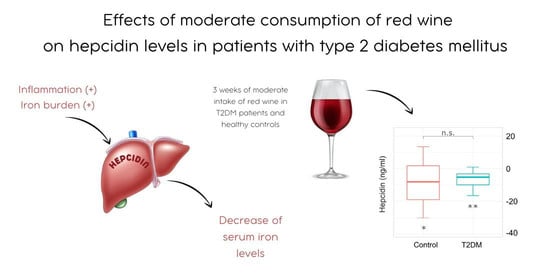
Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Wine Intervention
2.3. Anthropometric Assessments and Blood Sampling
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation Diabetes Atlas, 10th ed. 2021. Available online: www.diabetesatlas.org (accessed on 23 February 2022).
- Aregbesola, A.; Voutilainen, S.; Virtanen, J.K.; Aregbesola, A.; Tuomainen, T.P. Serum hepcidin concentrations and type 2 diabetes. World J. Diabetes 2015, 6, 978–982. [Google Scholar]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar]
- Armitage, A.E.; Eddowes, L.A.; Gileadi, U.; Cole, S.; Spottiswoode, N.; Selvakumar, T.A.; Ho, L.P.; Townsend, A.R.; Drakesmith, H. Hepcidin regulation by innate immune and infectious stimuli. Blood 2011, 118, 4129–4139. [Google Scholar]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Kakhlon, O.; Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar]
- Liu, J.F.; Li, Q.X.; Yang, Y.X.; Ma, L.H. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar]
- Lee, H.J.; Choi, J.S.; Lee, H.J.; Kim, W.H.; Park, S.I.; Song, J. Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J. Nutr. Biochem. 2015, 26, 1414–1423. [Google Scholar]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; SMART Study Group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar]
- McMillan, D.E. Increased levels of acute-phase serum proteins in diabetes. Metabolism 1989, 38, 1042–1046. [Google Scholar]
- Karamzad, N.; Eftekhari, A.; Ashrafi-Asgarabad, A.; Sullman, M.J.M.; Sahebkar, A.; Safiri, S. Serum hepcidin, the hepcidin/ferritin ratio and the risk of type 2 diabetes: A systematic review and meta-analysis. Curr. Med. Chem. 2021, 28, 1224–1233. [Google Scholar]
- Ndevahoma, F.; Mukesi, M.; Dludla, P.V.; Nkambule, B.B.; Nepolo, E.P.; Nyambuya, T.M. Body weight and its influence on hepcidin levels in patients with type 2 diabetes: A systematic review and meta-analysis of clinical studies. Heliyon 2021, 7, e06429. [Google Scholar]
- Harrison-Findik, D.D. Role of alcohol in the regulation of iron metabolism. World J. Gastroenterol. 2007, 13, 4925–4930. [Google Scholar]
- Milman, N.T. A review of nutrients and compounds, which promote or inhibit intestinal iron absorption: Making a platform for dietary measures that can reduce iron uptake in patients with genetic haemochromatosis. J. Nutr. Metab. 2020, 2020, 7373498. [Google Scholar]
- Omena, J.; Curioni, C.; Cople-Rodrigues, C.D.; Citelli, M. The effect of food and nutrients on iron overload: What do we know so far? Eur. J. Clin. Nutr. 2021, 75, 1771–1780. [Google Scholar]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar]
- Martin, M.A.; Goya, L.; Ramos, S. Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies. Food Chem. Toxicol. 2017, 109, 302–314. [Google Scholar]
- Blomster, J.I.; Zoungas, S.; Chalmers, J.; Li, Q.; Chow, C.K.; Woodward, M.; Mancia, G.; Poulter, N.; Williams, B.; Harrap, S.; et al. The relationship between alcohol consumption and vascular complications and mortality in individuals with type 2 diabetes. Diabetes Care 2014, 37, 1353–1359. [Google Scholar]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: A 2-year randomized, controlled trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar]
- Golan, R.; Shelef, I.; Shemesh, E.; Henkin, Y.; Schwarzfuchs, D.; Gepner, Y.; Harman-Boehm, I.; Witkow, S.; Friger, M.; Chassidim, Y.; et al. Effects of initiating moderate wine intake on abdominal adipose tissue in adults with type 2 diabetes: A 2-year randomized controlled trial. Public Health Nutr. 2017, 20, 549–555. [Google Scholar]
