Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus
Abstract
:1. Introduction
2. The Function of Protein in 3D Food Printing
2.1. Printable Function
2.2. Amino Acid Supplements Function
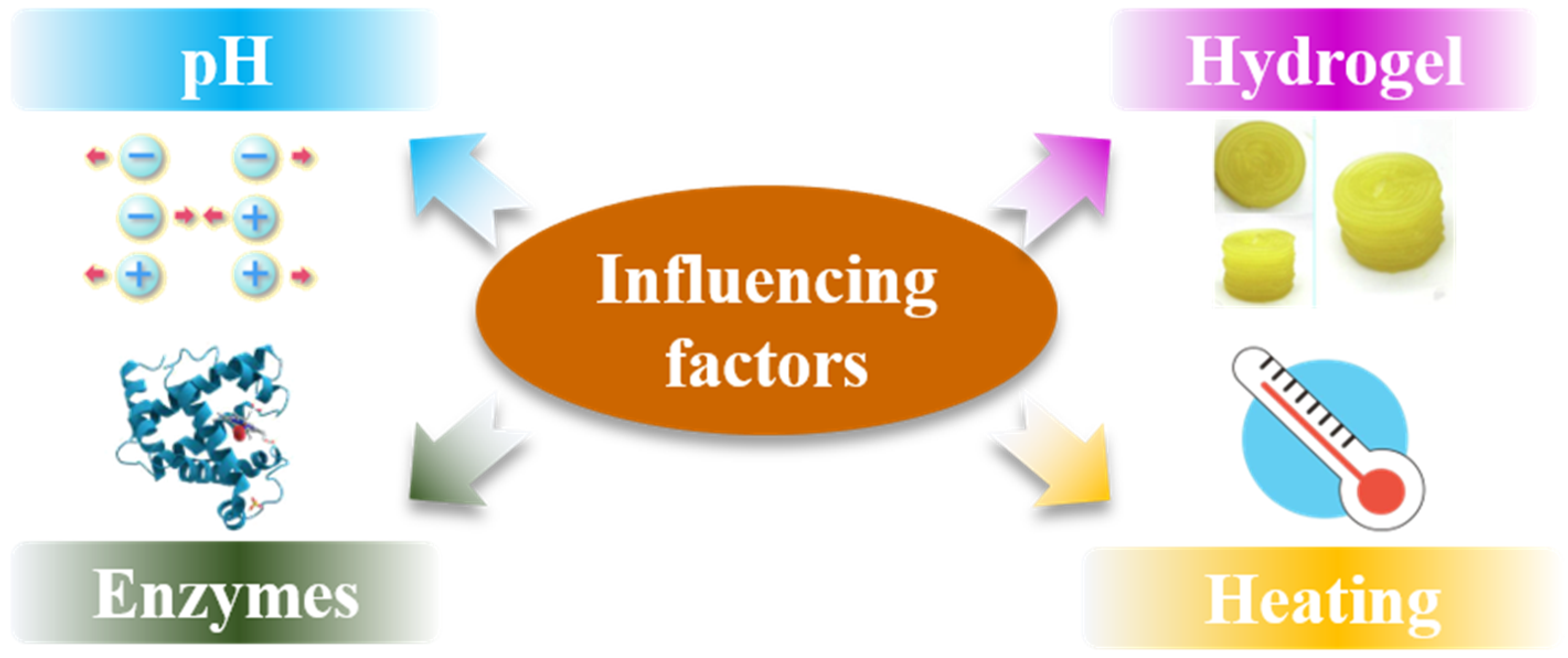
3. Influencing Factors of Protein Function in 3D Printing
3.1. pH of the Printing Ink
3.2. Functional Carbohydrates
3.3. Enzymes
3.4. Heating Treatment
4. The Application of Protein in 3D Food Printing
4.1. Animal Protein
4.1.1. Whey Protein
4.1.2. Egg Albumin
4.1.3. Gelatin
4.1.4. Surimi
4.1.5. Insect Protein
4.1.6. Other Protein
4.2. Plant Protein
4.2.1. Soy Protein
4.2.2. Pea Protein
4.2.3. Gluten Protein
4.2.4. Other Protein
5. Future Outlook of Protein Inks in 3D Food Printing
6. Conclusions
- Printable and nutritional function of protein are important in 3D food printing;
- Physical and chemical properties of protein have considerable impact on the final outcome;
- Animal and plant source protein as a function of 3D food printing ink is discussed;
- Robust extrusion-based 3D printers are needed to produce personalized foods
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; And, S.R.; Wright, P.K.; Montero, M.; Roundy, S. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp. J. 2002, 8, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zhang, M.; Bhandari, B. Model Building and Slicing in Food 3D Printing Processes: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Portanguen, S.; Tournayre, P.; Sicard, J.; Astruc, T.; Mirade, P.-S. Toward the design of functional foods and biobased products by 3D printing: A review. Trends Food Sci. Technol. 2019, 86, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsa-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3D printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Chen, H.; Zheng, B.; Xie, F.; Chen, L. Understanding the structure and rheological properties of potato starch induced by hot-extrusion 3D printing. Food Hydrocoll. 2020, 105, 105812. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, C.; Regenstein, J.M.; Liu, X.; Zhou, P. Rheological and mechanical behavior of milk protein composite gel for extrusion-based 3D food printing. LWT 2019, 102, 338–346. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R. Application of Pickering emulsions in 3D printing of personalized nutrition. Part II: Functional properties of reduced-fat 3D printed cheese analogues. Colloids Surf. Physicochem. Eng. Asp. 2021, 624, 126760. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Kaplan, D.L. Silk-based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Abascal, N.C.; Regan, L. The past, present and future of protein-based materials. Open Biol. 2018, 8, 180113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Vancauwenberghe, V.; Katalagarianakis, L.; Wang, Z.; Meerts, M.; Hertog, M.; Verboven, P.; Moldenaers, P.; Hendrickx, M.E.; Lammertyn, J.; Nicolaï, B. Pectin based food-ink formulations for 3-D printing of customizable porous food simulants. Innov. Food Sci. Emerg. Technol. 2017, 42, 138–150. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Joseph, A.; Ejaz, M.; Maniruzzaman, M. Zinc oxide/clove essential oil incorporated type B gelatin nanocomposite formulations: A proof-of-concept study for 3D printing applications. Food Hydrocoll. 2020, 98, 105256. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of recent advances in the preparation, properties, and applications of high internal phase emulsions. Trends Food Sci. Technol. