng, M.; Chen, J. Profiles of initial, intermediate, and advanced stages of harmful Maillard reaction products in whole-milk powders pre-treated with different heat loads during 18 months of storage. Food Chem. 2021, 351, 129361. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Fiore, A.; Colantuono, A.; Kokkinidou, S.; Peterson, D.G.; Fogliano, V. Effect of olive mill wastewater phenol compounds on reactive carbonyl species and Maillard reaction end-products in ultrahigh-temperature-treated milk. J. Agric. Food Chem. 2014, 62, 10092–10100. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Cui, H.; Hayat, K.; Hussain, S.; Tahir, M.U.; Yu, J.; Jia, C.; Zhang, X.; Ho, C.-T. Interaction of added l-cysteine with 2-threityl-thiazolidine-4-carboxylic acid derived from the xylose–cysteine system affecting its Maillard browning. J. Agric. Food Chem. 2019, 67, 8632–8640. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Visessanguan, W.; Phongkanpai, V.; Tanaka, M. Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Food Chem. 2005, 90, 231–239. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gu, D.; Sun, X.; Zhang, R. Original Paper Investigation of Processing Technology for Aged Black Jujube. Food Sci. Nutr. 2019, 3, 107. [Google Scholar]
- Milanovic, I.; Basic, J. Mechanism and Theory in Food Chemistry. Arch. Ind. Hyg. Toxicol. 2021, 72, A14. [Google Scholar]
- Thomsen, M.K.; Lauridsen, L.; Skibsted, L.H.; Risbo, J. Two types of radicals in whole milk powder. Effect of lactose crystallization, lipid oxidation, and browning reactions. J. Agric. Food Chem. 2005, 53, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Liu, P.; Xia, S.; Jia, C.; Mukunzi, D.; Zhang, X.; Xia, W.; Tian, H.; Xiao, Z. Temperature effect on the non-volatile compounds of Maillard reaction products derived from xylose–soybean peptide system: Further insights into thermal degradation and cross-linking. Food Chem. 2010, 120, 967–972. [Google Scholar] [CrossRef]