- Beulens, J.W.; van der Schouw, Y.T.; Bergmann, M.M.; Rohrmann, S.; Schulze, M.B.; Buijsse, B.; Grobbee, D.E.; Arriola, L.; Cauchi, S.; Tormo, M.J.; et al. Alcohol consumption and risk of type 2 diabetes in European men and women: Influence of beverage type and body size The EPIC-InterAct study. J. Intern. Med. 2012, 272, 358–370. [Google Scholar]
- Golan, R.; Gepner, Y.; Shai, I. Wine and health—New evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar]
- American Diabetes Association Professional Practice Care. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S17–S38. [Google Scholar]
- Boban, M.; Stockley, C.; Teissedre, P.L.; Restani, P.; Fradera, U.; Stein-Hammer, C.; Ruf, J.C. Drinking pattern of wine and effects on human health: Why should we drink moderately and with meals? Food Funct. 2016, 7, 2937–2942. [Google Scholar]
- Mollica, A.; Scioli, G.; Della Valle, A.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; Córdova, M.L.F.-D.; Castillo-López, R.; et al. Phenolic analysis and in vitro biological activity of red wine, pomace and grape seeds oil derived from Vitis vinifera L. cv. Montepulciano d’Abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar]
- Busbridge, M.; Griffiths, C.; Ashby, D.; Gale, D.; Jayantha, A.; Sanwaiya, A.; Chapman, R.S. Development of a novel immunoassay for the iron regulatory peptide hepcidin. Brit. J. Biomed. Sci. 2009, 66, 150–157. [Google Scholar]
- Ioannou, G.N.; Dominitz, J.A.; Weiss, N.S.; Heagerty, P.J.; Kowdley, K.V. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology 2004, 126, 1293–1301. [Google Scholar]
- Harrison-Findik, D.D.; Schafer, D.; Klein, E.; Timchenko, N.A.; Kulaksiz, H.; Clemens, D.; Fein, E.; Andriopoulos, B.; Pantopoulos, K.; Gollan, J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006, 281, 22974–22982. [Google Scholar]
- Harrison-Findik, D.D.; Klein, E.; Crist, C.; Evans, J.; Timchenko, N.; Gollan, J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology 2007, 46, 1979–1985. [Google Scholar]
- Ohtake, T.; Saito, H.; Hosoki, Y.; Inoue, M.; Miyoshi, S.; Suzuki, Y.; Fujimoto, Y.; Kohgo, Y. Hepcidin is down-regulated in alcohol loading. Alcohol. Clin. Exp. Res. 2007, 31, 2S–8S. [Google Scholar]
- Wang, X.M.; Li, Y.; Han, L.; Li, J.; Liu, C.; Sun, C.G. Role of flavonoids in the treatment of iron overload. Front. Cell Dev. Biol. 2021, 9, 685364. [Google Scholar]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar]
- Cook, J.D.; Reddy, M.B.; Hurrell, R.F. The effect of red and white wines on nonheme-iron absorption in humans. Am. J. Clin. Nutr. 1995, 61, 800–804. [Google Scholar]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; Van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; Heijer, M.D.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar]
- Sugiura, T.; Dohi, Y.; Takase, H.; Fujii, S.; Seo, Y.; Ohte, N. Analytical evaluation of serum non-transferrin-bound iron and its relationships with oxidative stress and cardiac load in the general population. Medicine 2021, 100, e24722. [Google Scholar]
- Vinchi, F. Non-transferrin-bound iron in the spotlight: Novel mechanistic insights into the vasculotoxic and atherosclerotic effect of iron. Antioxid. Redox Signal. 2021, 35, 387–414. [Google Scholar]
