The Impact of Innovative Plant Sources (Cordia myxa L. Fruit (Assyrian Plum) and Phoenix dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Collection of Raw Material
2.2. Preparation of the Assyrian Plum Fruit and Date Pit Flours
2.3. Preparation of the Flour Blends
2.4. Characterization of the Flour Blends
2.4.1. Proximate Composition of the Flour Blends
2.4.2. Functional Properties of the Flour Blends
2.4.3. Pasting Behavior of the Flour Blends
2.4.4. Differential Scanning Calorimetry of the Flour Blends
2.4.5. Antioxidant Activity of the Flour Blends
Radical Scavenging Activity by 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
Ferric/Ferricyanide (Fe3+) Reducing Antioxidant Power (FRAP)
2.4.6. Bioactive Compounds in the Flour Blends
Total Phenolic Content
Total Flavonoid Content
2.5. Gluten-Free Biscuit Preparation
2.6. Micromorphology of the Biscuits
2.7. Evaluation of the Quality Attributes of the Gluten-Free Biscuits
2.7.1. Antioxidant Activities and Bioactive Compounds
2.7.2. Dimensional Analysis
2.7.3. Textural Analysis
2.7.4. Color Analysis
2.7.5. Nutritional Analysis
2.7.6. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Functional Properties of the Flour Blends
3.3. Pasting Properties of the Flour Blends
3.4. Thermal Properties of the Flour Blends
3.5. Scanning Electron Micrograph of the Biscuits
3.6. Antioxidant Activity
3.7. Total Phenolic Content (TPC)
3.8. Total Flavonoid Content (TFC)
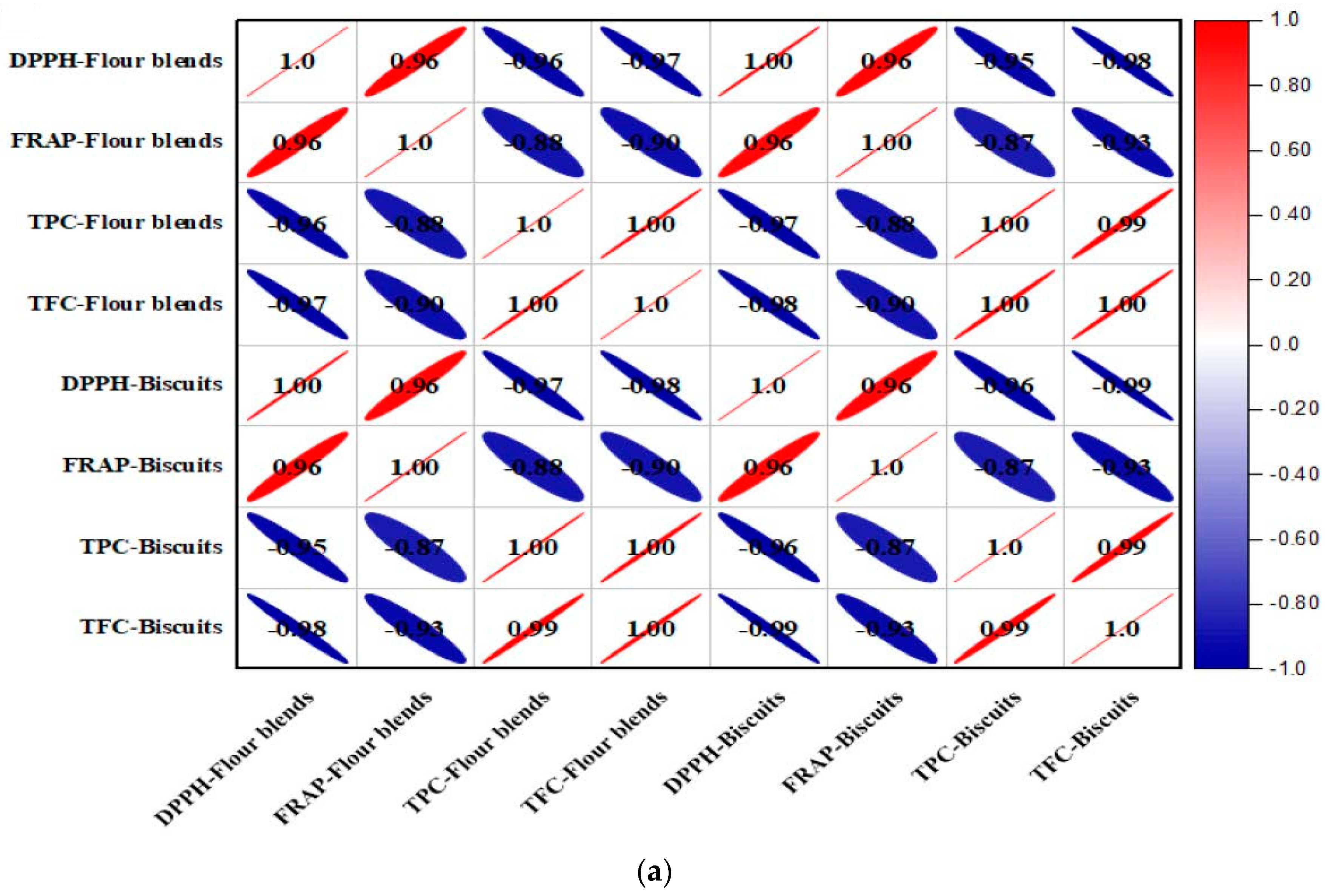
3.9. Correlation Studies of Antioxidants with Bioactive Compounds
3.10. Physical Properties of the Biscuits
3.11. Nutritional Analysis of the Biscuits
3.12. Sensory Analysis
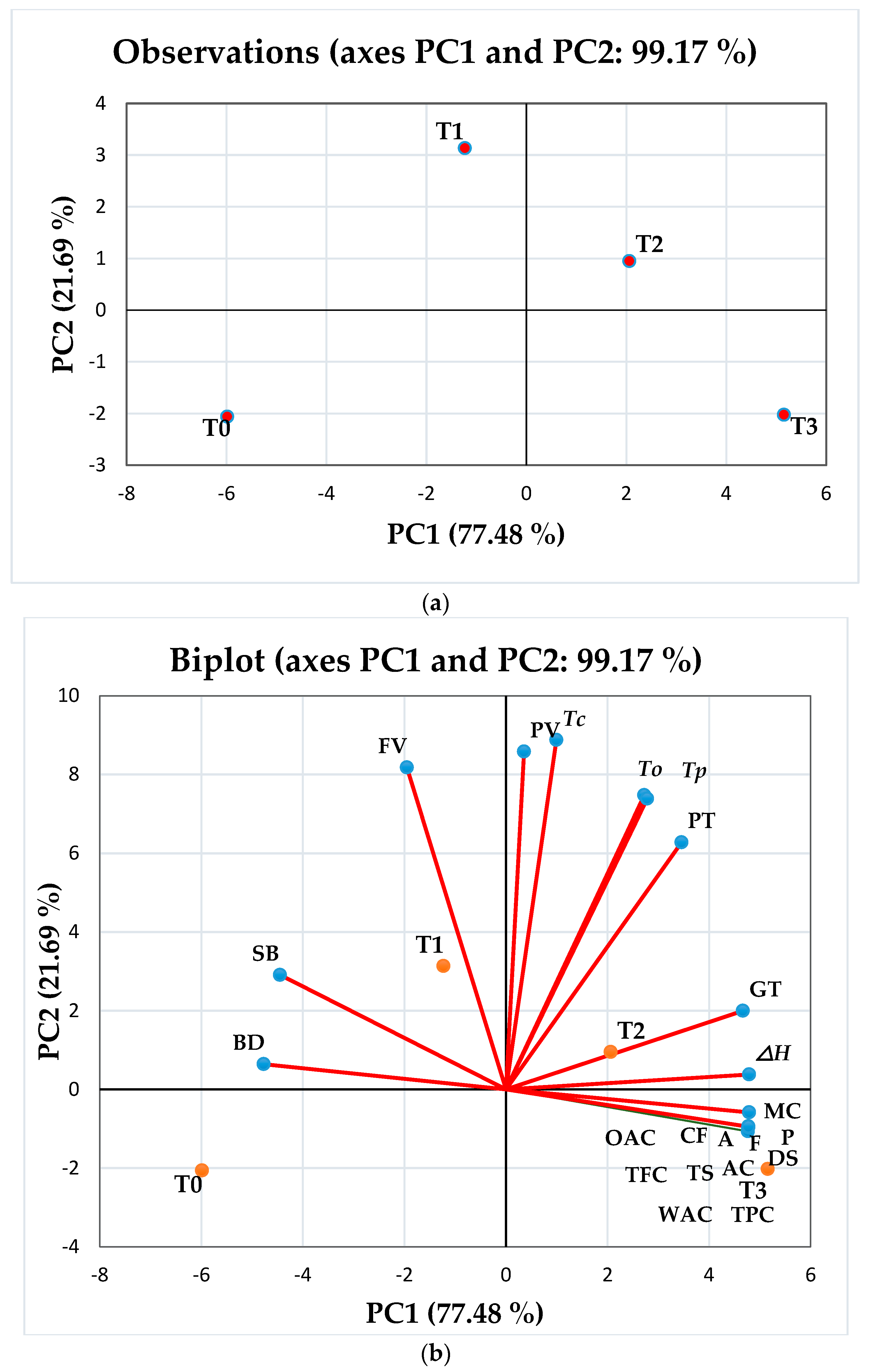
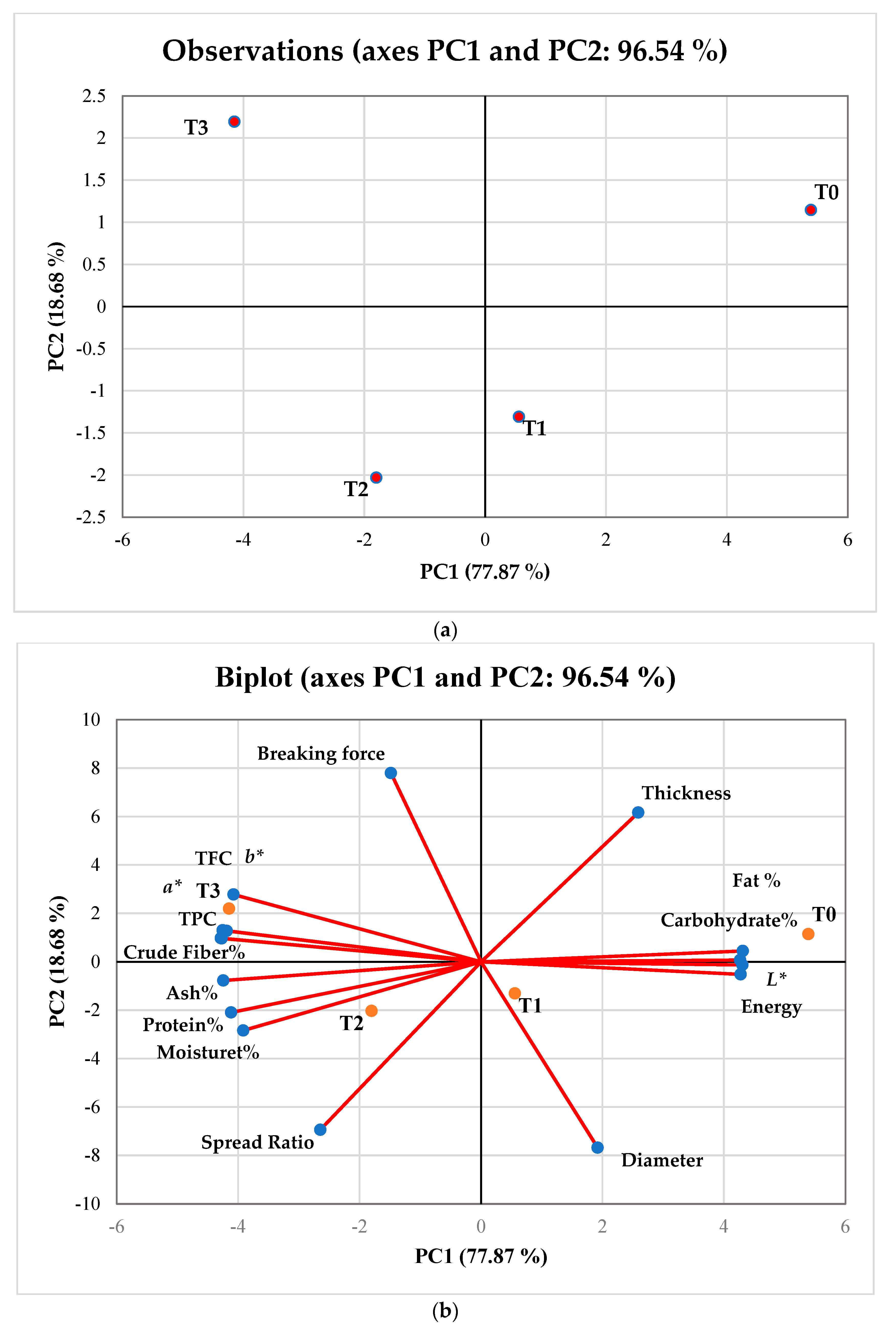
3.13. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cannas, M.; Pulina, S.; Conte, P.; Del Caro, A.; Urgeghe, P.P.; Piga, A. Effect of substitution of rice flour with Quinoa flour on the chemical-physical, nutritional, volatile and sensory parameters of gluten-free ladyfinger biscuits. Foods 2020, 9, 808. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 823–836. e822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaja-Bulsa, G.; Bulsa, M. Adherence to gluten-free diet in children with celiac disease. Nutrients 2018, 10, 1424. [Google Scholar] [CrossRef] [Green Version]