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Hussain, S.; Arora, V.K.; Malakar, S. Formulation of protein-enriched 3D printable food matrix and evaluation of textural, rheological characteristics, and printing stability. J. Food Process. Preserv. 2021, 45, e15182. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking rheology and printability of a multicomponent gel system of carrageenan-xanthan-starch in extrusion based additive manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- Mu, X.; Agostinacchio, F.; Xiang, N.; Pei, Y.; Khan, Y.; Guo, C.; Cebe, P.; Motta, A.; Kaplan, D.L. Recent advances in 3D printing with protein-based inks. Prog. Polym. Sci. 2021, 115, 101375. [Google Scholar] [CrossRef]
- Mantihal, S.; Kobun, R.; Lee, B.-B. 3D food printing of as the new way of preparing food: A review. Int. J. Gastron. Food Sci. 2020, 22, 100260. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of soy protein isolate and hydrocolloids based mixtures as promising food material in 3D food printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Z.; Pan, Y.; Jiang, P.; Pan, J.; Yu, C.; Dong, X. Effect of κ-carrageenan on quality improvement of 3D printed Hypophthalmichthys molitrix-sea cucumber compound surimi product. LWT 2022, 154, 112279. [Google Scholar] [CrossRef]
- Bareen, M.A.; Joshi, S.; Sahu, J.K.; Prakash, S.; Bhandari, B. Assessment of 3D printability of heat acid coagulated milk semi-solids ‘soft cheese’ by correlating rheological, microstructural, and textural properties. J. Food Eng. 2021, 300, 110506. [Google Scholar] [CrossRef]
- Du, J.; Dai, H.; Wang, H.; Yu, Y.; Zhu, H.; Fu, Y.; Ma, L.; Peng, L.; Li, L.; Wang, Q.; et al. Preparation of high thermal stability gelatin emulsion and its application in 3D printing. Food Hydrocoll. 2021, 113, 106536. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, R.; Feng, W.; Chen, Z.; Wang, T. High internal phase Pickering emulsions stabilized by co-assembled rice proteins and carboxymethyl cellulose for food-grade 3D printing. Carbohydr. Polym. 2021, 273, 118586. [Google Scholar] [CrossRef]
- Feng, T.; Fan, C.; Wang, X.; Wang, X.; Xia, S.; Huang, Q. Food-grade Pickering emulsions and high internal phase Pickering emulsions encapsulating cinnamaldehyde based on pea protein-pectin-EGCG complexes for extrusion 3D printing. Food Hydrocoll. 2022, 124, 107265. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Song, L.; Bi, A.; Wu, C.; Ma, Y.; Du, M.; Zhu, B. High Internal Phase Emulsion for Food-Grade 3D Printing Materials. ACS Appl. Mater. Interfaces 2020, 12, 45493–45503. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Ren, X.; Li, J.; Yang, Y.L.; Jin, X. Personalized Food Printing for Portrait Images. Comput. Graph. 2017, 70, 188–197. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Tai, B.C.U.; Du, C. Food security and nutrition—A systematic approach. Trends Food Sci. Technol. 2021, 109, 738–745. [Google Scholar] [CrossRef]
- Dick, A.; Bhandari, B.; Dong, X.; Prakash, S. Feasibility study of hydrocolloid incorporated 3D printed pork as dysphagia food. Food Hydrocoll. 2020, 107, 105940. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Zhang, M.; Rao, Z. Effect of ultrasound-assisted thawing on gelling and 3D printing properties of silver carp surimi. Food Res. Int. 2021, 145, 110405. [Google Scholar] [CrossRef] [PubMed]
