Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents for Wine Analysis
2.2. Chemicals and Reagents for Medical Analysis
2.3. Study Design, Subjects, and Materials and Methods
2.4. Standard Physico-Chemical Analysis
2.5. Analysis of Total Phenolic Content and Antioxidant Capacity
2.6. Analysis of Individual Phenolic Compounds
2.7. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) Analysis for Macro- and Microelements in Wine
2.8. Analysis of Vitamins in Wine
2.9. Medical Examination and Laboratory Tests
2.10. Statistical Data Analysis
3. Results and Discussion
3.1. Standard Physico-Chemical Analysis
| Physico-Chemical Parameters | Malvazija Istarska | Pošip | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| Alcoholic strength (%vol.) | 12.99 ± 0.010 d | 13.99 ± 0.010 b | 13.11 ± 0.020 c | 14.16 ± 0.020 a |
| Total dry extract (g/L) | 21.8 ± 0.0 ab | 21.5 ± 0.64 b | 20.6 ± 0.10 c | 22.1 ± 0.10 a |
| Reducing sugars (g/L) | 2.3 ± 0.1 a | 2.1 ± 0.0 b | 1.8 ± 0.1 c | 2.3 ± 0.0 a |
| Extract without sugars (g/L) | 18.5 ± 0.06 a | 18.4 ± 0.64 a | 17.7 ± 0.06 b | 18.8 ± 0.10 a |
| Ash (g/L) | 2.83 ± 0.01 a | 2.66 ± 0.02 b | 1.80 ± 0.01 d | 1.86 ± 0.01 c |
| pH | 3.63 ± 0.01 a | 3.34 ± 0.01 b | 3.20 ± 0.01 c | 3.07 ± 0.00 d |
| Total acidity (g/L) 1 | 5.0 ± 0.01 d | 5.6 ± 0.01 c | 5.8 ± 0.02 b | 7.0 ± 0.01 a |
| Volatile acidity (g/L) 2 | 0.59 ± 0.01 a | 0.44 ± 0.00 c | 0.39 ± 0.01 d | 0.56 ± 0.01 b |
| Physico-Chemical Parameters | Teran | Plavac Mali | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| Alcoholic strength (%vol.) | 13.20 ± 0.010 c | 13.79 ± 0.010 a | 13.18 ± 0.020 d | 13.57 ± 0.010 b |
| Total dry extract (g/L) | 29.1 ± 0.1 d | 34.5 ± 0.1 a | 29.8 ± 0.1 c | 33.6 ± 0.0 b |
| Reducing sugars (g/L) | 2.6 ± 0.3 b | 2.2 ± 0.2 c | 1.8 ± 0.1 d | 3.1 ± 0.2 a |
| Extract without sugars (g/L) | 25.5 ± 0.06 d | 31.3 ± 0.06 a | 26.9 ± 0.06 c | 29.5 ± 0.00 b |
| Ash (g/L) | 2.70 ± 0.01 d | 4.04 ± 0.02 a | 3.86 ± 0.01 b | 3.34 ± 0.01 c |
| pH | 3.42 ± 0.01 d | 3.61 ± 0.01 c | 3.95 ± 0.00 a | 3.71 ± 0.00 b |
| Total acidity (g/L) 1 | 6.0 ± 0.1 b | 6.5 ± 0.0 a | 4.3 ± 0.1 d | 5.3 ± 0.0 c |
| Volatile acidity (g/L) 2 | 0.44 ± 0.01 c | 0.88 ± 0.01 a | 0.37 ± 0.01 d | 0.6 ± 0.0 b |
3.2. Total Phenolic Content and Antioxidant Capacity
3.3. Individual Phenolic Compounds
3.4. Macro- and Microelements
3.5. Vitamins
3.6. Medical Examination and Laboratory Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis Vinifera (Grape) and Its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Gimeno-Mallench, L.; Olaso-Gonzalez, G.; Mastaloudis, A.; Traber, M.G.; Monleón, D.; Borrás, C.; Viña, J. Moderate Red Wine Consumption Increases the Expression of Longevity-Associated Genes in Controlled Human Populations and Extends Lifespan in Drosophila Melanogaster. Antioxidants 2021, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications; Elsevier: Amsterdam, Netherlands, 2014; ISBN 978-0-12-381469-2. [Google Scholar]