- Sulieman, A.A.; Zhu, K.X.; Peng, W.; Hassan, H.A.; Obadi, M.; Siddeeg, A.; Zhou, H.M. Rheological and quality characteristics of composite gluten-free dough and biscuits supplemented with fermented and unfermented Agaricus bisporus polysaccharide flour. Food Chem. 2019, 271, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Iorfida, D.; Anania, C.; Trovato, C.M.; Montuori, M.; Cucchiara, S.; Catassi, C. Cereal consumption among subjects with celiac disease: A snapshot for nutritional considerations. Nutrients 2017, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Di Cairano, M.; Galgano, F.; Tolve, R.; Caruso, M.C.; Condelli, N. Focus on gluten free biscuits: Ingredients and issues. Trends Food Sci. Technol. 2018, 81, 203–212. [Google Scholar] [CrossRef]
- Giuberti, G.; Bresciani, A.; Cervini, M.; Frustace, A.; Marti, A. Moringa oleifera L. leaf powder as ingredient in gluten-free biscuits: Nutritional and physicochemical characteristics. Eur. Food Res. Technol. 2021, 247, 687–694. [Google Scholar] [CrossRef]
- Wesley, S.D.; André, B.H.M.; Clerici, M.T.P.S. Gluten-free rice & bean biscuit: Characterization of a new food product. Heliyon 2021, 7, e05956. [Google Scholar] [CrossRef]
- Doğan, H.; Meral, R. The effects of locust bean gum and rhubarb on the physical and functional properties of the gluten-free biscuits. Int. J. Food Sci. 2019, 31, 1487–1492. [Google Scholar] [CrossRef]
- Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A.; Shahzad, S.A.; Ababtain, I.A. Use of Gum Cordia (Cordia myxa) as a Natural Starch Modifier; Effect on Pasting, Thermal, Textural, and Rheological Properties of Corn Starch. Foods 2020, 9, 909. [Google Scholar] [CrossRef]
- Duta, D.E.; Culetu, A.; Mohan, G. Sensory and physicochemical changes in gluten-free oat biscuits stored under different packaging and light conditions. J. Food Sci. Technol. 2019, 56, 3823–3835. [Google Scholar] [CrossRef] [PubMed]
- Giri, N.A.; Sakhale, B. Development of sweet potato flour based high protein and low calorie gluten free cookies. Curr. Res. Nutr. Food Sci. 2019, 7, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Ganino, T.; Rodolfi, M.; Chiancone, B.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and oxidative stability of gluten-free biscuits during storage. LWT Food Sci. Technol. 2018, 98, 451–457. [Google Scholar] [CrossRef]
- Alhassan, M.W.; Ojangba, T.; Amagloh, F.K. Development of gluten-free biscuits from peanut-pearl millet composite flour. Am. J. Food Technol. 2019, 7, 40–44. [Google Scholar] [CrossRef]
- Benkadri, S.; Salvador, A.; Sanz, T.; Nasreddine Zidoune, M. Optimization of Xanthan and Locust Bean Gum in a Gluten-Free Infant Biscuit Based on Rice-Chickpea Flour Using Response Surface Methodology. Foods 2021, 10, 12. [Google Scholar] [CrossRef]