- Bottiroli, R.; Troise, A.D.; Aprea, E.; Fogliano, V.; Gasperi, F.; Vitaglione, P. Understanding the effect of storage temperature on the quality of semi-skimmed UHT hydrolyzed-lactose milk: An insight on release of free amino acids, formation of volatiles organic compounds and browning. Food Res. Int. 2021, 141, 110120. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.L.; Chambers, D.H.; Kong, S.H.; Milliken, G.A.; Schmidt, K.A. Serving Temperature Effects on Milk Flavor, Milk Aftertaste, and Volatile Compound Quantification in Nonfat and Whole Milk. J. Food Sci. 2010, 70, s413–s418. [Google Scholar] [CrossRef]
- Moio, L.; Piombino, P.; Addeo, F. Odour-impact compounds of Gorgonzola cheese. J. Dairy Res. 2000, 67, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, A.M.M. Investigation into the Cooked Flavour in Heat-Treated Milk. Ph.D. Thesis, University of Reading, Reading, UK, 1987. [Google Scholar]
- Al-Attabi, Z.; D’Arcy, B.R.; Deeth, H.C. Volatile Sulphur Compounds in UHT Milk. Crit. Rev. Food Sci. Nutr. 2008, 49, 28–47. [Google Scholar] [CrossRef]
- Zabbia, A.; Buys, E.M.; De Kock, H.L. Undesirable Sulphur and Carbonyl Flavor Compounds in UHT Milk: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 21–30. [Google Scholar] [CrossRef] [PubMed]
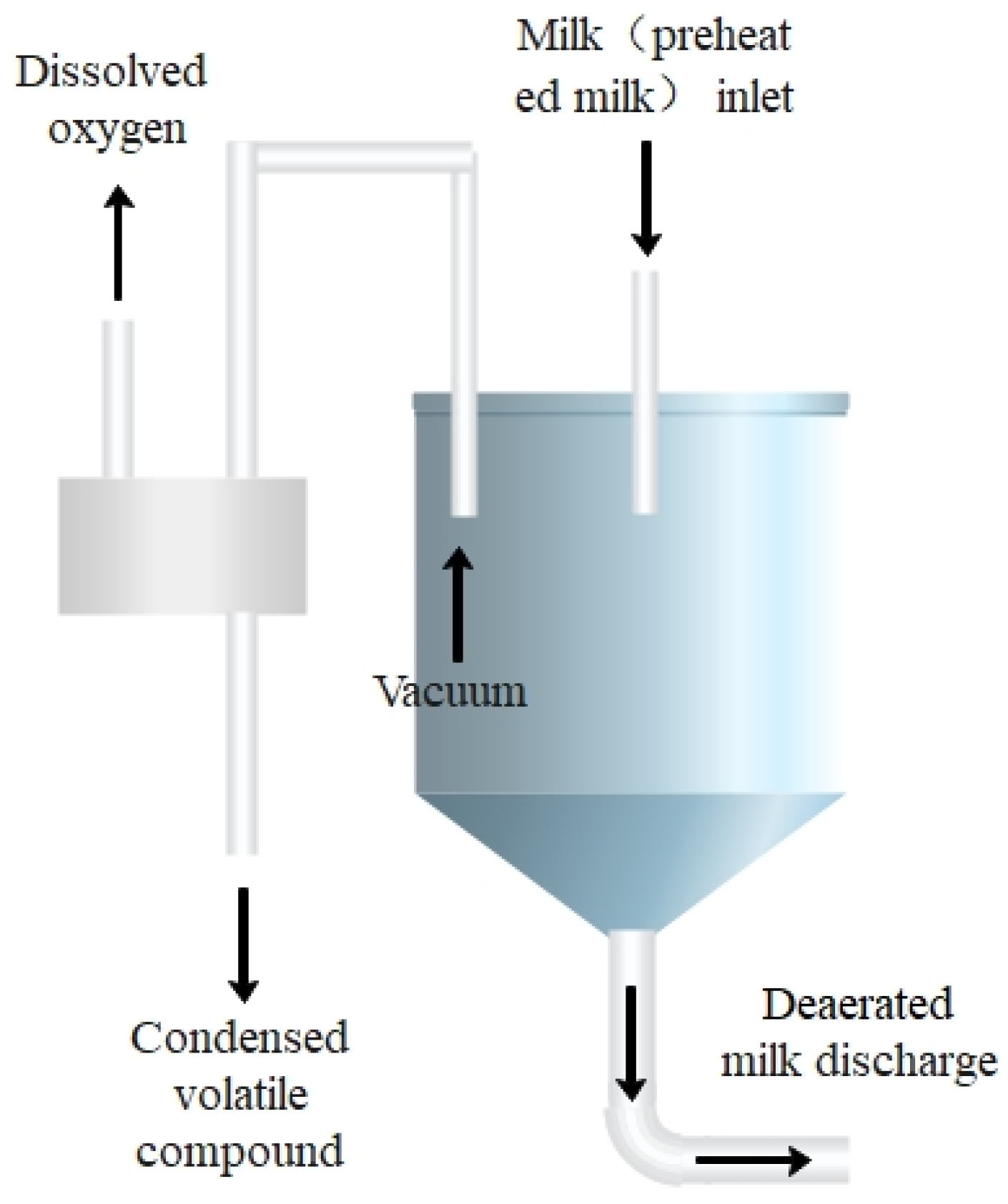
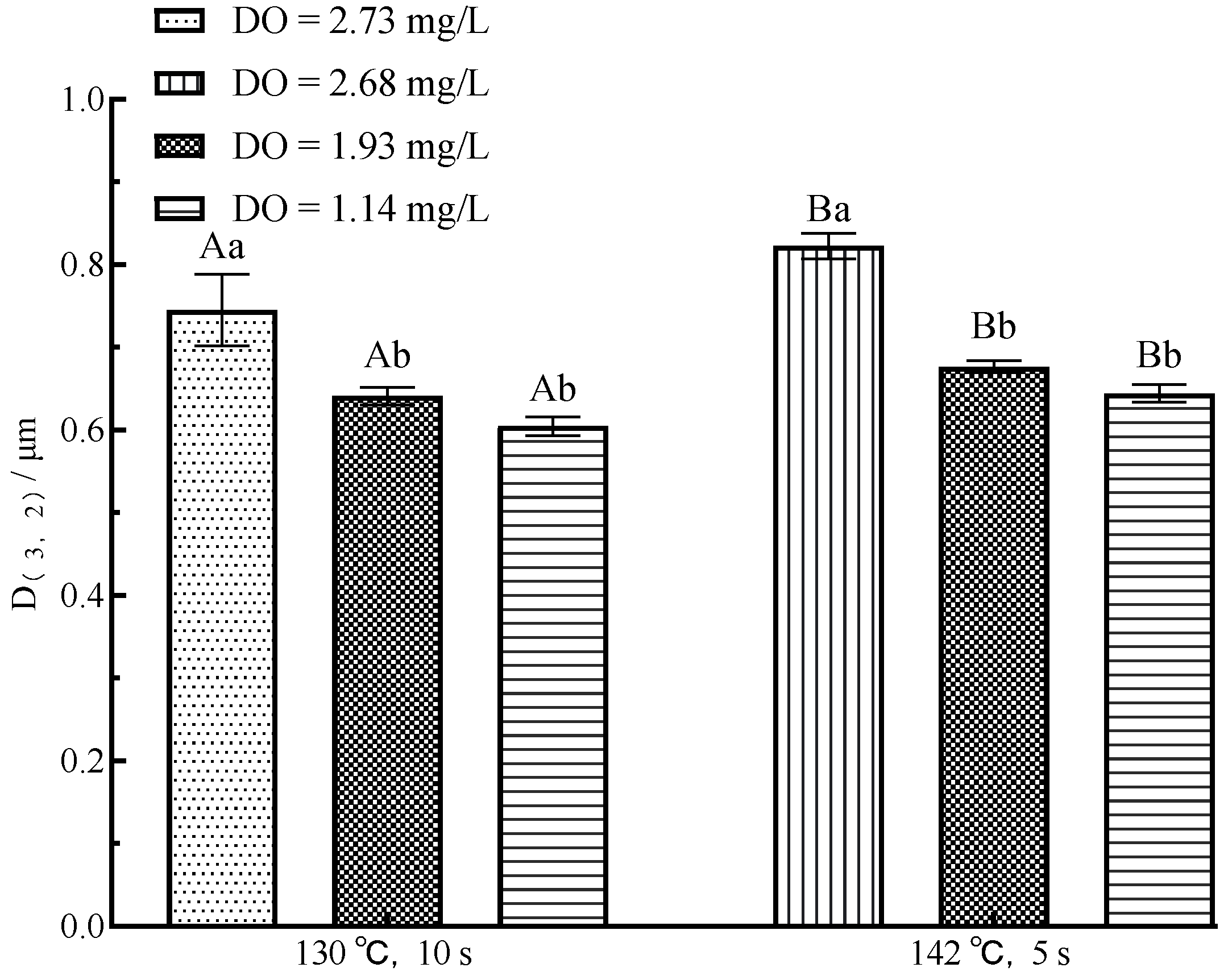
| Flash Condition | UHT Condition | Fat (%) | Protein (%) | Lactose (%) | Dry Matter (%) | DO (mg/L) |
|---|---|---|---|---|---|---|
| - | - | 2.85 ± 0.32 a | 3.03 ± 0.17 a | 4.05 ± 0.22 a | 11.24 ± 0.42 a | 2.93 ± 0.35 a |
