Abstract
With the growing popularity of the concept of healthy diet, modern obesity treatment is gradually shifting from surgical or pharmacological treatment to nutritional intervention. As a safe and effective measure, natural product interventions are a potential strategy of obesity management. The present study aimed to develop a kind of functional food rich in bioactive compounds (chenpi, kiwifruit, and pectin as raw materials) and investigate their bioactive effects on a mouse model. For development of functional kiwifruit jelly with chenpi (FKJ), the results of single-factor and response surface experiments showed that the optimized formulation was composed of a 30.26% addition of chenpi, 35% addition of kiwifruit juice, and 2.88% addition of pectin. The FKJ obtained with the optimal formulation could be used as a 3D printing raw material to print the desired food shapes successfully. For bioactivity evaluation of FKJ, the results with a mouse model showed that the food intake, liver weight, and adipose tissue weight were significantly decreased after administration of FKJ with dose-dependent effect compared to the CON group (p < 0.05). Meanwhile, the serum levels of several inflammatory factors (TG, IL-6, and TNF-α) were decreased and the activities of several antioxidant-related enzymes (SOD, GSH-PX, and CAT) were increased. In short, a functional kiwifruit jelly with chenpi was developed in this study. It is a functional snack food rich in active phenolic compounds, low in calories, with antioxidant and anti-inflammatory activity, and prevents fat accumulation. FKJ could well meet the needs of modern people for nutrition and health and also promote the processing and utilization of natural products, and has good development prospects in the functional food industry.
1. Introduction
The increasing incidence of obesity worldwide has become a significant public health problem that threatens the health of people around the world [1]. Obesity increases the risk of developing metabolic syndromes, such as heart disease, hypertension, dyslipidemia, and diabetes [2]. With the change in lifestyles, the treatment of obesity is gradually shifting from drug treatment to dietary intervention and prevention [3]. Studies have shown that obesity is closely related to oxidative stress [4,5] and that dietary supplementation with antioxidants has a potential therapeutic effect on weight loss or control [6,7]. Therefore, there is an urgent need to develop functional foods that offer long-term prevention of obesity and antioxidant activity.
Aged citrus peel, also known as chenpi, is usually made from the mature pericarp of Citrus reticulata. Chenpi contains various physiologically active ingredients, such as volatile oils, flavonoids, alkaloids, and polysaccharides [8]. A growing number of studies have found that chenpi can prevent obesity [9], hepatic steatosis, and metabolic syndrome [9,10], improve blood lipid profiles [11], and modulate liver and heart function parameters [12]. Kiwifruit (Actinidia chinensis Planch) is a ubiquitous and popular fruit in our daily life. It is rich in vitamin C [13] and other bioactive substances, such as phenolic compounds [14], insoluble fiber, carotenoids, flavonoids, and minerals [15]. Kiwifruit has a highly antioxidant effect and immunostimulatory activity in vitro and in vivo [16]. In addition to being eaten fresh, kiwifruit is often used to produce various functional foods, such as dried fruit snacks [17], fruit bars [18], gold kiwifruit leather products (a snack made of fruit puree and other ingredients) [19], kiwifruit milk powders [20], and kiwifruit jellies [15]. Jellies are considered an excellent substitute for fruit processing, which can greatly meet our requirements for the development of FKJ. Low methoxylated pectin (LMP) could form a gel state with calcium in an "ionotropic gelation" manner [21] and is often used as a raw material for production of jelly. Studies have shown that pectin could prevent and control obesity and diabetes [22], as well as improve lipid and cholesterol metabolism [23]. Pectin from citrus peel is an important by-product of citrus processing, thus its use in the production of jellies may be a good option for citrus waste management.
In the current lifestyle, people tend to prefer foods that are ready to eat and contain enough nutritional ingredients. As shown above, chenpi, kiwifruit, and LMP exhibit great potential for the development of functional food; thus, we expected to develop a kind of functional kiwifruit jelly with chenpi. Meanwhile, 3D food printing technology was used in the development process to meet the personalized needs of consumers. Further, the bioactivities of FKJ were explored. We expect that FKJ developed in this study will provide a good reference for the development of functional foods and provide a better choice for food intake that meets consumer demand.
2. Materials and Methods
2.1. Materials
Chenpi was purchased from Jiangmen, Guangdong Province, made by red Pericarpium Citri Reticulatae dried in natural sunlight and sealed for 3 years. Low methoxylated pectin (LMP) from citrus fruits (purity > 98%) was purchased from Yantai Andre pectin Co., Ltd. (Yantai, China). Kiwifruit (Zespri green kiwi) was purchased from Changsha, China. All were food-grade materials. Total antioxidant capacity kits (ABTS method, DPPH method, FRAP method) were purchased from Suzhou Comin Biotechnology Co., Ltd., Suzhou, China. All kits for the determination of serum biochemical indexes were purchased from Jiangsu Meimian Co., Ltd., Yancheng, China.
2.2. Development of FKJ
2.2.1. Processing of FKJ
FKJ was produced by modifying and combining the methods proposed by Nuramalia, D.R. et al. and Lima, M.B et al. [24,25]. In brief, 10 g of crushed chenpi was added to 200 mL of purified water and boiled for 40 min, then filtered with vacuum filter while hot. The filtrate was concentrated to 10 mL using a rotary evaporator to obtain 1 g/mL of chenpi decoction. The kiwifruit juice was obtained by squeezing and filtering after peeling the kiwifruit. Citrus pectin was added to 10 mL of purified water while stirring, while the pectin was dissolved in a water bath at 70 °C for 10–15 min, after which different volumes of chenpi decoction and kiwifruit juice were added, stirred well, and 1 mL of 0.14 g/mL CaCl2 solution was added. Then, purified water was added to reach a total volume of 100 mL [26]. Finally, the solutions were mixed evenly, subjected to pasteurization at 75 °C for 5 min, cooled, shaped, and stored at 4 °C.
2.2.2. Sensory Evaluation
The sensory analysis of FKJ was conducted by a sensory evaluation team, which consisted of ten food professionals. The total sensory evaluation score consisted of four components: color, tissue state, flavor, and taste. The sensory scoring criteria are shown in Table 1.

Table 1.
Indicators of sensory evaluation of FKJ.
2.2.3. Single-Factor Experiments (SFE)
Single-factor experiments were designed to investigate the effects of different additions of chenpi decoction, kiwifruit juice, and pectin on the sensory evaluation score of FKJ. They could provide a range of factor levels for the design of response surface methodology. In our study, the effect of calcium was not analyzed, since its primary role was to cross-link pectin to form a gel state, and 1 mL of 0.14 g/mL CaCl2 solution was added to all jelly formulations [27]. Chenpi decoction content, which was the main functional ingredient, was selected as 10–35%. Kiwifruit juice content, which could increase the flavor of FKJ and mask some of the bitterness of the chenpi, was chosen as 15–40%. The pectin content, which determines the gel state of FKJ and increases the toughness while maintaining the jelly shape, was chosen as 1–6% as referenced and adapted from Khouryieh, H.A, et al. [28]. Samples were prepared by adding different levels of chenpi decoction, kiwifruit juice, and pectin according to Section 2.2.1. The total score was given by the sensory evaluation team after sensory identification.