- Lu, L.W.; Venn, B.; Lu, J.; Monro, J.; Rush, E. Effect of cold storage and reheating of parboiled rice on postprandial glycaemic response, satiety, palatability and chewed particle size distribution. Nutrients 2017, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Carcea, M. Value of Wholegrain Rice in a Healthy Human Nutrition. Agriculture 2021, 11, 720. [Google Scholar] [CrossRef]
- Seyedian, R.; Isavi, F.; Najafiasl, M.; Zaeri, S. Electrospun fibers loaded with Cordia myxa L. fruit extract: Fabrication, characterization, biocompatibility and efficacy in wound healing. J. Drug Deliv. Sci. Technol. 2021, 63, 102528. [Google Scholar] [CrossRef]
- Echegaray, N.; Gullón, B.; Pateiro, M.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Date Fruit and Its By-products as Promising Source of Bioactive Components: A Review. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Alharbi, K.L.; Raman, J.; Shin, H.J. Date fruit and seed in nutricosmetics. Cosmetics 2021, 8, 59. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association Cereal Chemist Chemist; St. Pauls: London, UK, 2000. [Google Scholar]
- Ahn, H.; Kim, J.; Ng, P. Functional and thermal properties of wheat, barley, and soy flours and their blends treated with a microbial transglutaminase. J. Food Sci. Technol. 2005, 70, c380–c386. [Google Scholar] [CrossRef]
- Ye, L.; Wang, C.; Wang, S.; Zhou, S.; Liu, X. Thermal and rheological properties of brown flour from Indica rice. J. Cereal Sci. 2016, 70, 270–274. [Google Scholar] [CrossRef]
- Sayas-Barberá, E.; Martín-Sánchez, A.M.; Cherif, S.; Ben-Abda, J.; Pérez-Álvarez, J.Á. Effect of date (Phoenix dactylifera L.) pits on the shelf life of beef burgers. Foods 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Zheng, T.; Chen, Y.; Yang, G.Z. Chemical constituents with free-radical-scavenging activities from the stem of Fissistigma polyanthum. Pharmacogn. Mag. 2012, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sęczyk, Ł.; Złotek, U.; Różyło, R.; Kaszuba, K.; Ryszawy, D.; Czyż, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. BioMed Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S. Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of North Indian region. J. Food Meas. Charact. 2017, 11, 126–133. [Google Scholar] [CrossRef]
- Saeed, S.M.G.; Tayyaba, S.; Ali, S.A.; Tayyab, S.; Sayeed, S.A.; Ali, R.; Mobin, L.; Naz, S. Evaluation of the potential of Lotus root (Nelumbo nucifera) flour as a fat mimetic in biscuits with improved functional and nutritional properties. CyTA-J. Foods 2020, 18, 624–634. [Google Scholar] [CrossRef]
- Ali, R.; Saeed, S.M.G.; Ali, S.A.; Sayed, S.A.; Ahmed, R.; Mobin, L. Effect of black gram flour as egg replacer on microstructure of biscuit dough and its impact on edible qualities. J. Food Meas. Charact. 2018, 12, 1641–1647. [Google Scholar] [CrossRef]