| - | 135 °C, 10 s | 3.12 ± 0.15 a | 3.02 ± 0.09 a | 4.27 ± 0.12 a | 11.47 ± 0.23 a | 2.73 ± 0.27 b |
| 65 °C,−0.08 MPa | 135 °C, 10 s | 3.75 ± 0.14 b | 3.36 ± 0.10 b | 4.77 ± 0.15 b | 12.16 ± 0.11 b | 1.14 ± 0.15 c |
| 70 °C,−0.06 MPa | 135 °C, 10 s | 3.59 ± 0.18 b | 3.33 ± 0.12 b | 4.80 ± 0.17 b | 12.12 ± 0.17 b | 1.93 ± 0.23 d |
| - | 145 °C, 5 s | 2.77 ± 0.23 a | 3.05 ± 0.11 a | 4.22 ± 0.21 a | 11.34 ± 0.23 a | 2.68 ± 0.29 b |
| 65 °C,−0.08 MPa | 145 °C, 5 s | 3.79 ± 0.21 b | 3.42 ± 0.15 b | 4.72 ± 0.23 b | 12.19 ± 0.21 b | 1.14 ± 0.18 c |
| 70 °C,−0.06 MPa | 145 °C, 5 s | 3.63 ± 0.24 b | 3.41 ± 0.20 b | 4.75 ± 0.24 b | 12.05 ± 0.27 b | 1.93 ± 0.27 d |
| UHT Condition | Flash Condition | DO (mg/L) | TOTOX Value | PC (nmol/mg of Protein) | Dityrosine (AFU/mg of Protein) |
|---|---|---|---|---|---|
| 135 °C, 10 s | - | 2.73 | 6.73 ± 0.21 Aa | 3.17 ± 0.12 Aa | 0.84 ± 0.15 Aa |
| 70 °C, −0.06 MPa | 1.93 | 5.86 ± 0.11 Ab | 2.32 ± 0.07 Ab | 0.73 ± 0.13 Ab | |
| 65 °C, −0.08 MPa | 1.14 | 5.02 ± 0.06 Ac | 1.65 ± 0.11 Ac | 0.61 ± 0.12 Ac | |
| 145 °C, 5 s | - | 2.68 | 7.47 ± 0.25 Ba | 3.58 ± 0.06 Ba | 1.06 ± 0.10 Ba |
| 70 °C, −0.06 MPa | 1.93 | 6.81 ± 0.13 Bb | 2.99 ± 0.09 Bb | 0.93 ± 0.15 Bb | |
| 65 °C, −0.08 MPa | 1.14 | 6.07 ± 0.18 Bc | 2.43 ± 0.13 Bc | 0.81 ± 0.14 Bc |
| UHT Condition | Flash Condition | DO (mg/L) | L* | a* | b* | BI |
|---|---|---|---|---|---|---|
| 135 °C, 10 s | - | 2.73 | 82.97 ± 0.52 Aa | 9.52 ± 0.66 Aa | 22.01 ± 0.22 Aa | 69.23 ± 0.14 Aa |
| 70 °C, −0.06 MPa | 1.93 | 85.78 ± 0.27 Ab | 8.33 ± 0.10 Ab | 20.67 ± 0.11 Ab | 62.28 ± 0.26 Ab | |
| 65 °C, −0.08 MPa | 1.14 | 89.12 ± 0.49 Ac | 7.42 ± 0.22 Ac | 18.34 ± 0.36 Ac | 53.71 ± 0.19 Ac | |
| 145 °C, 5 s | - | 2.68 | 77.13 ± 0.04 Ba | 10.15 ± 0.12 Ba | 25.32 ± 0.11 Ba | 72.79 ± 0.25 Ba |