2.2.4. Experimental Design of Response Surface Methodology (RSM)
The response surface methodology can optimize the process by evaluating the factors affecting biological processes and their interactions [29]. The range of each factor in the response surface was selected from the results of the single-factor experiments. Three factors and three levels were used according to the central combination experimental design principle of the Box–Behnken model. Chenpi decoction, kiwifruit juice, and pectin addition were selected as the independent variables A, B, and C, respectively, and represented by 1, 0, and −1 for each of the three levels. The total score of the sensory evaluation was used as the response value (R) to determine the best formula for FKJ based on regression analysis. This production recipe was used in subsequent experiments. The specific parameters of RSM are shown in Table 2.

Table 2.
Variables of single-factor experiments and response surface methodology.
2.2.5. 3D Food Printing of FKJ
The aim of this assay was to obtain designed FKJ shapes through 3D printing and investigate whether the food formulations developed in our study could be personalized through 3D food printing. The best food recipe obtained by the response surface method was used to prepare samples, which were then used as food inks for printing. The food inks were sent to a specialized 3D food printing company (Hangzhou Shiyin Technology Co., Ltd.) for 3D printing tests. The 3D printing process is based on the extrusion of food inks at room temperature [26]. A 3D printer with a nozzle diameter of 0.84 mm was used for printing tests (Foodbot S2, Hangzhou Shiyin Technology Co., Ltd., Hangzhou, China). The exact shape of the print was designed by Cura15.02.1 software before printing (cuboid: 2.5 × 2.5 × 1.25 cm3). The parameters of the printing process on the printer were set (infill velocity V: 15 mm/s, infill density: 90%, infill pattern: rectilinear, print temperature: 40 °C) according to Vancauwenberghe V. et al. [26].
2.3. Determination of Nutrients and Bioactive Components of FKJ
The ash (using GB 5009.4-2016 method) [30], moisture (using GB 5009.3-2016 method) [31], protein (using GB 5009.5-2016 method) [32], fat (using GB 5009.6-2016 method) [33], carbohydrate, and sodium content (using GB 5009.91-2017 method) [34] of FKJ were determined to obtain the main nutritional composition.
The total flavonoids in FKJ were determined by the aluminum nitrate chromogenic method, which was adapted from Li X. et al. [35]. A 0.3 mL amount of FKJ solution, 4.7 mL of 30% ethanol, and 0.4 mL of 5% NaNO2 solution were mixed well and reacted for 6 min. After that, 0.4 mL of 10% Al(NO)3 solution was added and mixed well, standing for 6 min, then 4 mL of 4% NaOH solution was added and supplemented with 30% ethanol to make a total volume of 10 mL and mixed well. After 3 min, the absorbance values were measured by a UV-Vis spectrophotometer at 510 nm, and the total flavonoid content was presented as mg/g rutin equivalent (RE), using a rutin equivalent (0–0.24 mg/mL) standard curve.
The total phenolic concentrations were assayed using the Folin–Ciocalteu method modified from Yamin et al. [36]. A 10 µL amount of FKJ solution, 4.90 mL of distilled water, and 0.5 mL of Folin–Ciocalteu reagent were combined in a colorimetric tube and then shaken well. The mixture was placed in the dark for 5 min. After that, 2.5 mL of 5% Na2CO3 solution was added, then distilled water was added to make a total volume of 10 mL. The total solution was mixed well, then left for 60 min in the dark. The absorbance was measured at 765 nm by a UV-Vis spectrophotometer, and the total phenolic content was expressed as mg/g of gallic acid equivalent (GAE). Gallic acid solution in the range of 0–0.08 mg/mL was used to determine the standard curve.
2.4. Measurement of Antioxidant Capacity of FKJ
The total antioxidant capacity of FKJ was determined by three frequently used assays, including 2,2-diphenyl-1-octanohydrazide (DPPH) and 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, and ferric reducing antioxidant power (FRAP) [37]. The results were presented as µmol/g of Trolox equivalents (TE). In this experiment, FKJ used for the ABTS scavenging assay was diluted 80 times with extraction buffer from the kit, while FKJ used for the DPPH scavenging assay and FRAP reduction test was diluted 50 times. Finally, these assays were performed following the kit instructions.
2.5. Determination of Bioactivities of FKJ Using Mouse Model
2.5.1. Animal Administration and Treatment
Forty healthy male C57BL/6J mice (seven weeks old) were purchased from Hunan SJA laboratory animal Co., Ltd. (Changsha, China). They were housed in a controlled environment with a light/dark cycle of 12 h, temperature of 21–24 °C and relative humidity of 50–80%, with food and drinking water available ad libitum. The animal experiment was carried out after the review and approval of the Hunan Agricultural University Institutional Animal Care and Use Committee (202005).
After one week of acclimatization, all mice were randomly allocated into four groups (n = 10): Control (CON), High-dose (KH), Medium-dose (KM), and low-dose (KL). The mice in the KH, KM, and KL groups were administered 10 g/kg, 5 g/kg, and 2.5 g/kg FKJ daily by gavage, respectively. The CON group was gavaged with an equal volume of saline simultaneously. All groups were provided with a standard diet (composed of 54.9% corn, 18% soybean meal, 5.6% casein, 6.5% beer yeast, 0.7% lard, 0.8% bean oil, 0.5% salt, 1.4% fishmeal, and 1% premixture) during the experimentation. Body weight and food intake of each mouse were recorded weekly throughout the experiment. At the end of the experiment in week 8, the mouse blood was collected from the orbital sinus. Liver, subcutaneous adipose tissues (SAT), abdominal adipose tissues (AAT), and perirenal adipose tissues (PEAT) were collected and weighed.
2.5.2. Histopathological Observation
Paraformaldehyde-fixed AAT samples were dehydrated with ethanol, embedded with paraffin, and sectioned for hematoxylin and eosin (H&E) staining [38]. The pathological sections were observed and photographed using a light microscope (Nikon Eclipse E100, Tokyo, Japan) at 40× magnification.
2.5.3. Biochemical Analysis of Serum
Blood was allowed to stand for 4 h at 4 °C and then centrifuged at 3000 rpm for 15 min using a freezing centrifuge, and the supernatant was carefully separated and stored at −80 °C for testing [39]. The levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) in the serum were analyzed. The indicators of liver function were assessed, including alanine aminotransferase (ALT) and aspartate transaminase (AST). The serum concentrations of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were assessed, and markers of antioxidant capacity were measured, including malonaldehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and catalase (CAT). All of these biochemical indicators were determined according to the instructions of corresponding kits purchased from Jiangsu Meimian Co., Ltd. (Yancheng, China).
2.6. Statistical Analysis
Design-Expert (version 13) software was used for the design of response surface experiments. All of the data were plotted by Origin Pro 9.0 software. The IBM SPSS Statistics 26.0 software was used for comparative analysis. Student’s t-test was used to compare the data between the two groups, and the two-way analysis of variance (ANOVA) was used for the comparative analysis of multiple groups of data. p < 0.05 was indicated as a significant difference.