- Kuchtová, V.; Kohajdová, Z.; Karovicova, J.; Lauková, M. Physical, textural and sensory properties of cookies incorporated with grape skin and seed preparations. Pol. J. Food Nutr. Sci. 2018, 68, 309–317. [Google Scholar] [CrossRef]
- Heymann, H.; Lawless, H.T. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Saeed, S.M.G.; Urooj, S.; Ali, S.A.; Ali, R.; Mobin, L.; Ahmed, R.; Sayeed, S.A. Impact of the incorporation of date pit flour an underutilized biowaste in dough and its functional role as a fat replacer in biscuits. J. Food Process. Preserv. 2021, 45, e15218. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.; Wand O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Le Bouthillier, M.; Perron, J.; Pomerleau, S.; Gagnon, P.; Labonté, M.È.; Plante, C.; Guével, M.-H.; Provencher, V. Nutritional Content of Sliced Bread Available in Quebec, Canada: Focus on Sodium and Fibre Content. Nutrients 2021, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M. Date seeds: A promising source of oil with functional properties. Foods 2020, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Zheng, X. A review of milling damaged starch: Generation, measurement, functionality and its effect on starch-based food systems. Food Chem. 2020, 315, 126267. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.T.; Zhu, R.; Zhou, X.; Zhong, K.; Wang, L.; Liu, L.; Zhou, S. Soaking time of rice in semidry flour milling was shortened by increasing the grains cracks. J. Cereal Sci. 2017, 74, 121–126. [Google Scholar] [CrossRef]
- Asmeda, R.; Noorlaila, A.; Norziah, M. Relationships of damaged starch granules and particle size distribution with pasting and thermal profiles of milled MR263 rice flour. Food Chem. 2016, 191, 45–51. [Google Scholar] [CrossRef]
- Kaur, M.; Sandhu, K.S.; Arora, A.; Sharma, A. Gluten free biscuits prepared from buckwheat flour by incorporation of various gums: Physicochemical and sensory properties. LWT Food Sci. Technol. 2015, 62, 628–632. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food ingredients that inhibit cholesterol absorption. Prev. Nutr. Food Sci. 2017, 22, 67. [Google Scholar] [CrossRef]
- Hojjati, M.; Beirami-Serizkani, F. Structural characterization, antioxidant and antibacterial activities of a novel water soluble polysaccharide from Cordia myxa fruits. J. Food Meas. Charact. 2020, 14, 3417–3425. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Mahmood, K.; Alamri, M.S.; Abdellatif, M.A.; Hussain, S.; Qasem, A.A.A. Wheat flour and gum cordia composite system: Pasting, rheology and texture studies. Food Sci. Technol. 2018, 38, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Palabiyik, I.; Yildiz, O.; Toker, O.S.; Cavus, M.; Ceylan, M.M.; Yurt, B. Investigating the addition of enzymes in gluten-free flours–The effect on pasting and textural properties. LWT Food Sci. Technol. 2016, 69, 633–641. [Google Scholar] [CrossRef]