| 70 °C, −0.06 MPa | 1.93 | 83.42 ± 0.26 Bb | 9.17 ± 0.12 Bb | 23.82 ± 0.15 Bb | 67.56 ± 0.21 Bb | |
| 65 °C, −0.08 MPa | 1.14 | 87.69 ± 0.59 Bc | 8.37 ± 0.19 Bc | 20.25 ± 0.42 Bc | 60.25 ± 0.29 Bc |
| Compound | 135 °C, 10 s | 145 °C, 5 s | ||||
|---|---|---|---|---|---|---|
| DO = 2.73 mg/L | DO = 1.93 mg/L | DO = 1.14 mg/L | DO = 2.68 mg/L | DO = 1.93 mg/L | DO = 1.14 mg/L | |
| Ketones | ||||||
| 2-Pentanone | 6.59 ± 0.34 Aa | 5.65 ± 0.22 Ab | 4.83 ± 0.21 Ac | 6.97 ± 0.21 Ba | 6.30 ± 0.25 Bb | 5.59 ± 0.24 Bc |
| 2-Hexanone | 1.23 ± 0.11 Aa | 0.75 ± 0.08 Ab | 0.54 ± 0.09 Ac | 1.39 ± 0.13 Ba | 0.86 ± 0.15 Bb | 0.67 ± 0.09 Bc |
| 2-Heptanone | 43.01 ± 3.41 Aa | 39.43 ± 3.55 Ab | 37.43 ± 2.34 Ac | 46.69 ± 3.51 Ba | 43.33 ± 2.61 Bb | 40.69 ± 2.23 Bc |
| 2-Octanone | 4.51 ± 0.18 Aa | 3.78 ± 0.15 Ab | 3.24 ± 0.10 Ac | 4.87 ± 0.16 Ba | 4.28 ± 0.13 Bb | 3.64 ± 0.16 Bc |
| 2-Nonanone | 4.05 ± 0.22 Aa | 3.37 ± 0.13 Ab | 2.78 ± 0.19 Ac | 4.43 ± 0.22 Ba | 3.81 ± 0.17 Bb | 3.13 ± 0.13 Bc |
| 2-Decanone | 1.45 ± 0.17 Aa | 1.06 ± 0.12 Ab | 0.78 ± 0.11 Ac | 1.72 ± 0.22 Ba | 1.36 ± 0.18 Bb | 0.98 ± 0.15 Bc |
| 2-Undecanone | 9.99 ± 0.11 Aa | 9.38 ± 0.14 Ab | 8.74 ± 0.15 Ac | 10.33 ± 0.10 Ba | 9.62 ± 0.18 Bb | 9.03 ± 0.12 Bc |
| Aldehydes | ||||||
| Valeraldehyde | 0.69 ± 0.15 Aa | 0.42 ± 0.13 Ab | 0.35 ± 0.14 Ac | 0.79 ± 0.16 Ba | 0.62 ± 0.17 Bb | 0.55 ± 0.15 Bc |
| Hexanal | 12.31 ± 1.62 Aa | 10.27 ± 1.26 Ab | 9.18 ± 1.05 Ac | 13.49 ± 1.12 Ba | 11.22 ± 1.37 Bb | 10.38 ± 1.35 Bc |
| Heptanal | 5.71 ± 0.59 Aa | 4.61 ± 0.39 Ab | 3.79 ± 0.68 Ac | 6.54 ± 0.48 Ba | 5.79 ± 0.65 Bb | 4.83 ± 0.44 Bc |
| Octanal | 0.92 ± 0.11 Aa | 0.76 ± 0.14 Ab | 0.56 ± 0.07 Ac | 1.15 ± 0.12 Ba | 0.92 ± 0.12 Bb | 0.75 ± 0.14 Bc |