3. Results
3.1. Development of FKJ
3.1.1. Single-Factor Experiment
As shown in Figure 1a, increasing the addition of chenpi decoction resulted in increasing the sensory evaluation score of FKJ (Figure 1a). This growth trend continued up to 30%, whereas with higher additions of chenpi decoction, the sensory evaluation score declined. When we carried out the single-factor experiment with kiwifruit juice as the independent variable based on the addition of 30% chenpi decoction, the same trend as that of chenpi decoction was observed (Figure 1b). The results showed that the best addition of kiwifruit juice was 30%. Based on this result, we kept the additions of 30% chenpi decoction and 30% kiwifruit juice unchanged to investigate the effect of pectin concentration on sensory evaluation of FKJ. The sensory evaluation of FKJ showed an increasing trend with increased additions of pectin from 1 to 3%, whereas further increases in added pectin resulted in a decrease in the sensory evaluation score (Figure 1c). Therefore, the response surface design level of the chenpi decoction was 25–35%, the level of kiwifruit juice was 25–35%, and the level of pectin was 2–4%.
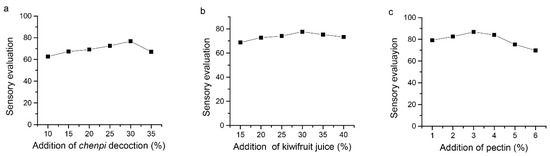
Figure 1.
Effects of chenpi decoction (a), kiwifruit juice (b), and pectin (c) on sensory evaluation of FKJ.
3.1.2. Response Surface Analysis
The development of FKJ was optimized using the response surface methodology (Box–Behnken design). The experimental design and related results are shown in Table 3. Using the total sensory evaluation score as the response variable (R), the regression analysis between R and three independent variables yielded the following regression equation:
R = −611.63 + 40.48 A − 1.85 B + 57.88 C + 0.01 AB − 0.1 AC − 0.05 BC − 0.67 A2 + 0.06 B2 −9.25 C2

Table 3.
Box–Behnken response surface factors and levels.
It was possible to predict the response values under different independent variables using the model equation. Table 4 shows that the model had a p-value < 0.0001, and the lack of fit had a p-value > 0.05, suggesting that the values predicted with the established regression model were extremely significant. In this model, the predicted R² of 0.9090 was in rational consistence with the adjusted R² of 0.9784, indicating that the actual sensory evaluation values fitted well with the predicted values. This model had a coefficient of variance (CV) of 2.49% (<10%) and adequate precision value of 23.96 (>4), which showed excellent reproducibility and accuracy.

Table 4.
Variance analysis of fitted model 1.
ANOVA analysis revealed that factor B (kiwifruit juice) showed an extremely significant difference (p < 0.0001) and factor C (pectin) was significantly different (p < 0.05), but factor A (chenpi decoction) was not significant (p = 0.0679). Larger F-values indicated a more significant effect of the factor on the response value. Therefore, the effect of each factor on the sensory evaluation of the product could be ranked as B-kiwifruit juice > C-pectin > A-chenpi decoction. Analysis of the p-values of the factors in the model showed that A2 and C2 had extremely significant differences (p < 0.0001), while the remaining effects were not significant (p > 0.1).
The 3D response surface and contour plots of the interaction effects of A-chenpi decoction (%), B-kiwifruit juice (%), and C-pectin (%) are shown in Figure 2. The steepness of the 3D response surface reflects the interaction between two factors, and the steeper the slope, the more significant the interaction. The shape of contour lines can reflect whether the interaction is significant or not; elliptical contour lines illustrated a significant interaction between two factors, and circular contour lines indicated little interaction. The 3D response surface of the sensory evaluation between A-chenpi decoction and C-pectin was steep, but the contours were close to circular, indicating that the interaction between these two factors was not significant (Figure 2b). Although the contours between A-chenpi decoction and B- kiwifruit juice were close to an ellipse, the 3D response surface was not significantly steep, indicating that the interaction between these two factors was not significant (Figure 2a). Similar results were observed between B-kiwifruit juice and C-pectin (Figure 2c). The results were consistent with the above ANOVA results.
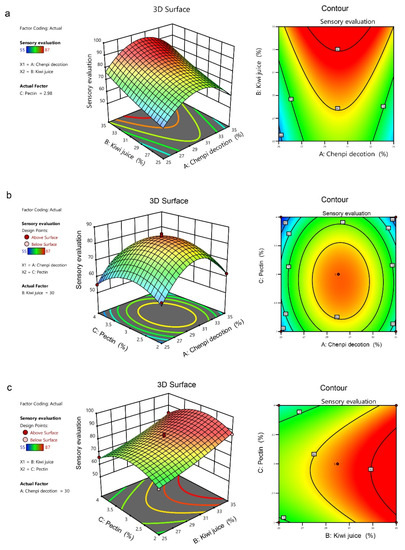
Figure 2.
Response 3D surfaces and contours of AB (a), AC (b), BC (c) interaction effects.
Finally, the best formulation was generated based on the regression model, and the results were validated. The highest sensory evaluation score of 95.20 was predicted with 30.26% addition of chenpi decoction (A), 35% addition of kiwifruit juice (B), and 2.88% addition of pectin (C). The actual score for the product based on the best formulation was 94.2 after evaluation by the sensory evaluation team members, which was similar to the predicted value.
3.1.3. 3D Printing of FKJ
3D food printing experiments were designed to investigate whether FKJ could be used as a printing material for extrusion printing. After the adjustment and testing of printing parameters, the expected food shape was printed successfully according to the food shape designed by the software. Figure 3a shows the design of the print shape (cuboid) of FKJ in the Cura 15.02.1 software. The cross-section of the print shape design is also shown (Figure 3b) and provides a visualization of the filling pattern during printing (rectilinear filling with a diagonal orientation). Finally, the printing of FKJ was completed (Figure 3c). Although the middle of the shape is slightly sunken, the printed FKJ sample had a high degree of matching with the designed shape.
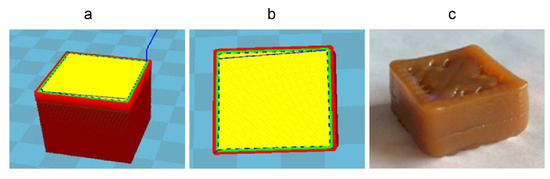
Figure 3.
Three-dimensional printing of FKJ. (a) Scheme of the 3D printed cuboid, 2.5 × 2.5 × 1.25 cm3, designed in Cura15.02.1 software. (b) Transversal view of the 3D designed object. Cross-section of one layer showing the printhead pathway; the yellow part in the middle is the printing infill part, and the slash in the middle represents the infill patterns. (c) 3D printing of a cuboid with food-ink made of best formulation.
3.2. Determination of Nutrients and Bioactive Components of FKJ
3.2.1. Nutritional Evaluation
The main nutritional composition of FKJ made with the optimal formula was deter-mined (Table 5). The results showed that FKJ was almost fat-free (0.1%), contained a small amount of protein (0.61%), and had a carbohydrate content of 20.5%, with a total energy of 369 kJ (88.28 cal) in 100 g of FKJ.

Table 5.
Nutritional composition of the optimal FKJ 1.
3.2.2. Determination of Total Flavonoids (TF) and Phenols (TP) of FKJ
In order to analyze the content of biologically active substances in FKJ, TP and TF contents were tested as shown in Figure 4a. The results showed that the TP and TF contents were 7.58 ± 0.32 mg GAE/g and 2.47 ± 0.13 mg RE/g, respectively.