- Di Cairano, M.; Condelli, N.; Caruso, M.C.; Cela, N.; Tolve, R.; Galgano, F. Use of Underexploited Flours for the Reduction of Glycaemic Index of Gluten-Free Biscuits: Physicochemical and Sensory Characterization. Food Bioprocess Technol. 2021, 14, 1490–1502. [Google Scholar] [CrossRef]
- Dickinson, E.; Wedlock, D. Controlled Particle, Droplet and Bubble Formation; Butterworth-Heinemann: Oxford, UK, 1994. [Google Scholar] [CrossRef]
- Chusak, C.; Adisakwattana, S. Physicochemical and Functional Characteristics of RD43 Rice Flour and Its Food Application. Foods 2020, 9, 1912. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Safdarianghomsheh, R.; Zamanifar, P.; Salehi, S.; Niakousari, M.; Hosseini, S.M.H. Characterization of novel edible films and coatings for food preservation based on gum cordia. J. Food Qual. 2020, 2020, 8883916. [Google Scholar] [CrossRef]
- Hussein, A.M.; Yaseen, A.A.; Esmail, R.M.; Mohammad, A.A. Gluten-free biscuits produced from new drought tolerant corn hybrids: Processing and evaluation. Bull. Natl. Res. Cent. 2020, 44, 25. [Google Scholar] [CrossRef]
- Tavares, B.O.; Da Silva, E.P.; Da Silva, V.S.N.; Soares, M.S.; Ida, E.I.; Damiani, C. Stability of gluten free sweet biscuit elaborated with rice bran, broken rice and okara. Food Sci. Technol. 2016, 36, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Habib, H.M.; Ibrahim, W.H. Effect of date seeds on oxidative damage and antioxidant status in vivo. J. Sci. Food Agric. 2011, 91, 1674–1679. [Google Scholar] [CrossRef]
- El-Massry, K.F.; Farouk, A.; Mahmoud, K.F.; El-Ghorab, A.H.; Musa, A.; Mostafa, E.M.; Ghoneim, M.M.; Naguib, I.A.; Abdelgawad, M.A. Chemical characteristics and targeted encapsulated Cordia myxa fruits extracts nanoparticles for antioxidant and cytotoxicity potentials. Saudi J. Biol. Sci. 2021, 28, 5349–5358. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Cookie making behavior of wheat–barley flour blends and effects on antioxidant properties. LWT Food Sci. Technol. 2014, 55, 301–307. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. Effects of baking conditions and dough formulations on phenolic compound stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. LWT Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.M.; Hashim, I.B.; Kamal, H.; AlMaqbali, F.; Souka, U.; Ibrahim, W.H. Production of functional pita bread using date seed powder. J. Food Sci. Technol. 2015, 52, 6375–6384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef] [PubMed]