- Lorieau, L.; Halabi, A.; Ligneul, A.; Hazart, E.; Dupont, D.; Floury, J. Impact of the dairy product structure and protein nature on the proteolysis and amino acid bioaccessiblity during in vitro digestion. Food Hydrocoll. 2018, 82, 399–411. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Influence of Surface pH on Color, Texture and Flavor of 3D Printed Composite Mixture of Soy Protein Isolate, Pumpkin, and Beetroot. Food Bioprocess Technol. 2020, 13, 1600–1610. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Oliveira, S.; Bengoechea, C.; Sousa, I.; Raymundo, A.; Guerrero, A. A rheological approach to 3D printing of plasma protein based doughs. J. Food Eng. 2021, 288, 110255. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Zhang, L.; Noort, M.W.J.; Schutyser, M.A.I.; García-Segovia, P.; Martínez-Monzó, J. Printability and Physicochemical Properties of Microalgae-Enriched 3D-Printed Snacks. Food Bioprocess Technol. 2020, 13, 2029–2042. [Google Scholar] [CrossRef]
- Noh, H.; Yohe, S.T.; Vogler, E.A. Volumetric interpretation of protein adsorption: Ion-exchange adsorbent capacity, protein pI, and interaction energetics. Biomaterials 2008, 29, 2033–2048. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, M.E.; Dang, H.; Dahlhauser, S.D.; Simmonds, S.P.; Reuther, J.F.; Wyse, J.M.; Vandewalle, A.N.; Anslyn, E.V.; Peppas, N.A. Nanogel receptors for high isoelectric point protein detection: Influence of electrostatic and covalent polymer–protein interactions. Chem. Commun. 2020, 56, 6141–6144. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, Y.; Jin, H.; Sui, X.; Jiang, L. Deciphering the Structural Network That Confers Stability to High Internal Phase Pickering Emulsions by Cross-Linked Soy Protein Microgels and Their In Vitro Digestion Profiles. J. Agric. Food Chem. 2020, 68, 9796–9803. [Google Scholar] [CrossRef] [PubMed]
- Nöbel, S.; Seifert, B.; Daffner, K.; Schäfer, J.; Hinrichs, J. Instantaneous gelation of acid milk gels via customized temperature-time profiles: Screening of concentration and pH suitable for temperature triggered gelation towards 3D-printing. Food Hydrocoll. 2021, 113, 106450. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Guo, Y.; Sun, L. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.; Chen, H. Effect of whey protein on the 3D printing performance of konjac hybrid gel. LWT 2021, 140, 110716. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Bhandari, B. 4D printing of products based on soy protein isolate via microwave heating for flavor development. Food Res. Int. 2020, 137, 109605. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Fasolin, L.H.; Vicente, A.A.; Fuciños, P.; Pastrana, L.M. Printability, microstructure, and flow dynamics of phase-separated edible 3D inks. Food Hydrocoll. 2020, 109, 106120. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, L.W.; Xue, H.; Lu, Y. Cheese whey protein recovery by ultrafiltration through transglutaminase (TG) catalysis whey protein cross-linking. Food Chem. 2017, 215, 31–40. [Google Scholar]
- Dong, X.; Pan, Y.; Zhao, W.; Huang, Y.; Qu, W.; Pan, J.; Qi, H.; Prakash, S. Impact of microbial transglutaminase on 3D printing quality of Scomberomorus niphonius surimi. LWT 2020, 124, 109123. [Google Scholar] [CrossRef]
- Cortez-Trejo, M.C.; Gaytán-Martínez, M.; Reyes-Vega, M.L.; Mendoza, S. Protein-gum-based gels: Effect of gum addition on microstructure, rheological properties, and water retention capacity. Trends Food Sci. Technol. 2021, 116, 303–317. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Liu, Z.; Ye, Y. Effect of microwave-salt synergetic pre-treatment on the 3D printing performance of SPI-strawberry ink system. LWT 2020, 122, 109004. [Google Scholar] [CrossRef]