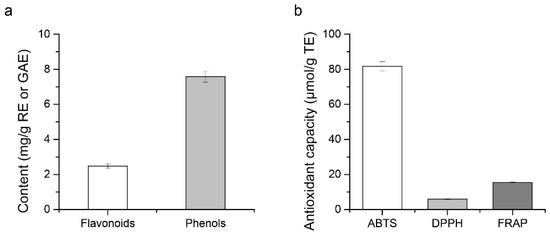
Figure 4.
Bioactive substances (a) of FKJ and its antioxidant ability (b).
3.3. Determination of Antioxidant Capacity of FKJ In Vitro
As shown in Figure 4b, FKJ possessed 81.65 ± 2.65 μmol/g TE ABTS and 5.94 ± 0.21 μmol/g TE DPPH radical scavenging capacity, and 15.49 ± 0.21 μmol/g TE FRAP reducing capacity.
3.4. Bioactive Effects of FKJ on Healthy Mice
3.4.1. FKJ Alleviated Body Weight Gain and Fat Accumulation in Mice
As shown in Figure 5, body weight was significantly reduced in the KH group compared to the CON group (p < 0.05). Interestingly, mice showed a dose-dependent decrease in food intake, liver weight, SAT weight, AAT weight, and PEAT weight after administration of FKJ compared to the CON group (Figure 5a–f). The liver weights in all three FKJ treatment groups were significantly lower than those in the CON group (p < 0.05, p < 0.001, and p < 0.001, respectively). The food intake of mice in the KM and KH groups was significantly lower than that of mice in the CON group (p < 0.001 and p < 0.001), while there was no remarkable effect in the KL group (Figure 5b). Compared with the CON group, the weights of SAT, AAT, and PEAT showed a significant decline in the KH group (p < 0.05, p < 0.05, and p < 0.01, respectively). The weights of AAT and PEAT in the KM group were significantly lower than those in the CON group (p < 0.05 and p < 0.05), while the weight change in the KL group was not significant (Figure 5e, f). Consistently, the histopathological examination of AAT also revealed a dose-dependent inhibition of adipocyte enlargement after FKJ supplementation in mice, as shown in Figure 5g. These results suggested that FKJ could slow down weight gain and fat accumulation in mice, which might be related to the control of appetite in mice by FKJ supplement.
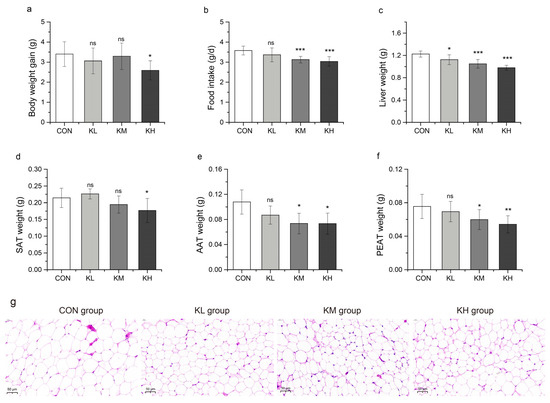
Figure 5.
FKJ alleviated body weight gain and fat accumulation in mice (n = 7–10). (a) Body weight gain (in grams) after 8 weeks of FKJ supplementation (the control group was expressed as CON, and the groups supplemented with low-dose, medium-dose, and high-dose FKJ were expressed as KL, KM, and KH, respectively). (b) The average daily food intake of mice during the whole experimental period. (c) Liver weight, (d) subcutaneous adipose tissues (SAT) weight, (e) abdominal adipose tissues (AAT) weight, (f) perirenal adipose tissues (PEAT) weight. (g) The observation of AAT by H&E staining of four treatment groups (40×). * p < 0.05; ** p < 0.01; *** p < 0.001; and ns p > 0.05. Compared with CON group.
3.4.2. FKJ Reduced the Risk of Hyperlipidemia and Inflammation
Abnormally elevated serum levels of TC, TG, and LDL-C, as well as reduced serum levels of HDL-C caused by disorders of lipid metabolism are always associated with a high prevalence of hyperlipidemia [40,41]. A noticeable phenomenon was observed in the results for the serum biochemical indexes (Figure 6a–f). Among the three different doses of FKJ, the medium dose (KM group) showed a remarkable effect in regulating lipid metabolism and was significantly different from the other two groups. Compared with the CON group, the level of serum TG in the KM group was significantly decreased (p < 0.05), while changes in TC, HDL-C, and LDL-C were not significant (Figure 6a–d).
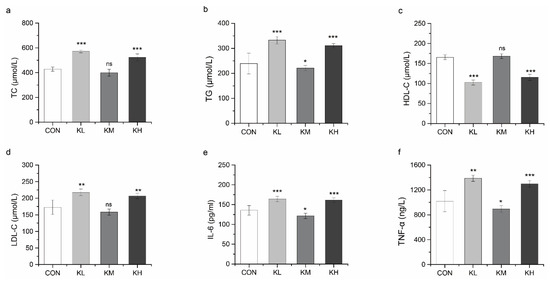
Figure 6.
FKJ reduced the risk of hyperlipidemia and inflammation. Concentrations of total cholesterol (TC) (a), triglycerides (TG) (b), high-density lipoprotein cholesterol (HDL-C) (c), low-density lipoprotein cholesterol (LDL-C) (d), interleukin-6 (IL-6) (e), and tumor necrosis factor-alpha (TNF-α) (f) in serum (n = 7–10). The control group was expressed as CON, and the groups supplemented with low-dose, medium-dose, and high-dose FKJ were expressed as KL, KM, and KH, respectively. * p < 0.05; ** p < 0.01; *** p < 0.001; and ns p > 0.05. Compared with CON group.
IL-6 and TNF-α are pro-inflammatory factors that cause inflammatory responses in the body [42]. The concentrations of serum IL-6 and TNF-α in the KM group were significantly lower than those in the CON group (p < 0.05) (Figure 6e,f). In contrast, the KL and KH groups showed the opposite results in comparison with the KM group.
3.4.3. FKJ Enhanced Antioxidant Capacity In Vivo
ALT and AST are representative indicators of liver function that reflect the health conditions of the liver. Compared with the CON group, the serum AST levels in the KM group were significantly decreased (p < 0.05), but the serum levels of ALT (Figure 7a,b) were not affected by the medium FKJ dose. In contrast, the KL and KH groups showed a significant increase compared to the CON group, in both ALT and AST. These results suggested that FKJ supplement at the medium dose had a significant preventive effect against liver injury.
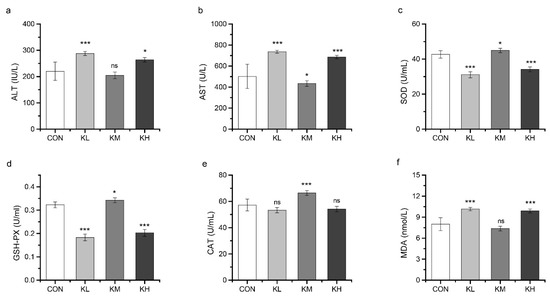
Figure 7.