- Cervini, M.; Frustace, A.; Garrido, G.D.; Rocchetti, G.; Giuberti, G. Nutritional, physical and sensory characteristics of gluten-free biscuits incorporated with a novel resistant starch ingredient. Heliyon 2021, 7, e06562. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Glibetic, M. Polyphenols and their interactions with other dietary compounds: Implications for human health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar] [CrossRef]
- Panyoo, A.E.; Emmambux, M.N. Amylose–lipid complex production and potential health benefits: A mini-review. Stärke 2017, 69, 1600203. [Google Scholar] [CrossRef]
- Molinari, R.; Costantini, L.; Timperio, A.M.; Lelli, V.; Bonafaccia, F.; Bonafaccia, G.; Merendino, N. Tartary buckwheat malt as ingredient of gluten-free cookies. J. Cereal Sci. 2018, 80, 37–43. [Google Scholar] [CrossRef]
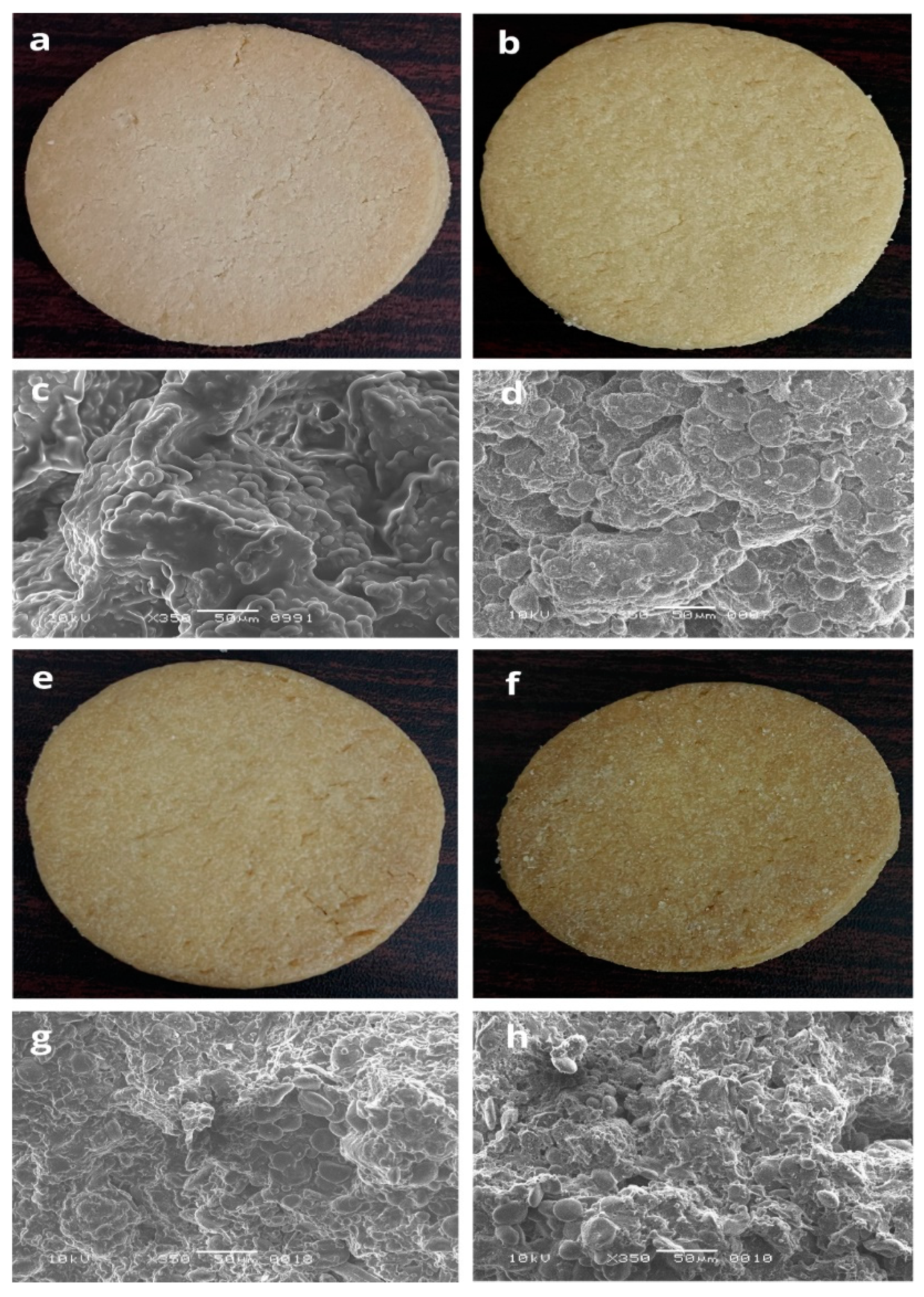


| Ingredients | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Refined rice flour (g) | 100 | 90 | 80 | 70 |
| Butter (g) | 40 | 40 | 40 | 40 |
| Sugar (g) | 40 | 40 | 40 | 40 |
| Egg (g) | 25 | 25 | 25 | 25 |
| Milk powder (g) | 5 | 5 | 5 | 5 |
| Baking powder (g) | 1.5 | 1.5 | 1.5 | 1.5 |
| Distilled water (mL) | 5 ± 2 | 7 ± 2 | 9 ± 2 | 11 ± 2 |
| Assyrian plum flour (g) | 0 | 5 | 10 | 15 |
| Date pit flour (g) | 0 | 5 | 10 | 15 |
| Proximate compositions | Functional Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Moisture % | Protein % | Fat % | Ash % | Crude Fiber % | Damaged Starch % | Amylose % | Total Starch % | Water Absorption Capacity % | Oil Absorption Capacity % |
| T0 | 7.04 ± 0.11 a | 5.78 ± 0.12 a | 0.92 ± 0.01 a | 1.25 ± 0.03 a | 0.52 ± 0.02 a | 7.92 ± 0.14 f | 5.88 ± 0.12 a | 78.67 ± 1.10 f | 138.32 ± 2.01 a | 104.11 ± 2.02 b |
| T1 | 7.16 ± 0.14 b | 5.94 ± 0.13 b | 1.20 ± 0.07 b | 2.14 ± 0.05 c | 3.71 ± 0.07 b | 7.41 ± 0.16 e | 6.05 ± 0.15 b | 74.50 ± 1.24 e | 154.01 ± 2.11 b | 103.19 ± 2.06 d |
| T2 | 7.30 ± 0.17 c | 6.09 ± 0.15 c | 1.48 ± 0.08 c | 3.04 ± 0.07 d | 6.90 ± 0.10 c | 6.89 ± 0.18 d | 6.17 ± 0.17 c | 70.19 ± 1.30 d | 140.14 ± 2.14 c | 102.28 ± 2.09 e |
| T3 | 7.42 ± 0.19 d | 6.26 ± 0.17 d | 1.76 ± 0.10 d | 3.94 ± 0.08 e | 10.09 ± 1.03 d | 6.43 ± 0.19 c | 6.35 ± 0.19 e | 66.05 ± 1.37 c | 185.30 ± 2.17 d | 101.35 ± 2.10 f |
| DPF | 7.21 ± 0.13 e | 6.53 ± 0.14 e | 5.64 ± 0.12 f | 2.11 ± 0.02 b | 48.32 ± 0.21 f | 2.32 ± 0.13 a | 6.23 ± 0.09 d | 32.21 ± 0.21 a | 268.32 ± 2.19 e | 117.43 ± 2.04 c |
