Characterization, Classification and Authentication of Spanish Blossom and Honeydew Honeys by Non-Targeted HPLC-UV and Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Honey Samples
2.3. Sample Preparation
2.3.1. Honey Samples Analyzed by the Non-Targeted HPLC-UV Fingerprinting Method
2.3.2. Honey Samples Analyzed by the Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Method
2.4. Instrumentation
2.4.1. Non-Targeted HPLC-UV Fingerprinting Method
2.4.2. Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Method
2.5. Data Analysis
3. Results and Discussion
3.1. Non-Targeted HPLC-UV Fingerprinting Methodology
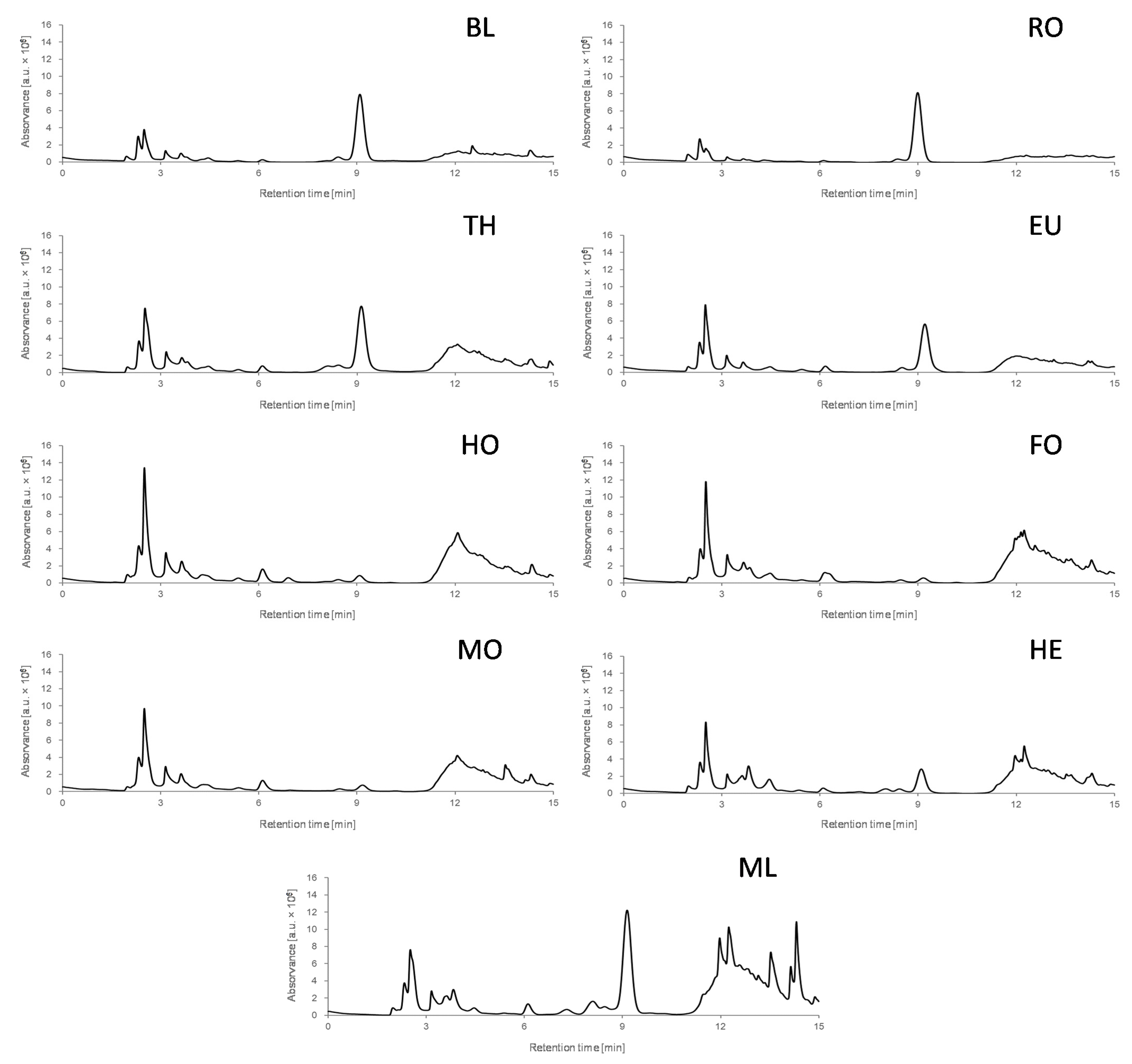
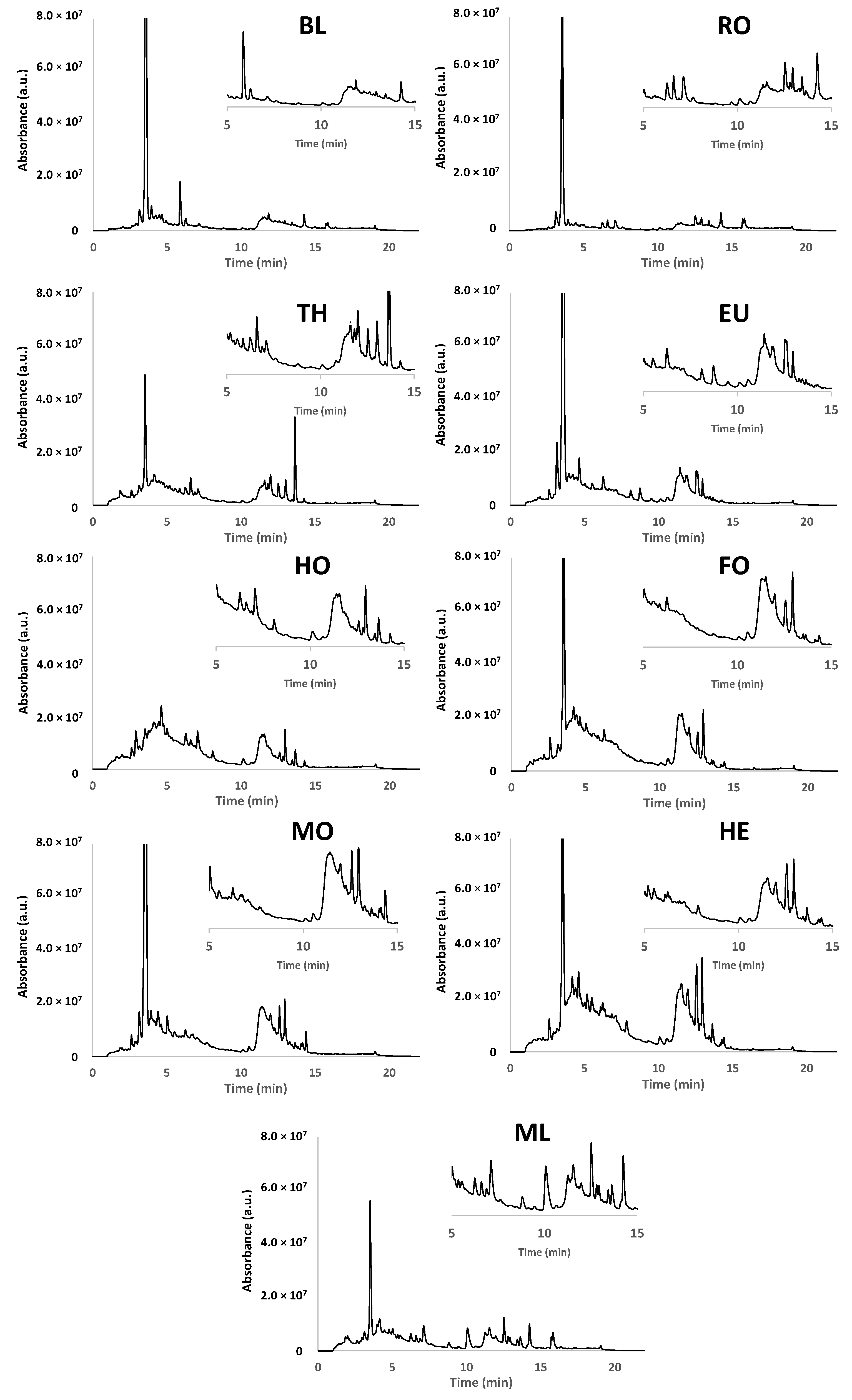
3.1.1. Chromatographic Fingerprints
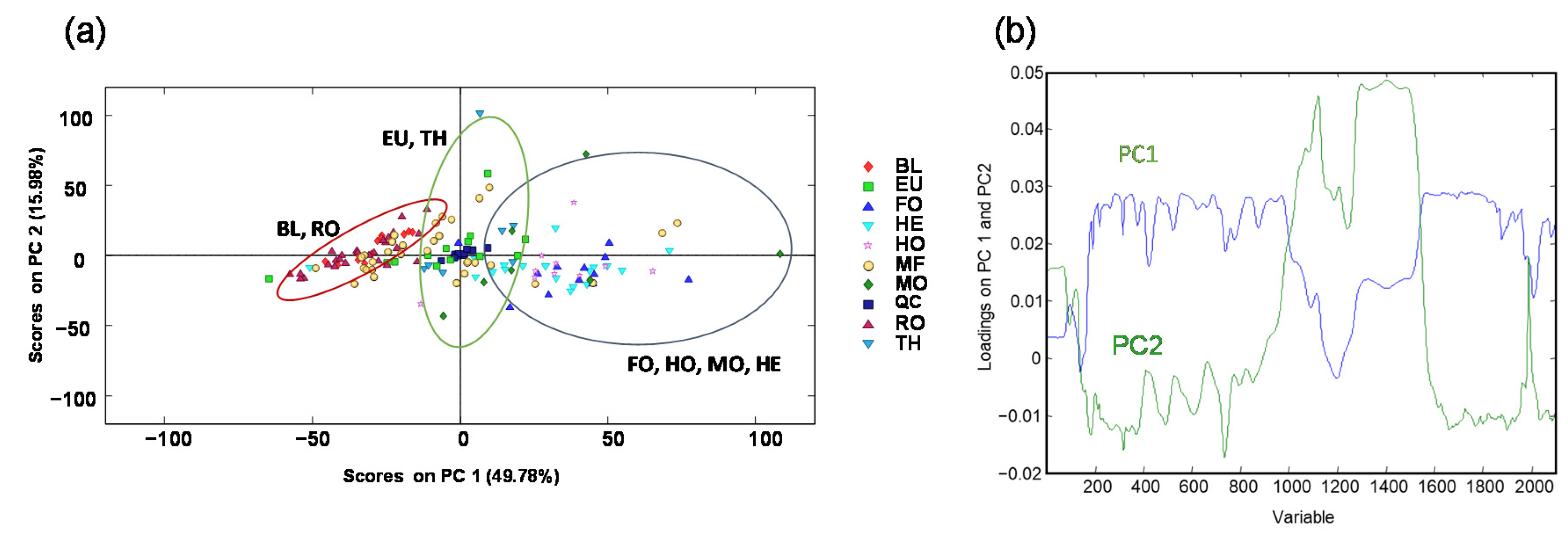
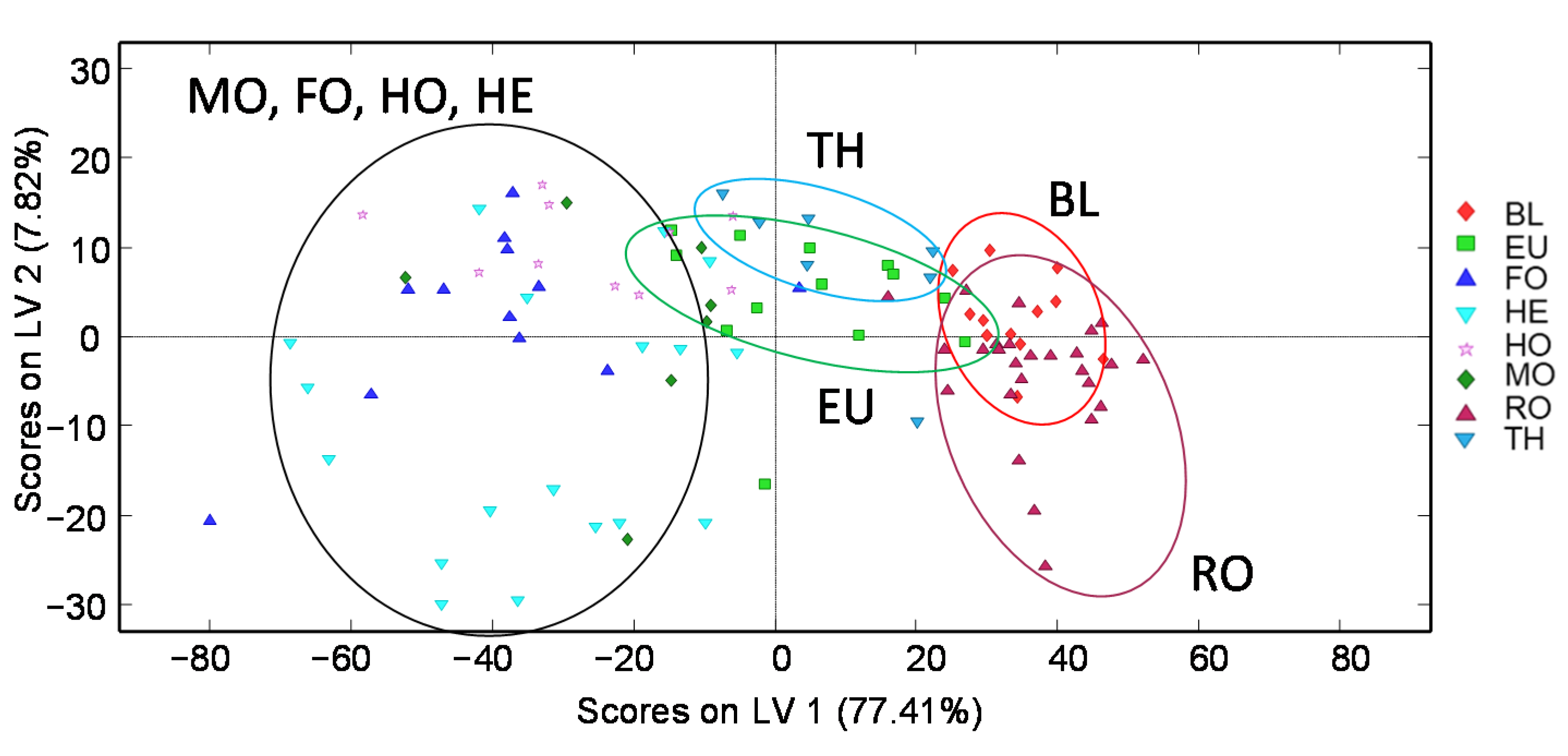
3.1.2. Chemometric Data Analysis
3.2. Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Methodology
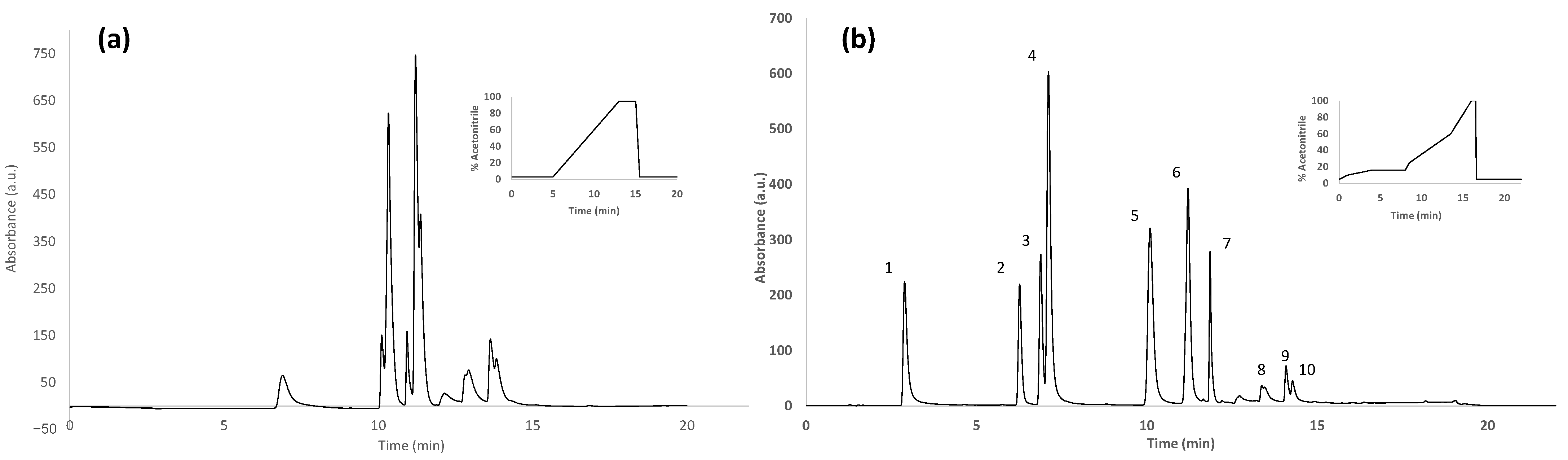
3.2.1. Optimization of the Chromatographic Separation
3.2.2. Optimization of the Off-Line SPE Extraction of Polyphenols in Honey Samples
3.2.3. Analysis of Honey Samples with the Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Methodology
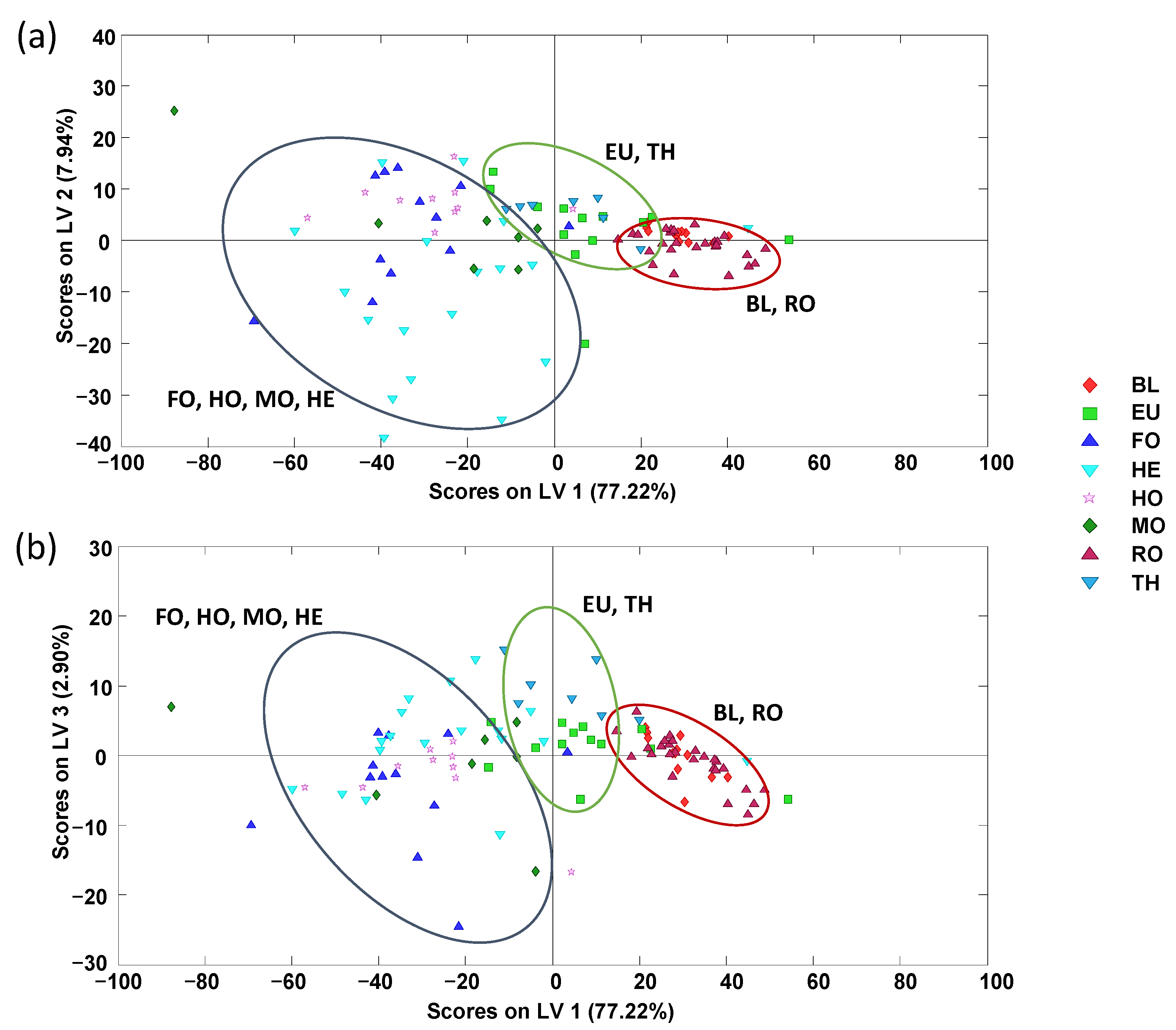
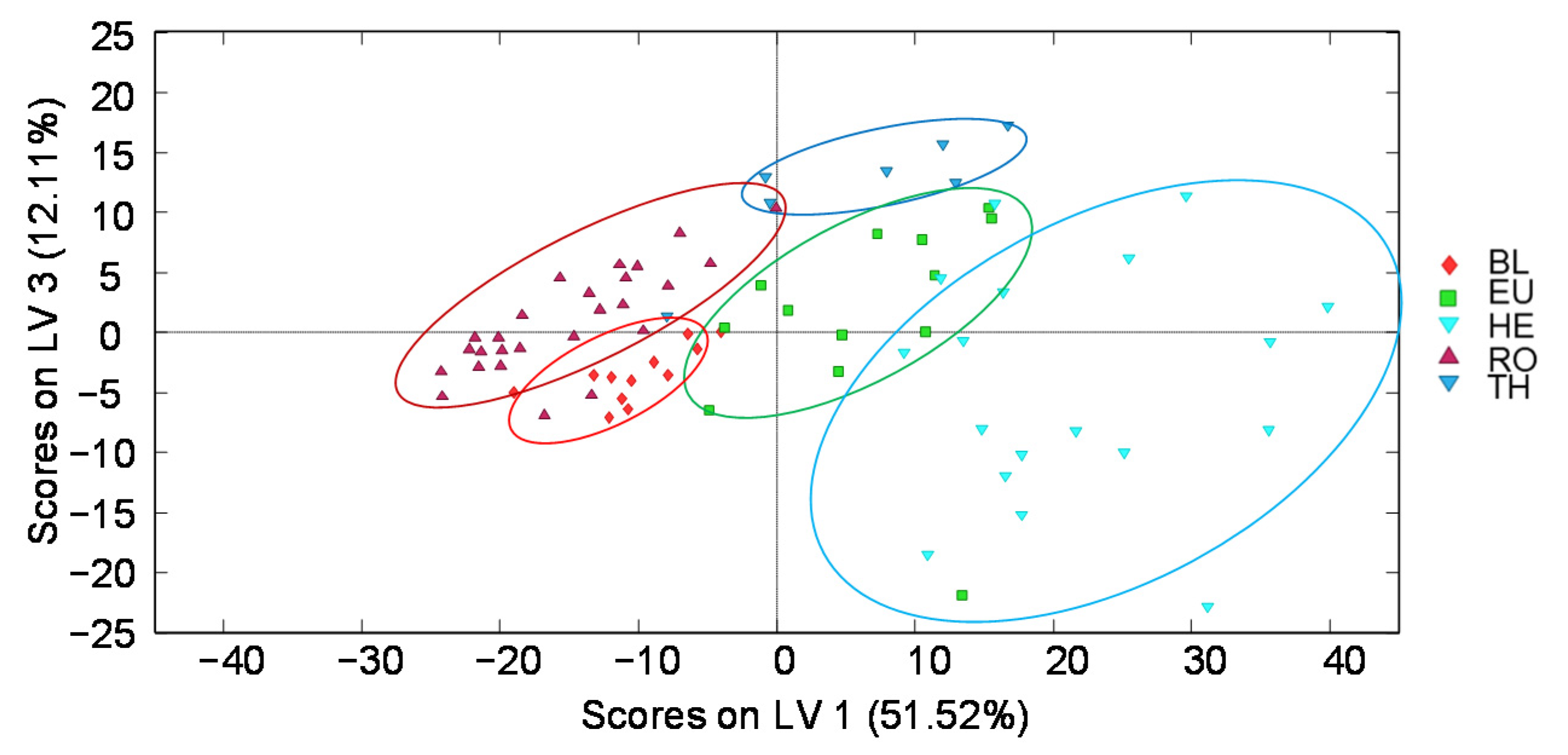
3.2.4. Chemometric Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vázquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Recklies, K.; Peukert, C.; Kölling-Speer, I.; Speer, K. Differentiation of Honeydew Honeys from Blossom Honeys and According to Their Botanical Origin by Electrical Conductivity and Phenolic and Sugar Spectra. J. Agric. Food Chem. 2021, 69, 1329–1347. [Google Scholar] [CrossRef]
- Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, L10, 47–52.
- Pita-Calvo, C.; Vázquez, M. Honeydew Honeys: A Review on the Characterization and Authentication of Botanical and Geographical Origins. J. Agric. Food Chem. 2018, 66, 2523–2537. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. Recent developments in honey characterization. RSC Adv. 2015, 5, 59696–59714. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Wang, L.; Lin, L.; Shi, H.; Cao, H.; Cao, B. Detection of honey adulteration with starch syrup by high performance liquid chromatography. Food Chem. 2015, 172, 669–674. [Google Scholar] [CrossRef]
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC—Trends Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Du, B.; Wu, L.; Xue, X.; Chen, L.; Li, Y.; Zhao, J.; Cao, W. Rapid Screening of Multiclass Syrup Adulterants in Honey by Ultrahigh-Performance Liquid Chromatography/Quadrupole Time of Flight Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6614–6623. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Hu, Y.; Zhou, J.; Chen, L.; Lu, X. Systematic Review of the Characteristic Markers in Honey of Various Botanical, Geographic, and Entomological Origins. ACS Food Sci. Technol. 2022, 2, 206–220. [Google Scholar] [CrossRef]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey phenolic compound profiling and authenticity assessment using hrms targeted and untargeted metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of phenolic compounds in honey using dispersive liquid-liquid microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef]
- Kawashima, H.; Suto, M.; Suto, N. Determination of carbon isotope ratios for honey samples by means of a liquid chromatography/isotope ratio mass spectrometry system coupled with a post-column pump. Rapid Commun. Mass Spectrom. 2018, 32, 1271–1279. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geană, E.I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Férez-Melgarejo, G.; Hernández-Córdoba, M. Dispersive liquid-liquid microextraction for the determination of flavonoid aglycone compounds in honey using liquid chromatography with diode array detection and time-of-flight mass spectrometry. Talanta 2015, 131, 185–191. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Li, Y.; Chen, L.; Wu, L.; Zhao, J. Floral classification of honey using liquid chromatography-diode array detection-tandem mass spectrometry and chemometric analysis. Food Chem. 2014, 145, 941–949. [Google Scholar] [CrossRef]
- Dong, H.; Luo, D.; Xian, Y.; Luo, H.; Guo, X.; Li, C.; Zhao, M. Adulteration Identification of Commercial Honey with the C-4 Sugar Content of Negative Values by an Elemental Analyzer and Liquid Chromatography Coupled to Isotope Ratio Mass Spectroscopy. J. Agric. Food Chem. 2016, 64, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC-MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, M.; Rogers, K.M.; Jamin, E.; Thomas, F.; Guyader, S.; Lees, M.; Rutledge, D.N. Combination of 1H NMR and chemometrics to discriminate manuka honey from other floral honey types from Oceania. Food Chem. 2017, 217, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Fan, C.; Gao, S.; Zhang, Z.; Bo, L. Classification of the botanical and geographical origins of Chinese honey based on 1H NMR profile with chemometrics. Food Res. Int. 2020, 137, 109714. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef]
- Latorre, C.H.; Crecente, R.M.P.; Martín, S.G.; García, J.B. A fast chemometric procedure based on NIR data for authentication of honey with protected geographical indication. Food Chem. 2013, 141, 3559–3565. [Google Scholar] [CrossRef]
- Guelpa, A.; Marini, F.; du Plessis, A.; Slabbert, R.; Manley, M. Verification of authenticity and fraud detection in South African honey using NIR spectroscopy. Food Control 2017, 73, 1388–1396. [Google Scholar] [CrossRef]
- Valinger, D.; Longin, L.; Grbeš, F.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Detection of honey adulteration—The potential of UV-VIS and NIR spectroscopy coupled with multivariate analysis. LWT 2021, 145, 111316. [Google Scholar] [CrossRef]
- Lenhardt, L.; Bro, R.; Zeković, I.; Dramićanin, T.; Dramićanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef]