FKJ enhanced antioxidant capacity in vivo. The serum levels of aminotransferase (ALT) (a), aspartate transaminase (AST) (b), superoxide dismutase (SOD) (c), glutathione peroxidase (GSH-PX) (d), catalase (CAT) (e), and malonaldehyde (MDA) (f). The control group was expressed as CON, and FKJ supplemented with low-dose, medium-dose, and high-dose were expressed as KL, KM, and KH. * p < 0.05; *** p < 0.001; and ns p > 0.05. Compared with CON group.
The MAD level, SOD activity, GSH-PX activity, and CAT activity in serum are related with the body’s antioxidant capacity. As shown in Figure 7, SOD, GSH-PX, and CAT were significantly increased in the KM group compared to their levels in the CON group (p < 0.05), whereas MDA levels were not significantly different between the two groups (Figure 7c–f). In contrast, MDA was significantly increased and the SOD, GSH-PX, and CAT were significantly decreased in the KL and KH groups. These results indicated that the antioxidant capacity of mice might be effectively improved by supplementation with FKJ at medium dose.
4. Discussion
In this study, a kind of functional kiwifruit jelly snack with low calories and antioxidant activity was developed using chenpi, kiwifruit juice, and citrus pectin. Single-factor experiments were used to explore the effects of various components and determine the range of addition of each ingredient. Then, a response surface experiment was designed to obtain an optimal food production formulation, which was combined with 3D food printing technology to obtain jelly products of specific shapes rapidly.
Chenpi is the main functional ingredient, which might contribute to an FKJ product with better health benefits. In the single-factor experiment, we observed that with increases in the concentration of chenpi, the sensory evaluation score of FKJ was the highest at a concentration of 30%, and then decreased significantly. This may be because the bitter substances (limonoids) contained in chenpi increased [43]. Kiwifruit juice is the source of most vitamin C and phenolic substances, which neutralize the bitterness of chenpi. LMP can form a gel in the presence of calcium ions [44], and the effect of pectin concentration on FKJ was determined in this study. Acosta, O. et al. have examined the effect of calcium concentration on jelly [45], which was not discussed in this study because changes in calcium concentration did not have a significant impact on our products (in the preliminary experimental results, not shown in this study).
Currently, jelly products are produced using traditional plastic molds or silicon molds [46]. However, these molds are often used to produce large quantities of simple product shapes, and might also cause environmental pollution problems [47]. Compared to molds, 3D food printing technology offers the advantage of customizing jelly products according to consumers’ needs for shape, color, flavor and even texture, which is difficult to achieve in the production of molds [48]. Three-dimensional food printing is an achievement of the application of 3D printing technology in the food manufacturing industry [49] that allows for personalized food customization based on consumer gender, age, physical/health status and nutritional needs, while also maximizing time and material savings [50,51]. Extrusion printing is generally the most common mode today in this industry [52], which has been successfully printing paste-like foods such as mashed potatoes for dysphagic patients [53] and fruit-based snacks that can meet the nutritional needs of children [54]. In our study, the optimized FKJ met the requirements of extrusion printing and enabled successful printing of the expected food shape. Although there was a slight deviation between the printed sample and the target shape (a slight depression in the middle), this may have been due to the deposition deformation of the printed material [55]. At present, we are still in the early stage of exploratory experiments and have only designed a simple jelly shape for 3D printing; more complex and diversified jelly shapes will be obtained in future studies. At the same time, with the increasing popularity of 3D printing technology in the food industry, it will be easier and faster to obtain customized food in the future. Personalized customization will be a development trend in the future. While the production of ordinary molds to design jelly shapes is more affordable at this stage, 3D food printing is more advantageous for customized foods, as even trained masters require more time and effort to satisfy complex customization requirements.
The Code of Federal Regulations of The U.S. (Title 21, part 101, chapter 1, subchapter B, 2022) states that the term “low-calorie” is used provided that the reference amount of the food is normally 30 g or less, or 2 tbsp or less, and does not exceed 40 calories in each reference amount [56]. In the low-calorie jelly studies, Khouryieh, H. A. et al. developed a low-calorie grape jelly offering 10 calories in one serving (1 tbsp or 15 g) [28]; Acosta, O. et al. developed a kind of blackberry jelly that provided less than 8 calories per serving [57] and a mixed fruit jelly that provided less than 12 calories per serving (1 tbsp or 15 mL) [45]. In our study, based on the comprehensive consideration of various components of FKJ, the recommended daily intake was less than 30 g given that 100 g of FKJ provided 88.28 calories, and each serving (less than 30 g) provided less than 26 cal. Therefore, FKJ could be considered as a low-calorie food.
Tylewicz, U. et al. developed two kinds of healthy snacks (fruit leathers and bars) based on kiwifruit with a TP content of about 922–1346 mg GAE/100 g dry weight (DW) and a TF content of about 0.45–4.92 ± 0.33 mg quercetin/100 g DW [18]. Similarly, Donno, D. et al. developed a freeze-dried fruit product from kiwifruit containing TP of 210.9 mg GAE/100g DW [17]. Our results showed that FKJs were rich in phenolic compounds (7.58 ± 0.32 mg GAE/g) and flavonoids (2.47 ± 0.13 mg RE/g). Both chenpi decoction [11] and kiwifruit juice [58,59] are rich in phenolic compounds, and flavonoids are the most abundant phenols [60]. Ercan Bursal et al. [61] produced a lyophilized aqueous extract of kiwifruit and determined a TP content of 16.67 ± 2.83 mg GAE/g. In comparison, the TP content of FKJ was 7.58 ± 0.32 mg GAE/g, probably due to the use of kiwifruit juice in our preparation, which may have lower TP content than the aqueous extract of kiwifruit. Jin Wang et al. [62] determined that the TP content of kiwifruit juice was 0.58 ± 0.01 mg GAE/mL, which was much lower than that of fresh kiwifruit. The TF content of fresh kiwifruit ranged from 0.82–1.61 mg RE/g fresh weight [58], while 2.47 ± 0.13 mg RE/g was detected in FKJ. The possible reason might be that another material in FKJ, chenpi decoction, also contains large amounts of flavonoids [63].
ABTS, DPPH, and FRAP assays are widely used methods for the determination of antioxidant activity in vitro [64]. The ABTS assay is based on the production of blue/green ABTS+, which is suitable for hydrophilic and lipophilic antioxidant systems; the DPPH analysis uses free radicals dissolved in organic media and is, therefore, suitable for hydrophobic systems [65]; the FRAP method depends on the ability of the antioxidant to reduce Fe3+ to Fe2+, which is bound to the ligand, producing an intense navy blue color [66]. Due to the different principles of the three determination methods, it is necessary to use them together to obtain more accurate results for antioxidant capacity. Our results showed that FKJ possessed 81.65 ± 2.65 μmol/g TE ABTS and 5.94 ± 0.21 μmol/g TE DPPH radical scavenging capacity, and 15.49 ± 0.21 μmol/g TE FRAP reducing capacity. The obvious differences in the results of the three methods may be related to the different reactions of various antioxidants in FKJ with the radicals or iron ions used [64]. In addition, since the ABTS assay better reflected the highly pigmented and hydrophilic antioxidants than the DPPH assay [67], the scavenging of ABTS radicals by FKJ was significantly higher than that of DPPH radicals. A similar phenomenon was also observed in the experiments of Thaipong, K. et al. [37] and Anna Floegel et al. [67]. The antioxidant capacity of fruit or vegetable extracts measured by ABTS was significantly higher than that measured by DPPH and FRAP in their results. Antioxidant activity is positively correlated with phenolic content [8] and vitamin C, which are abundant in kiwifruit juice [13] and chenpi [68]. Kiwifruit is generally considered to be a fruit with high antioxidant activity [69]. The antioxidant capacity of different kiwifruit varieties varies considerably, and studies by Y.-S. Park et al. showed that the ABTS radical scavenging capacity of kiwifruit ranged from 22.9–109.0 μmol TE/g DW, 8.49–102 μmol TE/g DW in the DPPH assay, and 11.0–94.4 μmol TE/g DW in the FRAP assay [70]. Most of our results fall in this range, and these results were expressed as fresh weight of FKJ. Thus, it can be suggested that FKJ has certain antioxidant activity in vitro.