| APF | 9.32 ± 0.10 f | 8.20 ± 0.11 f | 1.78 ± 0.04 e | 18.32 ± 1.03 f | 16.56 ± 0.17 e | 3.32 ± 0.11 b | 8.60 ± 0.10 f | 40.32 ± 0.36 b | 321.32 ± 2.21 f | 72.42 ± 1.01 a |
| Pasting Properties | Thermal Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Gelatinization Time (Min) | Peak Viscosity (BU) | Final Viscosity (BU) | Break Down Viscosity (BU) | Setback Viscosity (BU) | Pasting Temperature (°C) | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
| T0 | 24.01 ± 0.13 a | 1361.02 ± 10.10 d | 1542.06 ± 18.11 d | 378.11 ± 4.13 d | 479.13 ± 6.10 d | 67.01 ± 0.23 a | 75.32 ± 0.16 d | 77.24 ± 0.24 d | 79.81 ± 0.33 d | 6.11 ± 0.01 a |
| T1 | 30.11 ± 0.33 b | 1354.03 ± 14.11 c | 1528.11 ± 16.03 c | 367.13 ± 8.30 c | 472.19 ± 7.41 c | 71.11 ± 0.33 bc | 73.43 ± 0.38 c | 75.21 ± 0.42 c | 78.30 ± 0.47 c | 6.43 ± 0.04 b |
| T2 | 33.12 ± 0.42 c | 1333.07 ± 15.03 b | 1488.10 ± 56.01 b | 354.09 ± 7.51 b | 464.21 ± 5.31 b | 71.12 ± 0.35 bc | 72.31 ± 0.35 b | 74.57 ± 0.37 b | 76.41 ± 0.40 b | 6.67 ± 0.06 c |
| T3 | 34.01 ± 0.61 d | 1310.04 ± 18.12 a | 1442.09 ± 13.01 a | 346.08 ± 7.20 a | 442.12 ± 5.10 a | 70.03 ± 0.32 b | 70.11 ± 0.31 a | 72.43 ± 0.34 a | 73.56 ± 0.36 a | 6.82 ± 0.09 d |
| Flour blends | Biscuits | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | DPPH Scavenging Activity IC50 (mg/)mL | FRAP IC50 (mg/)mL | TPC (mg GAE/100 g DW) | TFC (mg CE/100 g DW) | DPPH Scavenging Activity IC50 (mg/)mL | FRAP IC50 (mg/)mL | TPC (mg GAE/100 g DW) | TFC (mg CE/100 g DW) | Diameter (mm) | Thickness (mm) | Spread Ratio (mm) | Breaking Force (n) | L* | a* | b* |
| T0 | 378.21 ± 0.15 f | 347.13 ± 4.52 f | 38.43 ± 0.11 a | 18.67 ± 0.11 a | 360.10 ± 3.15 d | 340.01 ± 4.52 d | 34.73 ± 0.12 a | 20.41 ± 0.13 a | 41.27 ± 0.56 c | 8.17 ± 0.52 d | 5.05 ± 0.10 d | 21.90 ± 0.39 c | 76.25 ± 0.32 d | 3.34 ± 0.01 a | 25.26 ± 0.10 a |
| T1 | 210.40 ± 1.29 e | 108.35 ± 0.51 e | 75.02 ± 0.18 b | 47.30 ± 0.78 b | 200.40 ± 1.29 c | 102.45 ± 0.4 c | 68.74 ± 2.18 b | 64.53 ± 1.03 b | 41.34 ± 0.40 d | 7.76 ± 0.2 a | 5.32 ± 0.14 e | 19.82 ± 0.2 a | 70.14 ± 0.27 c | 3.86 ± 0.03 b | 25.64 ± 0.12 b |
| T2 | 117.62 ± 1.21 d | 86.34 ± 0.21 d | 102.61 ± 2.31 c | 67.94 ± 1.02 c | 111.62 ± 1.21 b | 78.64 ± 0.3 b | 96.21 ± 2.21 c | 94.21 ± 1.20 c | 41.56 ± 0.88 e | 7.06 ± 0.20 b | 5.46 ± 0.12 c | 20.87 ± 0.23 b | 64.21 ± 0.13 b | 4.67 ± 0.05 c | 26.09 ± 0.17 c |
| T3 | 96.04 ± 1.01 c | 63.32 ± 0.45 c | 132.20 ± 3.42 d | 87.27 ± 1.12 d | 80.04 ± 1.01 a | 58.39 ± 0.15 a | 126.49 ± 3.12 d | 122.54 ± 1.27 d | 40.63 ± 0.51 b | 7.78 ± 0.21 c | 5.22 ± 0.13 b | 23.77 ± 0.30 d | 60.34 ± 0.10 a | 5.21 ± 0.08 d | 26.84 ± 0.19 d |