- Daffner, K.; Vadodaria, S.; Ong, L.; Nöbel, S.; Gras, S.; Norton, I.; Mills, T. Design and characterization of casein–whey protein suspensions via the pH–temperature-route for application in extrusion-based 3D-Printing. Food Hydrocoll. 2021, 112, 105850. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Wei, S.; Xia, Q.; Pan, Y.; Ji, H.; Deng, C.; Hao, J.; Liu, S. Insight into the correlations among rheological behaviour, protein molecular structure and 3D printability during the processing of surimi from golden pompano (Trachinotus ovatus). Food Chem. 2022, 371, 131046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Q.; Yan, B.; Gao, W.; Jiao, X.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Synergistic effect of microwave 3D print and transglutaminase on the self-gelation of surimi during printing. Innov. Food Sci. Emerg. Technol. 2021, 67, 102546. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant Protein and Animal Proteins: Do They Differentially Affect Cardiovascular Disease Risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Chitrakar, B.; Adhikari, B. Recent advances in functional 3D printing of foods: A review of functions of ingredients and internal structures. Crit. Rev. Food Sci. Nutr. 2020, 61, 3489–3503. [Google Scholar] [CrossRef]
- Papenburg, R.; Bounous, G.; Fleiszer, D.; Gold, P. Dietary milk proteins inhibit the development of dimethylhydrazine-induced malignancy. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 1990, 11, 129. [Google Scholar] [CrossRef]
- Anema, S.G. On heating milk, the dissociation of κ-casein from the casein micelles can precede interactions with the denatured whey proteins. J. Dairy Res. 2008, 75, 415–421. [Google Scholar] [CrossRef]
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Co-gels of whey protein isolate with crosslinked waxy maize starch: Analysis of solvent partition and phase structure by polymer blending laws. Food Hydrocoll. 2008, 22, 468–484. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Zhang, L.; Niu, P.; Sha, X.M. Promotion of foam properties of egg white protein by subcritical water pre-treatment and fish scales gelatin. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 171–177. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Dai, X.; Chen, K.; Zhu, Y. 3D Printing Complex Egg White Protein Objects: Properties and Optimization. Food Bioprocess Technol. 2019, 12, 267–279. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Bhandari, B.; Meng, Y.; Prakash, S. Optimization of the Formulation and Properties of 3D-Printed Complex Egg White Protein Objects. Foods 2020, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zhai, X.; Hu, X.; Liang, N.; Zou, X. Rapid and highly sensitive detection of Salmonella typhimurium in lettuce by using magnetic fluorescent nanoparticles. Anal. Methods 2020, 12, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Jining Med. Univ. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strother, H.; Moss, R.; Mcsweeney, M.B. Comparison of 3D printed and moulded carrots produced with gelatin, guar gum and xanthan gum. J. Texture Stud. 2020, 51, 852–860. [Google Scholar] [CrossRef]