We observed a slowdown in weight gain and a decrease in fat accumulation in a dose-dependent manner in healthy mice after long-term intake of FKJ. One of the main effects may have been influenced by chenpi [71]. Guo, J et al. illustrated that chenpi extract can reduce adipogenesis effectively during the differentiation of 3T3-L1 adipocytes [72]. Moreover, a study showed that chenpi extract (rich in PMF) could effectively attenuate obesity and hepatic steatosis caused by a high-fat diet [73]. In addition, kiwifruit juice, one of the main ingredients in FKJ, may play an essential role because of its abundance of vitamin C and flavonoids [74,75]. A study carried out by Alim, A. et al. showed that intervention with two kiwis per day was effective in reducing body weight and liver weight in healthy rats [76]. Furthermore, pectin is a soluble dietary fiber that improves metabolic homeostasis while enhancing satiety [77,78], which may explain the decrease in food intake during FKJ supplementation [79]. In short, the anti-obesity effects and health benefits exerted by FKJ in healthy mice may be the result of the combined effect of several main components.
A growing number of studies have shown that kiwifruit and its related processed products are able to improve lipid metabolism and enhance antioxidant capacity [80]. The study of Alim, A. et al. suggested that supplementation with kiwifruit significantly reduced TC and TG and increased HDL-C levels in rats; meanwhile, the lipid peroxidation was improved by decreasing MDA concentration and increasing GSH-PX and SOD activity [76]. Similarly, the results of a clinical study also showed that consuming kiwifruit juice increased the activity of antioxidant-related enzymes in the serum of patients and reduced markers of inflammation [81]. Further, kiwifruit juice fermented with probiotics also showed hypolipidemic and anti-oxidative effects in mice on a high-fat diet by improving the corresponding indexes in the serum [82]. In our study, similar effects were also observed in mice administered with a medium dose of FKJ, and chenpi may also play an important role. Chenpi is rich in polymethoxyflavones (PMFs) [83], which could exert anti-inflammatory and antioxidant effects [8]. In our study, low doses of FKJ did not show similar health effects because they fell below the threshold of usefulness. Notably, the same effect was not obtained in mice ingesting high doses of FKJ, which was supposed to be a counter effect of high doses. Thus, supplementation with moderate doses of FKJ (medium dose in this study) can effectively improve the lipid profile and anti-inflammatory and antioxidant abilities.
5. Conclusions
In conclusion, functional kiwifruit jelly with chenpi was developed through single-factor and response surface experiments. FKJ was rich in phenols and flavonoids, while being low in calories, and possessed good antioxidant capacity. FKJ can also be personalized using 3D food printing technology. In addition, in vivo experimental results illustrated that daily supplementation with FKJ at an appropriate dose (medium dose) could moderately reduce weight gain and fat accumulation, improving serum lipid profiles, relieving inflammation and liver damage, and improving antioxidant capacity. However, it should be noted that FKJ intake is not recommended above the high dose in this study, which may cause adverse effects. FKJ could meet the needs of modern people for nutrition and health and also promote the processing and utilization of natural products, and has good development prospects in the functional food industry.
Author Contributions
Conceptualization, J.G.; methodology, M.P. and Z.G.; software, M.P.; validation, J.G. and Z.G.; writing—original draft preparation, M.P. and Z.G.; writing—review and editing, M.P., Z.G. and J.G.; visualization, M.P., Z.G. and Y.L.; supervision, J.G., and Y.S.; project administration, Y.S.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Agricultural Science and Technology Innovation Project of Hunan Province, China (2021CX05), the Changsha Municipal Natural Science Foundation (kq2202332, kq2014070), the Agricultural Science and Technology Innovation Fund of Hunan (2020CX47), the Key Laboratory of Agro-Products Processing, Ministry of Agriculture and Rural Affairs of China (S2021KFKT-22), the National Natural Science Foundation of China (32073020), and the Hunan innovative province construction project (2019NK2041).
Institutional Review Board Statement
The animal study was approved by the Hunan Agricultural University Institutional Animal Care and Use Committee (202005).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carrera-Quintanar, L.; Lopez Roa, R.I.; Quintero-Fabian, S.; Sanchez-Sanchez, M.A.; Vizmanos, B.; Ortuno-Sahagun, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, S.; Ho, C.-T.; Huang, Q. Citrus polymethoxyflavones as regulators of metabolic homoeostasis: Recent advances for possible mechanisms. Trends Food Sci. Technol. 2021, 110, 743–753. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef]
- Hosseini, B.; Saedisomeolia, A.; Allman-Farinelli, M. Association Between Antioxidant Intake/Status and Obesity: A Systematic Review of Observational Studies. Biol. Trace Elem. Res. 2017, 175, 287–297. [Google Scholar] [CrossRef]
- Anigboro, A.A.; Avwioroko, O.J.; Akeghware, O.; Tonukari, N.J. Anti-obesity, antioxidant and in silico evaluation of Justicia carnea bioactive compounds as potential inhibitors of an enzyme linked with obesity: Insights from kinetics, semi-empirical quantum mechanics and molecular docking analysis. Biophys. Chem. 2021, 274, 106607. [Google Scholar] [CrossRef]