- Ansari, M.J.; Al-Ghamdi, A.; Khan, K.A.; Adgaba, N.; El-Ahmady, S.H.; Gad, H.A.; Roshan, A.; Meo, S.A.; Kolyali, S. Validation of botanical origins and geographical sources of some Saudi honeys using ultraviolet spectroscopy and chemometric analysis. Saudi J. Biol. Sci. 2018, 25, 377–382. [Google Scholar] [CrossRef]
- Suhandy, D.; Yulia, M. The use of UV spectroscopy and SIMCA for the authentication of Indonesian honeys according to botanical, entomological and geographical origins. Molecules 2021, 26, 915. [Google Scholar] [CrossRef]
- Cavazza, A.; Corradini, C.; Musci, M.; Salvadeo, P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J. Sci. Food Agric. 2013, 93, 1169–1175. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Yang, S.; Zhang, W.; Zhang, J.; Zhao, W.; Chen, L.; Wen, Y.; Zhang, Y.; Lu, K.; et al. Strategy for comparative untargeted metabolomics reveals honey markers of different floral and geographic origins using ultrahigh-performance liquid chromatography-hybrid quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2017, 1499, 78–89. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey volatiles as a fingerprint for botanical origin—a review on their occurrence on monofloral honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.K.; Kaparakou, E.; Kanakis, C.; Pappas, C.; Tarantilis, P. The use of spme-gc-ms ir and raman techniques for botanical and geographical authentication and detection of adulteration of honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef]