- Van Bussel, B.C.T.; Henry, R.M.A.; Schalkwijk, C.G.; Dekker, J.M.; Nijpels, G.; Feskens, E.J.M.; Stehouwer, C.D.A. Alcohol and red wine consumption, but not fruit, vegetables, fish or dairy products, are associated with less endothelial dysfunction and less low-grade inflammation: The Hoorn Study. Eur. J. Nutr. 2018, 57, 1409–1419. [Google Scholar]
- Imhof, A.; Woodward, M.; Doering, A.; Helbecque, N.; Loewel, H.; Amouyel, P.; Lowe, G.; Koenig, W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: Results from three MONICA samples (Augsburg, Glasgow, Lille). Eur. Heart J. 2004, 25, 2092–2100. [Google Scholar]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the US. J. Am. Coll. Cardiol. 2020, 76, 2181–2193. [Google Scholar]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar]
- Adams, P.C. Diabetes: Serum ferritin levels and T2DM—Are body iron stores elevated? Nat. Rev. Endocrinol. 2012, 8, 573–575. [Google Scholar]
- Pham, N.M.; Nanri, A.; Yi, S.; Kurotani, K.; Akter, S.; Foo, L.H.; Nishi, N.; Sato, M.; Hayabuchi, H.; Mizoue, T. Serum ferritin is associated with markers of insulin resistance in Japanese men but not in women. Metabolism 2013, 62, 561–567. [Google Scholar]
- Vari, I.S.; Balkau, B.; Kettaneh, A.; André, P.; Tichet, J.; Fumeron, F.; Caces, E.; Marre, M.; Grandchamp, B.; Ducimetière, P.; et al. Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population—Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2007, 30, 1795–1801. [Google Scholar]
- Park, S.K.; Kim, M.G.; Ryoo, J.H.; Shin, J.Y. Association of serum ferritin and the development of metabolic syndrome in middle-aged Korean men: A 5-year follow-up study. Diabetes Care 2012, 35, 2521–2526. [Google Scholar]
- Peng, Y.Y.; Uprichard, J. Ferritin and iron studies in anaemia and chronic disease. Ann. Clin. Biochem. 2017, 54, 43–48. [Google Scholar]
- Justin, C.L.; Stevic, I.; Chan, A.; Lau, K.K.H.; Chan, H.H.W. Serum ferritin is not sensitive or specific for the diagnosis of iron deficiency in patients with normocytic anemia. Blood 2015, 126, 955. [Google Scholar]
- Garcia-Casal, M.N.; Pena-Rosas, J.P.; Pasricha, S.R. Rethinking ferritin cutoffs for iron deficiency and overload. Lancet Haematol. 2014, 1, E92–E94. [Google Scholar]
- Speeckaert, M.M.; Speeckaert, R.; Delanghe, J.R. Biological and clinical aspects of soluble transferrin receptor. Crit. Rev. Clin. Lab. Sci. 2010, 47, 213–228. [Google Scholar]
- Skikne, B.S.; Punnonen, K.; Caldron, P.H.; Bennett, M.T.; Rehu, M.; Gasior, G.H.; Chamberlin, J.S.; Sullivan, L.A.; Bray, K.R.; Southwick, P.C. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: A prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am. J. Hematol. 2011, 86, 923–927. [Google Scholar]
- Andrews, M.; Soto, N.; Arredondo-Olguin, M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition 2015, 31, 51–57. [Google Scholar]
- Coimbra, S.; Catarino, C.; Santos-Silva, A. The role of adipocytes in the modulation of iron metabolism in obesity. Obes. Rev. 2013, 14, 771–779. [Google Scholar]
- Vuppalanchi, R.; Troutt, J.S.; Konrad, R.J.; Ghabril, M.; Saxena, R.; Bell, L.N.; Kowdley, K.V.; Chalasani, N. Serum hepcidin levels are associated wih obesity but not liver disease. Obesity 2014, 22, 836–841. [Google Scholar]
- Hawula, Z.J.; Wallace, D.F.; Subramaniam, V.N.; Rishi, G. Therapeutic advances in regulating the hepcidin/ferroportin axis. Pharmaceuticals 2019, 12, 170. [Google Scholar]



| Parameter | Control Group (n = 13) | T2D Group (n = 18) | p-Value |
|---|---|---|---|
| Age (years) | 50.5 ± 5.9 | 54.6 ± 6.2 | 0.075 |
| Age at T2D onset (years) | N/A | 50.6 ± 6.7 | N/A |