- Puchau, B.; Ochoa, M.C.; Zulet, M.A.; Marti, A.; Martinez, J.A.; Members, G. Dietary total antioxidant capacity and obesity in children and adolescents. Int. J. Food Sci. Nutr. 2010, 61, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, L.; Ruan, Z.; Han, P.; Yu, Y. The Regulatory Effects of Citrus Peel Powder on Liver Metabolites and Gut Flora in Mice with Non-Alcoholic Fatty Liver Disease (NAFLD). Foods 2021, 10, 3022. [Google Scholar] [CrossRef]
- Ashraf, H.; Butt, M.S.; Iqbal, M.J.; Suleria, H.A.R. Citrus peel extract and powder attenuate hypercholesterolemia and hyperglycemia using rodent experimental modeling. Asian Pac. J. Trop. Biomed. 2017, 7, 870–880. [Google Scholar] [CrossRef]
- Qian, Y.; Gao, Z.; Wang, C.; Ma, J.; Li, G.; Fu, F.; Guo, J.; Shan, Y. Effects of Different Treatment Methods of Dried Citrus Peel (Chenpi) on Intestinal Microflora and Short-Chain Fatty Acids in Healthy Mice. Front. Nutr. 2021, 8, 702559. [Google Scholar] [CrossRef] [PubMed]
- Green, C.O.; Wheatley, A.O.; McGrowder, D.A.; Dilworth, L.L.; Asemota, H.N. Citrus peel polymethoxylated flavones extract modulates liver and heart function parameters in diet induced hypercholesterolemic rats. Food Chem. Toxicol. 2013, 51, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Carr, A.C.; Pullar, J.M.; Bozonet, S.M. The bioavailability of vitamin C from kiwifruit. Adv. Food Nutr. Res. 2013, 68, 125–147. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, Y.; Chen, X.; He, H.; Liu, Z.; Zhang, Z.; Ren, Y.; Ren, X. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Res. Int. 2019, 116, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.L.; Santos, N.C.; Almeida, R.L.J.; Silva, S.d.N.; Nascimento, A.P.S.; Almeida, R.D.; Ribeiro, V.H.d.A.; Silva, W.P.; Gomes, J.P.; Silva, V.M.d.A.; et al. Influence of Pulp, Sugar and Maltodextrin Addiction in the Formulation of Kiwi Jellies With Lemon Grass Tea. J. Agric. Sci. 2019, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Iwasawa, H.; Morita, E.; Yui, S.; Yamazaki, M. Anti-oxidant Effects of Kiwi Fruit in Vitro and in Vivo. Biol. Pharm. Bull. 2011, 34, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G.L. Traditional and Unconventional Dried Fruit Snacks as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef]
- Vatthanakul, S.; Jangchud, A.; Jangchud, K.; Therdthai, N.; Wilkinson, B. Gold kiwifruit leather product development using Quality function deployment approach. Food Qual. Prefer. 2010, 21, 339–345. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N. Spray-Drying of Green or Gold Kiwifruit Juice–Milk Mixtures; Novel Formulations and Processes to Retain Natural Fruit Colour and Antioxidants. Food Bioprocess Technol. 2014, 8, 191–207. [Google Scholar] [CrossRef]
- Padma Ishwarya, S.; Sandhya, R.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2021, 62, 4393–4417. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Pan, L.L.; Luo, Y.; Niu, W.; Fang, X.; Liang, W.; Li, J.; Li, H.; Pan, X.; Yang, G.; et al. Low Methoxyl Pectin Protects against Autoimmune Diabetes and Associated Caecal Dysfunction. Mol. Nutr. Food Res. 2019, 63, e1900307. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Pan, L.L.; Niu, W.; Fang, X.; Liang, W.; Li, J.; Li, H.; Pan, X.; Chen, W.; Zhang, H.; et al. Modulation of Gut Microbiota by Low Methoxyl Pectin Attenuates Type 1 Diabetes in Non-obese Diabetic Mice. Front. Immunol. 2019, 10, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuramalia, D.R.; Damayanthi, E. Effect of green okra and strawberry ratio on antioxidant activity, total phenolic content, and organoleptic properties of jelly drink. IOP Conf. Ser. Earth Environ. Sci. 2018, 196, 012005. [Google Scholar] [CrossRef]
- Lima, M.B.; Domingos, F.M.; de Jesus Lima, J.J.F.; de Souza Monteiro, R.; dos Santos, O.D.H.; Pereira, P.A.P. Characterization and influence of hydrocolloids on low caloric orange jellies. Emir. J. Food Agric. 2019, 31, 7–15. [Google Scholar] [CrossRef]
- Vancauwenberghe, V.; Katalagarianakis, L.; Wang, Z.; Meerts, M.; Hertog, M.; Verboven, P.; Moldenaers, P.; Hendrickx, M.E.; Lammertyn, J.; Nicolaï, B. Pectin based food-ink formulations for 3-D printing of customizable porous food simulants. Innov. Food Sci. Emerg. 2017, 42, 138–150. [Google Scholar] [CrossRef]
- Duan, H.; Wang, X.; Azarakhsh, N.; Wang, C.; Li, M.; Fu, G.; Huang, X. Optimization of calcium pectinate gel production from high methoxyl pectin. J. Sci. Food Agric. 2022, 102, 757–763. [Google Scholar] [CrossRef]
- Khouryieh, H.A.; Aramouni, F.M.; Herald, T.J. Physical, chemical and sensory properties of sugar-free jelly. J. Food Qual. 2005, 28, 179–190. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- GB 5009.4-2016; National standard for food safety-Determination of ash in food. China Food and Drug Administration: Beijing, China, 2016. Available online: http://down.foodmate.net/standard/sort/3/49326.html (accessed on 1 June 2022).
- GB 5009.3-2016; National standard for food safety-Determination of moisture in foods. China Food and Drug Administration: Beijing, China, 2016. Available online: http://mall.foodmate.net/goods-84973.html (accessed on 1 June 2022).
- GB 5009.5-2016; National standard for food safety-Determination of protein in foods. China Food and Drug Administration: Beijing, China, 2016. Available online: http://down.foodmate.net/standard/sort/3/50381.html (accessed on 1 June 2022).
- GB 5009.6-2016; National standard for food safety-Determination of fat in foods. China Food and Drug Administration: Beijing, China, 2016. Available online: http://down.foodmate.net/standard/sort/3/50382.html (accessed on 1 June 2022).
- GB 5009.91-2017; National standard for food safety-Determination of potassium and sodium in food. China Food and Drug Administration: Beijing, China, 2017. Available online: http://down.foodmate.net/standard/sort/3/50752.html (accessed on 1 June 2022).