- Pan, J.; Jia, H.; Shang, M.; Xu, C.; Lian, H.; Li, H.; Dong, X. Physiochemical properties and tastes of gels from Japanese Spanish mackerel (Scomberomorus niphonius) urimi by different washing processes. J. Texture Stud. 2018, 49, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Zhang, M.; Yang, C.-H. Comparative analysis of 3D printability and rheological properties of surimi gels via LF-NMR and dielectric characteristics. J. Food Eng. 2021, 292, 110278. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of deacetylation of konjac glucomannan on Alaska Pollock surimi gels subjected to high-temperature (120 °C) treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Pan, Y.; Wang, K.; Prakash, S.; Zhu, B. Investigation of sweet potato starch as a structural enhancer for three-dimensional printing of Scomberomorus niphonius surimi. J. Texture Stud. 2019, 50, 316–324. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Javier Borderias, A.; Moreno, H.M. Effect of high pressure on reduced sodium chloride surimi gels. Food Hydrocoll. 2015, 51, 176–187. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Severini, C.; Azzollini, D.; Albenzio, M.; Derossi, A. On printability, quality and nutritional properties of 3D printed cereal based snacks enriched with edible insects. Food Res. Int. 2018, 106, 666–676. [Google Scholar] [CrossRef]
- Soares, S.; Forkes, A. Insects Au Gratin—An Investigation into the Experiences of Developing a 3D Printer that uses Insect Protein Based Flour as a Building Medium for the Production of Sustainable Food. In Proceedings of the DS 78: Proceedings of the 16th International Conference on Engineering and Product Design Education (E&PDE14), Design Education and Human Technology Relations, Enschede, The Netherlands, 11 February 2014. [Google Scholar]
- Pan, Y.; Sun, Q.; Liu, Y.; Wei, S.; Xia, Q.; Zheng, O.; Liu, S.; Ji, H.; Deng, C.; Hao, J. The relationship between rheological and textural properties of shrimp surimi adding starch and 3D printability based on principal component analysis. Food Sci. Nutr. 2021, 9, 2985–2999. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zhai, X.; Hu, X.; Zou, X. Employing CuInS2 quantum dots modified with vancomycin for detecting Staphylococcus aureus and iron(III). Anal. Methods 2021, 13, 1517–1526. [Google Scholar] [CrossRef]
- Shan, H.; Lu, S.W.; Jiang, L.Z.; Wang, L.K.; Liao, H.; Zhang, R.Y.; Dai, C.J.; Yao, X.M.; Zhang, Y.L.; Su, P. Gelation Property of Alcohol-Extracted Soy Protein Isolate and Effects of Various Reagents on the Firmness of Heat-Induced Gels. Int. J. Food Prop. 2015, 18, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Investigation on 3D printing ability of soybean protein isolate gels and correlations with their rheological and textural properties via LF-NMR spectroscopic characteristics. LWT 2020, 122, 109019. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Q.; Li, H.; Zhou, Q.; Meng, W. Effects of Pea Protein on the Properties of Potato Starch-Based 3D Printing Materials. Int. J. Food Eng. 2018, 14, 20170297. [Google Scholar]
- Oyinloye, T.M.; Yoon, W.B. Stability of 3D printing using a mixture of pea protein and alginate: Precision and application of additive layer manufacturing simulation approach for stress distribution. J. Food Eng. 2021, 288, 110127. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Han, D.; Li, D. Nutritional components and functional properties of yellow pea. Food Sci. Technol. 2012, 37, 141–144. [Google Scholar]
- Fan, Y.; Min, Z.; Fang, Z.; Liu, Y. Impact of processing parameters and post-treatment on the shape accuracy of 3D-printed baking dough. Int. J. Food Sci. Technol. 2019, 54, 68–74. [Google Scholar]
- Letang, C.; Piau, M.; Verdier, C. Characterization of wheat flour–water doughs. Part I: Rheometry and microstructure. J. Food Eng. 1999, 41, 121–132. [Google Scholar] [CrossRef]