| Weight (kg) | 100.3 (84.7–105.5) | 98.5 (87.2–107.0) | 0.617 |
| Height (cm) | 186.8 ± 5.6 | 184.5 ± 10.1 | 0.427 |
| Waist circumference (cm) | 106.0 (95.8–109.5) | 107.0 (98.8–112.4) | 0.458 |
| Hip circumference (cm) | 108.0 ± 5.6 | 105.0 ± 7.5 | 0.237 |
| Upper arm circumference (cm) | 35.2 ± 2.9 | 32.2 ± 3.4 | 0.015 |
| Neck circumference (cm) | 42.0 (40.3–44.0) | 38.5 (37.0–41.2) | 0.051 |
| BMI (kg/m2) | 27.2 ± 2.7 | 29.8 ± 4.1 | 0.062 |
| Fasting glucose (mmol/L) | 5.3 ± 0.4 | 7.5 ± 1.4 | <0.0001 |
| AST (IU/L) | 27.1 ± 7.1 | 22.1 ± 4.9 | 0.029 |
| ALT (IU/L) | 25.0 (19.5–39.2) | 25.5 (16.4–29.6) | 0.674 |
| GGT (IU/L) | 28.0 (23.5–43.7) | 25.5 (20.0–32.2) | 0.307 |
| Albumin (g/L) | 45.0 (45.0–46.0) * | 43.0 (41.4–44.0) | 0.002 |
| Total bilirubin (µmol/L) | 13.8 ± 4.0 † | 12.4 ± 4.6 | 0.388 |
| Urates (µmol/L) | 328.9 ± 43.8 | 359.6 ± 81.2 | 0.188 |
| hsCRP (mg/L) | 1.3 (0.7–1.9) | 1.5 (0.8–2.9) | 0.317 |
| Laboratory Parameter | Control Group (n = 13) | T2D Group (n = 18) | ||||
|---|---|---|---|---|---|---|
| After Drive-In | After Red Wine | p-Value | After Drive-In | After Red Wine | p-Value | |
| RBC (×1012/L) | 5.2 ± 0.5 | 5.1 ± 0.4 | 0.077 | 5.0 ± 0.3 | 4.9 ± 0.3 | 0.888 |
| Hematocrit (L/L) | 0.45 ± 0.039 | 0.45 ± 0.034 | 0.388 | 0.44 ± 0.019 | 0.44 ± 0.021 | 0.749 |
| Hemoglobin (g/L) | 154.2 ± 12.6 | 153.0 ± 10.6 | 0.348 | 151.4 ± 6.2 | 151.5 ± 6.5 | 0.969 |
| RDW (%) | 13.0 ± 0.5 | 13.2 ± 0.5 | 0.013 | 13.5 ± 0.4 | 13.6 ± 0.5 | 0.033 |
| MCH (pg) | 29.6 ± 1.0 | 29.9 ± 1.0 | 0.047 | 30.7 ± 1.3 | 30.7 ± 1.4 | 0.709 |
| MCHC (g/L) | 337.5 ± 7.3 | 339.5 ± 8.8 | 0.261 | 341.4 ± 5.5 | 342.7 ± 6.8 | 0.349 |
| MCV (fL) | 87.1 (84.9–90.1) | 87.2 (85.2–91.5) | 0.675 | 89.8 ± 3.6 | 89.6 ± 3.5 | 0.495 |
| Serum iron (µmol/L) | 21.8 ± 7.1 | 19.6 ± 6.9 | 0.328 | 15.0 (13.8–18.7) | 16.4 (14.6–20.9) | 0.177 |
| TIBC (µmol/L) | 54.2 ± 9.3 | 53.8 ± 12.1 | 0.767 | 57.8 ± 7.2 | 58.2 ± 7.6 | 0.434 |
| UIBC (µmol/L) | 31.6 ± 11.6 | 34.2 ± 11.4 | 0.109 | 39.4 (37.5–45.5) | 41.6 (36.8–46.5) | 0.453 |
| Transferrin saturation (%) * | 40.6 ± 12.9 | 37.0 ± 11.3 | 0.307 | 26.7 (22.6–29.9) | 29.3 (24.5–32.7) | 0.265 |
| sTfR (mg/L) † | 1.20 ± 0.22 | 1.24 ± 0.25 | 0.734 | 1.05 ± 0.17 | 1.04 ± 0.20 | 0.830 |
| Ferritin (ng/mL) | 173.0 (126.4–259.8) | 118.0 (90.5–232.6) | 0.017 | 209.5 ± 141.5 | 198.8 ± 139.4 | 0.215 |
| Hepcidin (ng/mL) ‡ | 30.0 ± 17.3 | 21.0 ± 12.1 | 0.045 | 17.9 (11.9–25.2) | 13.2 (8.2–18.3) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazlić, J.; Jurić, D.; Mudnić, I.; Boban, Z.; Dželalija, A.M.; Tandara, L.; Šupe-Domić, D.; Gugo, K.; Boban, M. Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus. Foods 2022, 11, 1881. https://doi.org/10.3390/foods11131881
Nazlić J, Jurić D, Mudnić I, Boban Z, Dželalija AM, Tandara L, Šupe-Domić D, Gugo K, Boban M. Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus. Foods. 2022; 11(13):1881. https://doi.org/10.3390/foods11131881
Chicago/Turabian StyleNazlić, Jurica, Diana Jurić, Ivana Mudnić, Zvonimir Boban, Ana Marija Dželalija, Leida Tandara, Daniela Šupe-Domić, Katarina Gugo, and Mladen Boban. 2022. "Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus" Foods 11, no. 13: 1881. https://doi.org/10.3390/foods11131881
APA StyleNazlić, J., Jurić, D., Mudnić, I., Boban, Z., Dželalija, A. M., Tandara, L., Šupe-Domić, D., Gugo, K., & Boban, M. (2022). Effects of Moderate Consumption of Red Wine on Hepcidin Levels in Patients with Type 2 Diabetes Mellitus. Foods, 11(13), 1881. https://doi.org/10.3390/foods11131881