- Puscas, A.; Hosu, A.; Cimpoiu, C. Application of a newly developed and validated high-performance thin-layer chromatographic method to control honey adulteration. J. Chromatogr. A 2013, 1272, 132–135. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics. J. Chem. Inf. Comput. Sci. 1997, 38, 1254. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomás-Barberán, F.A. Flavonoid Composition of Tunisian Honeys and Propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC—Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef] [Green Version]
| Honey | Botanical Variety | Number of Samples | Geographical Origin | Number of Samples |
|---|---|---|---|---|
| Blossom honeys | Orange/Lemon Blossom (BL) | 12 | Aragon | 1 |
| Balearic Islands | 2 | |||
| Cantabria | 1 | |||
| Castile and Leon | 2 | |||
| Catalonia | 3 | |||
| Extremadura | 1 | |||
| Spain a | 2 | |||
| Eucalyptus (EU) | 13 | Andalusia | 1 | |
| Asturias | 2 | |||
| Cantabria | 1 | |||
| Castile and Leon | 2 | |||
| Catalonia | 1 | |||
| Extremadura | 4 | |||
| Navarre | 1 | |||
| Spain and others b | 1 | |||
| Rosemary (RO) | 26 | Andalusia | 1 | |
| Aragon | 4 | |||
| Asturias | 1 | |||
| Balearic Islands | 4 | |||
| Castile and Leon | 1 | |||
| Cantabria | 2 | |||
| Catalonia | 6 | |||
| Extremadura | 4 | |||
| Navarre | 1 | |||
| Spain a | 2 | |||
| Thyme (TH) | 7 | Castile and Leon | 1 | |
| Castile La Mancha | 2 | |||
| Catalonia | 1 | |||
| Extremadura | 2 | |||
| Spain a | 1 | |||
| Heather (HE) | 18 | Asturias | 2 | |
| Basque Country | 1 | |||