| DPF | 40.11 ± 0.92 b | 26.81 ± 0.13 b | 359.37 ± 3.55 e | 278.31 ± 1.23 e | - | - | - | - | |||||||
| APF | 32.14 ± 0.42 a | 15.62 ± 0.11 a | 372.48 ± 3.82 f | 292.42 ± 1.34 f | - | - | - | - | |||||||
| Nutritional Information | Sensory | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Moisture Content (%) | Ash (%) | Protein (%) | Fat (%) | Fiber (%) | Carbohydrate (%) | Energy (Kcal/100 g) | Appearance (9*) | Color (9*) | Taste (9*) | Texture (9*) | Overall Acceptability (9*) |
| T0 | 4.53 ± 0.12 a | 1.63 ± 0.01 a | 10.20 ± 0.10 a | 22.12 ± 0.20 d | 0.69 ± 0.26 a | 60.83 ± 0.80 d | 483 | 8.35 ± 0.15 c | 7.22 ± 0.17 a | 8.35 ± 0.13 d | 8.23 ± 0.15 b | 8.07 ± 0.17 b |
| T1 | 4.67 ± 0.14 b | 2.50 ± 0.03 b | 12.81 ± 0.12 b | 19.41 ± 0.14 c | 3.88 ± 0.20 b | 56.73 ± 0.42 c | 453 | 8.33 ± 0.12 b | 8.43 ± 0.15 c | 8.32 ± 0.15 b | 8.32 ± 0.13 c | 8.36 ± 0.16 c |
| T2 | 4.67 ± 0.10 b | 3.29 ± 0.01 c | 13.11 ± 0.11 c | 18.21 ± 0.10 b | 7.02 ± 0.11 c | 53.07 ± 0.57 b | 429 | 8.36 ± 0.17 cd | 8.62 ± 0.15 d | 8.34 ± 0.12 cd | 8.38 ± 0.17 d | 8.42 ± 0.19 d |
| T3 | 4.68 ± 0.11 bc | 4.11 ± 0.02 d | 13.73 ± 0.15 d | 17.18 ± 0.21 a | 10.20 ± 0.21 d | 50.10 ± 0.64 a | 410 | 7.52 ± 0.13 a | 7.89 ± 0.14 b | 7.25 ± 0.10 a | 7.73 ± 0.12 a | 7.13 ± 0.14 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.M.G.; Ali, S.A.; Faheem, K.; Ali, R.; Giuffrè, A.M. The Impact of Innovative Plant Sources (Cordia myxa L. Fruit (Assyrian Plum) and Phoenix dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits. Foods 2022, 11, 2346. https://doi.org/10.3390/foods11152346
Saeed SMG, Ali SA, Faheem K, Ali R, Giuffrè AM. The Impact of Innovative Plant Sources (Cordia myxa L. Fruit (Assyrian Plum) and Phoenix dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits. Foods. 2022; 11(15):2346. https://doi.org/10.3390/foods11152346
Chicago/Turabian StyleSaeed, Syed Muhammad Ghufran, Syed Arsalan Ali, Khizra Faheem, Rashida Ali, and Angelo Maria Giuffrè. 2022. "The Impact of Innovative Plant Sources (Cordia myxa L. Fruit (Assyrian Plum) and Phoenix dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits" Foods 11, no. 15: 2346. https://doi.org/10.3390/foods11152346
APA StyleSaeed, S. M. G., Ali, S. A., Faheem, K., Ali, R., & Giuffrè, A. M. (2022). The Impact of Innovative Plant Sources (Cordia myxa L. Fruit (Assyrian Plum) and Phoenix dactylifera L. Biowaste (Date Pit)) on the Physicochemical, Microstructural, Nutritional, and Sensorial Properties of Gluten-Free Biscuits. Foods, 11(15), 2346. https://doi.org/10.3390/foods11152346