- Li, X.; Chen, D.; Mai, Y.; Wen, B.; Wang, X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi). Nat. Prod. Res. 2012, 26, 1050–1053. [Google Scholar] [CrossRef]
- Yamin, R.; Mistriyani, S.; Ihsan, S.; Armadany, F.I.; Sahumena, M.H.; Fatimah, W.O.N. Determination of total phenolic and flavonoid contents of jackfruit peel and in vitro antiradical test. Food Res. 2020, 5, 84–90. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Zhao, Z.; Zhao, Y.; Xu, X.; Li, Y.; Tan, S.; Huang, C.; Zhou, Z. PMFs-rich Citrus extract prevents the development of non-alcoholic fatty liver disease in C57BL/6J mice induced by a high-fat diet. J. Funct. Foods 2018, 47, 28–39. [Google Scholar] [CrossRef]
- Feng, K.; Zhu, X.; Liu, G.; Kan, Q.; Chen, T.; Chen, Y.; Cao, Y. Dietary citrus peel essential oil ameliorates hypercholesterolemia and hepatic steatosis by modulating lipid and cholesterol homeostasis. Food Funct. 2020, 11, 7217–7230. [Google Scholar] [CrossRef]
- Manzoni, A.G.; Passos, D.F.; da Silva, J.L.G.; Bernardes, V.M.; Bremm, J.M.; Jantsch, M.H.; de Oliveira, J.S.; Mann, T.R.; de Andrade, C.M.; Leal, D.B.R. Rutin and curcumin reduce inflammation, triglyceride levels and ADA activity in serum and immune cells in a model of hyperlipidemia. Blood Cells Mol. Dis. 2019, 76, 13–21. [Google Scholar] [CrossRef]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lu, S. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- Fraeye, I.; Duvetter, T.; Doungla, E.; Van Loey, A.; Hendrickx, M. Fine-tuning the properties of pectin–calcium gels by control of pectin fine structure, gel composition and environmental conditions. Trends Food Sci. Technol. 2010, 21, 219–228. [Google Scholar] [CrossRef]
- Acosta, O.; Víquez, F.; Cubero, E. Optimisation of low calorie mixed fruit jelly by response surface methodology. Food Qual. Prefer. 2008, 19, 79–85. [Google Scholar] [CrossRef]
- Darade, A.D.; Mundada, A.S. Oral medicated jellies as a emerging platform for oral drug delivery in pediatrics. World J. Pharm. Res. 2021, 10, 1628–1647. [Google Scholar] [CrossRef]
- Kuo, R.L.; Laiy, W.Y.; Kao, Y.H. Application of Digital Modeling in the Elementary School Digital Dessert Workshop. In Proceedings of the 2018 1st IEEE International Conference on Knowledge Innovation and Invention (ICKII), Jeju, Korea, 23–27 July 2018; pp. 165–167. [Google Scholar] [CrossRef]
- Lee, B.; Hong, J.; Surh, J.; Saakes, D. Ori-mandu: Korean Dumpling into Whatever Shape You Want. In Proceedings of the 2017 Conference on Designing Interactive Systems, Edinburgh, UK, 10–14 June 2017; pp. 929–941. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, L.; Zou, Y.; Tong, Z.; Han, S.; Wang, S. 3D food printing: Main components selection by considering rheological properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S. Minimally Processed Functional Foods: Technological and Operational Pathways. J. Food Sci. 2016, 81, R2309–R2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kewuyemi, Y.O.; Kesa, H.; Adebo, O.A. Trends in functional food development with three-dimensional (3D) food printing technology: Prospects for value-added traditionally processed food products. Crit. Rev. Food Sci. Nutr. 2021, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, M.; Devahastin, S. Improvement of 3D printability of buckwheat starch-pectin system via synergistic Ca2+-microwave pretreatment. Food Hydrocoll. 2021, 113, 106483. [Google Scholar] [CrossRef]
- Mirazimi, F.; Saldo, J.; Sepulcre, F.; Gràcia, A.; Pujola, M. Enriched puree potato with soy protein for dysphagia patients by using 3D printing. Food Front. 2022, 1–10. [Google Scholar] [CrossRef]
- Derossi, A.; Caporizzi, R.; Azzollini, D.; Severini, C. Application of 3D printing for customized food. A case on the development of a fruit-based snack for children. J. Food Eng. 2018, 220, 65–75. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Zhang, M. Creation of internal structure of mashed potato construct by 3D printing and its textural properties. Food Res. Int. 2018, 111, 534–543. [Google Scholar] [CrossRef]
- CPO U.S. Government Printing Office. Code of Federal Regulations Title 21—Food and Drugs; Department of Health and Human Services: Washington, DC, USA, 2022; Part 101. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101 (accessed on 1 June 2022).
- Acosta, O.; Víquez, F.; Cubero, E.; Morales, I. Ingredient Levels Optimization and Nutritional Evaluation of a Low-calorie Blackberry (Rubus irasuensis Liebm.) Jelly. J. Food Sci. 2006, 71, S390–S394. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly)phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT-Food Sci. Technol. 2019, 107, 299–307. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Zhang, X.; Zhao, D.G.; Ma, Y.Y.; Li, D.; Ho, C.T.; Huang, Q. Aged citrus peel (chenpi) extract causes dynamic alteration of colonic microbiota in high-fat diet induced obese mice. Food Funct. 2020, 11, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Modi, H.A. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Liang, P.-L.; Chen, X.-L.; Gong, M.-J.; Xu, Y.; Tu, H.-S.; Zhang, L.; Liao, B.-s.; Qiu, X.-H.; Zhang, J.; Huang, Z.-H.; et al. Guang Chen Pi (the pericarp of Citrus reticulata Blanco’s cultivars ‘Chachi’) inhibits macrophage-derived foam cell formation. J. Ethnopharmacol. 2022, 293, 115328. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.-l.; Li, J.-y.; Liang, Y.-j.; Yang, R.-q.; Liu, J.-y.; Ma, Z.; Wu, L. Evaluation of biochemical components and antioxidant capacity of different kiwifruit (Actinidia spp.) genotypes grown in China. Biotechnol. Biotechnol. Equip. 2018, 32, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Suhaj, M.; Cvikrová, M.; Martincová, O.; Weisz, M.; Gorinstein, S. Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. J. Food Compos. Anal. 2011, 24, 963–970. [Google Scholar] [CrossRef]
- Liu, D.; Chen, C.; Li, R. Protective effect of flavonoids from pericarpium citri reticulatae (chenpi) against oxidative stress induced by exhaustive exercise. Afr. J. Microbiol. Res. 2011, 5, 50–56. [Google Scholar] [CrossRef]
- Guo, J.; Cao, Y.; Ho, C.-T.; Jin, S.; Huang, Q. Aged citrus peel (chenpi) extract reduces lipogenesis in differentiating 3T3-L1 adipocytes. J. Funct. Foods 2017, 34, 297–303. [Google Scholar] [CrossRef]
- Falduto, M.; Smedile, F.; Zhang, M.; Zheng, T.; Zhu, J.; Huang, Q.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Anti-obesity effects of Chenpi: An artificial gastrointestinal system study. Microb. Biotechnol. 2022, 15, 874–885. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Quan, W.; Tao, Y.; Lu, M.; Yuan, B.; Chen, J.; Zeng, M.; Qin, F.; Guo, F.; He, Z. Stability of the phenolic compounds and antioxidant capacity of five fruit (apple, orange, grape, pomelo and kiwi) juices during in vitro-simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2018, 53, 1131–1139. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Liu, Y.; Yang, X. Consumption of two whole kiwifruit (Actinide chinensis) per day improves lipid homeostasis, fatty acid metabolism and gut microbiota in healthy rats. Int. J. Biol. Macromol. 2020, 156, 186–195. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef]
- Schwartz, S.E.; Levine, R.A.; Singh, A.; Scheidecker, J.R.; Track, N.S. Sustained Pectin Ingestion Delays Gastric Emptying. Gastroenterology 1982, 83, 812–817. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis Planch.: A Review of Chemistry and Pharmacology. Front Pharmacol. 2019, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, X.; Li, G.; Dai, B.; Tan, W. Actinidia chinensis Planch. Improves the Indices of Antioxidant and Anti-Inflammation Status of Type 2 Diabetes Mellitus by Activating Keap1 and Nrf2 via the Upregulation of MicroRNA-424. Oxid. Med. Cell. Longev. 2017, 2017, 7038789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, H.; Ren, Y.; Wang, Y.; Yaopeng, R.; Xiaowei, W.; Tianli, Y.; Zhouli, W.; Zhenpeng, G. Preparation, model construction and efficacy lipid-lowering evaluation of kiwifruit juice fermented by probiotics. Food Biosci. 2022, 47, 101710. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Z.; Wen, M.; Wu, Z.; Zha, M.; Xu, W.; Zhang, L. Chemical Variation of Chenpi (Citrus Peels) and Corresponding Correlated Bioactive Compounds by LC-MS Metabolomics and Multibioassay Analysis. Front. Nutr. 2022, 9, 825381. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).