| Cantabria | 3 | |||
| Castile and Leon | 5 | |||
| Catalonia | 2 | |||
| Extremadura | 5 | |||
| Honeydew honeys | Mountain (MO) | 6 | Asturias | 2 |
| Castile and Leon | 1 | |||
| Castile La Mancha | 1 | |||
| Catalonia | 2 | |||
| Forest (FO) | 10 | Balearic Islands | 2 | |
| Cantabria | 2 | |||
| Castile and Leon | 1 | |||
| Catalonia | 1 | |||
| Spain a | 4 | |||
| Holm Oak (HO) | 10 | Aragon | 2 | |
| Castile and Leon | 2 | |||
| Extremadura | 6 | |||
| Other honeys | Multifloral (MF) | 34 | Asturias | 1 |
| Balearic Islands | 4 | |||
| Cantabria | 1 | |||
| Castile and Leon | 7 | |||
| Castile La Mancha | 2 | |||
| Catalonia | 8 | |||
| Extremadura | 4 | |||
| Navarre | 4 | |||
| Spain a | 1 | |||
| Spain and others b | 2 |
| Compound | Recoveries (%) a (Standard Deviation Provided within Parenthesis) | |||||
|---|---|---|---|---|---|---|
| Methanol | Acetonitrile | |||||
| 2 mL | 4 mL | 6 mL | 2 mL | 4 mL | 6 mL | |
| gallic acid | 82 (7) | 83 (4) | 72 (9) | 1 (2) | 14 (16) | 14 (13) |
| p-hydroxybenzoic acid | 93 (4) | 110 (10) | 97 (12) | 46 (8) | 81 (7) | 110 (8) |
| vanillic acid | 72 (1) | 74 (2) | 67 (5) | 77 (1) | 73 (1) | 87 (8) |
| p-coumaric acid | 87 (2) | 87.3 (0.9) | 84 (6) | 42 (11) | 77 (2) | 90 (5) |
| apigenin | 57 (3) | 50 (11) | 110 (8) | 0.2 (0.1) | 0 (0) | 2 (4) |
| kaempferol | 52 (4) | 112 (12) | 142 (22) | 7 (8) | 1 (2) | 60 (22) |
| Class | Sensitivity (%) | Specificity (%) | Classification Error (%) |
|---|---|---|---|
| BL | 91.7 | 98.4 | 2.6 |