| Nonanal | 3.76 ± 0.31 Aa | 2.47 ± 0.25 Ab | 1.75 ± 0.16 Ac | 3.96 ± 0.37 Ba | 2.72 ± 0.27 Bb | 1.96 ± 0.25 Bc |
| Decanal | 6.63 ± 0.25 Aa | 5.51 ± 0.18 Ab | 4.42 ± 0.21 Ac | 6.97 ± 0.36 Ba | 5.83 ± 0.27 Bb | 4.88 ± 0.22 Bc |
| Sulphur compounds | ||||||
| Hydrogen sulfide | 11.83 ± 1.83 Aa | 10.79 ± 1.52 Aa | 9.31 ± 1.39 Aa | 12.46 ± 1.77 Aa | 11.59 ± 1.48 Aa | 10.21 ± 1.65 Aa |
| Methyl mercaptan | 23.17 ± 2.50 Aa | 22.03 ± 2.42 Aa | 21.93 ± 2.39 Aa | 25.53 ± 2.32 Aa | 24.33 ± 2.41 Aa | 23.23 ± 2.61 Aa |
| Methyl sulfide | 21.32 ± 1.92 Aa | 19.98 ± 2.13 Aa | 19.17 ± 1.72 Aa | 24.52 ± 2.88 Aa | 23.95 ± 1.76 Aa | 23.27 ± 1.73 Aa |
| Dimethyl sulfoxide | 1.43 ± 0.12 Aa | 1.28 ± 0.11 Aa | 1.11 ± 0.15 Aa | 1.53 ± 0.22 Aa | 1.41 ± 0.21 Aa | 1.29 ± 0.14 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Luo, W.; Peng, Q.; Wu, Z.; Li, H.; Li, H.; Yu, J. Effects of Flash Evaporation Conditions on the Quality of UHT Milk by Changing the Dissolved Oxygen Content in Milk. Foods 2022, 11, 2371. https://doi.org/10.3390/foods11152371
Jiang S, Luo W, Peng Q, Wu Z, Li H, Li H, Yu J. Effects of Flash Evaporation Conditions on the Quality of UHT Milk by Changing the Dissolved Oxygen Content in Milk. Foods. 2022; 11(15):2371. https://doi.org/10.3390/foods11152371
Chicago/Turabian StyleJiang, Shiyao, Wenjing Luo, Qiuqi Peng, Zhengyan Wu, Hongbo Li, Hongjuan Li, and Jinghua Yu. 2022. "Effects of Flash Evaporation Conditions on the Quality of UHT Milk by Changing the Dissolved Oxygen Content in Milk" Foods 11, no. 15: 2371. https://doi.org/10.3390/foods11152371
APA StyleJiang, S., Luo, W., Peng, Q., Wu, Z., Li, H., Li, H., & Yu, J. (2022). Effects of Flash Evaporation Conditions on the Quality of UHT Milk by Changing the Dissolved Oxygen Content in Milk. Foods, 11(15), 2371. https://doi.org/10.3390/foods11152371