- Zhang, L.; Lou, Y.; Schutyser, M.A.I. 3D printing of cereal-based food structures containing probiotics. Food Struct. 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Villanueva, M.; Harasym, J.; Munoz, J.M.; Ronda, F. Rice flour physically modified by microwave radiation improves viscoelastic behavior of doughs and its bread-making performance. Food Hydrocoll. 2019, 90, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, M.; Chen, H. LF-NMR intelligent evaluation of rheology and printability for 3D printing of cookie dough pretreated by microwave. LWT 2020, 132, 109752. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Phuhongsung, P. 3D printing of protein-based composite fruit and vegetable gel system. LWT 2021, 141, 110978. [Google Scholar] [CrossRef]
- Chaunier, L.; Leroy, E.; Valle, G.D.; Dalgalarrondo, M.; Bakan, B.; Marion, D.; Madec, B.; Lourdin, D. 3D printing of maize protein by fused deposition modeling. AIP Conf. Proc. 2017, 1914, 190003. [Google Scholar]
- Chaunier, L.; Leroy, E.; Della Valle, G.; Lourdin, D. Plasticized protein for 3D printing by fused deposition modeling. AIP Conf. Proc. 2016, 1769, 190001. [Google Scholar]

| Category | Other Materials | Experimental Conditions | Optimal Conditions | Actual Application Results | Reference |
|---|---|---|---|---|---|
| Whey protein | Casein | Acid environment, heating treatment | pH: 4.8–5.0; T: 80 °C | Mixture suspension is printable and forms a stable gel | [1] |
| Konjac | Continuous mixing at heating treatment | T: 85 °C | Gelling property performed best as an additive (20%) | [2] | |
| Canola oil, corn starch | Continuous mixing at room temperature | - | Excessive whey protein impaired the printing inks’ printability | [3] | |
| NaCl, fat, protein | Severe mixing at neutral condition | pH: 7 | Effect of whey protein as printing inks and additives on printing results was explored | [4] | |
| Starch, beeswax | Continuous mixing at heating treatment | T: 90 °C | As a printing ink component to meet the nutritional function of the product | [5] | |
| Egg albumin | Rice flour | Severe mixing, heating conditions | T: 70 °C | Egg yolk has a higher binding capacity to starch compared to egg albumin, and the effect of 3D printing was good | [6] |
| Sesbania gum | Continuous mixing at heating condition | T: 75 °C | The 0.1% SG protein gel mixture structure was stable, there was no covalent bond between them | [7] | |
| Gelatin, sucrose cornstarch | Severe mixing, heating conditions | pH: 6.5, T: 55 °C | EWP can improve the hardness and elasticity of the gel sample | [8] | |
| Gelatin, sucrose cornstarch | Severe mixing, heating conditions | pH: 6.5, T: 55 °C | An optimization plan for a new 3D printed formula containing EWP system | [9] | |
| Gelatin | TGase, soy oil | Mixing at heating conditions | pH = 5.0, T: 60 °C, TGase: 2 mg/mL | TGase cross-linking of gelatin effectively improved the thermal stability of HIPEs. | [10] |
| Zinc oxide, clove essential oil | Extrusion at room temperature | Gelatin/zinc oxide/clove oil nano-packaging was developed for food preservation. | [11] | ||
| Pureed carrots | Mixing at heating conditions | T: 45 °C | Samples made with gelatin were the hardest (texture profile analysis) product | [12] | |
| TGase | Mixing at heating conditions | T: 40 °C TGase: 5% | Preheating of gelatin improves its printability with TGase | [13] | |
| Surimi | NaCl | Mixing at room temperature | NaCl: 1 g/100 g | Better prediction obtained for multiple rheological parameters by LF-NMR. | [14] |
| NaCl | Mixing at room temperature | NaCl: 3% | SEM showed that 3% salt was suitable for 3D printing ink using fish surimi. | [15] | |