| EU | 92.3 | 90.5 | 7.9 |
| HE | 100 | 94.8 | 3.9 |
| RO | 96.2 | 94.0 | 3.9 |
| TH | 71.4 | 89.9 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Seval, V.; Martínez-Alfaro, C.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization, Classification and Authentication of Spanish Blossom and Honeydew Honeys by Non-Targeted HPLC-UV and Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Strategies. Foods 2022, 11, 2345. https://doi.org/10.3390/foods11152345
García-Seval V, Martínez-Alfaro C, Saurina J, Núñez O, Sentellas S. Characterization, Classification and Authentication of Spanish Blossom and Honeydew Honeys by Non-Targeted HPLC-UV and Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Strategies. Foods. 2022; 11(15):2345. https://doi.org/10.3390/foods11152345
Chicago/Turabian StyleGarcía-Seval, Víctor, Clàudia Martínez-Alfaro, Javier Saurina, Oscar Núñez, and Sònia Sentellas. 2022. "Characterization, Classification and Authentication of Spanish Blossom and Honeydew Honeys by Non-Targeted HPLC-UV and Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Strategies" Foods 11, no. 15: 2345. https://doi.org/10.3390/foods11152345
APA StyleGarcía-Seval, V., Martínez-Alfaro, C., Saurina, J., Núñez, O., & Sentellas, S. (2022). Characterization, Classification and Authentication of Spanish Blossom and Honeydew Honeys by Non-Targeted HPLC-UV and Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Strategies. Foods, 11(15), 2345. https://doi.org/10.3390/foods11152345