| MTGase | Mixing at room temperature | MTGase: 3% | Microbial TGase impacts 3D printability and extrudability of surimi. | [16] | |
| TGase | Mixing, microwave printing | TGase: 5 U/g, power: 50 w/g | MW3DP and TGase can be used for 3D printing of heat-induced gel food. | [17] | |
| Yellow mealworm | Dough | Mixing at heating treatment | - | Changed the printability of the dough, improving the texture, digestibility, and microstructure of snacks | [18] |
| Pig plasma protein | Glycerin, dough | Mixing at room temperature | - | Dough with pig plasma protein content between 42.5 and 47.5% weight could be printed successfully. | [19] |
| Category | Other Materials | Experimental Conditions | Optimal Conditions | Actual Application Results | Reference |
|---|---|---|---|---|---|
| Soy protein | Xanthan gum, NaCl | Severe mixing and microwave | pH:7; mixing: 6400 rpm, 5 min; microwave:100 W, 5 min | SPI gel with xanthan gum and NaCl solution at 1 g/100 mL could be successfully printed | [20] |
| κ-carrageenan, vanilla flavor | Severe mixing and heating treatment | Mixing: 6400 rpm, 5 min; T:70 °C | SPI gel made with 3% (w/v) carrageenan was the most suitable for 3D printing | [21] | |
| Pumpkin, beetroot | Severe mixing and microwave | Mixing: 6400 rpm, 5 min; microwave: 100 W, 5 min | The best printing results were obtained when stimulated with pH = 6 | [22] | |
| Strawberry | Mixing and microwave | microwave: 70 W | Salt and microwave treatment improved the printing accuracy and self-supporting performance of the ink system | [23] | |
| Pea Protein | Alginate | Mixing and heating treatment | T: 43 °C | Alginate solution 80% and pea protein solution 20% were found to be the most suitable for 3D printing | [24] |
| Potato starch | Mixing | - | Pea protein improved the texture, thermal property, and structural properties of the ink | [25] | |
| Gluten protein | Milk, fat | Mixing | - | Cookie dough formulations with reduced sugar content were more printable | [26] |
| …. | Mixing and heating treatment | T: 55 °C | The heating condition affected the protein structure and improved the printing effect of printing ink | [27] | |
| Peanut protein | Hawthorn powder | Mixing and storage | Storage temperature and time: 4 °C, 12 h | The mixture has good 3D printing properties and applies to other fruit and vegetable inks | [28] |
| Oat protein & fava bean protein | - | Severe mixing | - | Mixture improved the printability of printing materials, but the forming effect was defective | [29] |
| Zein | - | Mixing | - | Realize the control of the three-position structure by adjusting the printable ingredients | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Arslan, M.; Li, Z.; Cen, S.; Shi, J.; Huang, X.; Xiao, J.; Zou, X. Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods 2022, 11, 1902. https://doi.org/10.3390/foods11131902
Guo Z, Arslan M, Li Z, Cen S, Shi J, Huang X, Xiao J, Zou X. Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods. 2022; 11(13):1902. https://doi.org/10.3390/foods11131902
Chicago/Turabian StyleGuo, Ziang, Muhammad Arslan, Zhihua Li, Shaoyi Cen, Jiyong Shi, Xiaowei Huang, Jianbo Xiao, and Xiaobo Zou. 2022. "Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus" Foods 11, no. 13: 1902. https://doi.org/10.3390/foods11131902
APA StyleGuo, Z., Arslan, M., Li, Z., Cen, S., Shi, J., Huang, X., Xiao, J., & Zou, X. (2022). Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods, 11(13), 1902. https://doi.org/10.3390/foods11131902