- Yang, J.; Xiao, Y.-Y. Grape Phytochemicals and Associated Health Benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Budić-Leto, I.; Corral-Jara, K.F.; Ajdžanović, V.; Arola-Arnal, A.; Bravo, F.I.; Deligiannidou, G.-E.; Havlik, J.; Janeva, M.; Kistanova, E.; et al. Systematic Bioinformatic Analyses of Nutrigenomic Modifications by Polyphenols Associated with Cardiometabolic Health in Humans—Evidence from Targeted Nutrigenomic Studies. Nutrients 2021, 13, 2326. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Jager, G.; Van Zyl, H.; Voss, H.-P.; Pintado, M.; Hogg, T.; De Graaf, C. Cheers, Proost, Saúde: Cultural, Contextual and Psychological Factors of Wine and Beer Consumption in Portugal and in the Netherlands. Crit. Rev. Food Sci. Nutr. 2017, 57, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.L.; Jensen, H.H.; Sanders, S.A.; Reinisch, J.M. Better Psychological Functioning and Higher Social Status May Largely Explain the Apparent Health Benefits of Wine: A Study of Wine and Beer Drinking in Young Danish Adults. Arch. Intern. Med. 2001, 161, 1844–1848. [Google Scholar] [CrossRef] [Green Version]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.J. Wine as a Functional Food: Chemical, Sensory, and Consumer Perception Considerations. Doctoral Thesis, Charles Sturt University, Wagga Wagga, NSW, Australia, 2013. [Google Scholar]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of Red Wine Polyphenols and Alcohol on Glucose Metabolism and the Lipid Profile: A Randomized Clinical Trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Petropulos, V.; Hermosín-Gutiérrez, I.; Boros, B.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Dörnyei, Á.; Kilár, F. Phenolic Compounds and Antioxidant Activity of Macedonian Red Wines. J. Food Compos. Anal. 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Influence of Enological Practices on the Antioxidant Activity of Wines. Food Chem. 2006, 95, 394–404. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol Content, Antioxidant Activity and Metal Composition of Croatian Wines Deriving from Organically and Conventionally Grown Grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Caruso, M.; Scarpa, T.; Palma, A. Analysis of Trace Elements in Southern Italian Wines and Their Classification According to Provenance. LWT Food Sci. Technol. 2008, 41, 1808–1815. [Google Scholar] [CrossRef]
- Tariba, B. Metals in Wine—Impact on Wine Quality and Health Outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- OIV Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine (OIV): Paris, France, 2018; Volume 1, Available online: https://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts (accessed on 29 April 2022).
- Official Gazette—Regulation on Wine Production, No 2. 2005. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2005_01_2_17.html (accessed on 29 April 2022).
- Official Gazette—Regulation on Toxins, Metals, Metalloids and Other Harmful Substances That Can Be Found in Food, No 16. 2005. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2005_02_16_283.html (accessed on 29 April 2022).
- Núñez, M.; Peña, R.M.; Herrero, C.; García-Martín, S. Analysis of Some Metals in Wine by Means of Capillary Electrophoresis. Application to the Differentiation of Ribeira Sacra Spanish Red Wines. Analusis 2000, 28, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.A.; Aslam, M.; Akbar, W.; Iqbal, Z. Biological Importance of Vitamins for Human Health: A Review. J. Agric. Basic Sci. 2018, 2, 50–58. [Google Scholar]
- Combs, G.F.; McClung, J.P. Chapter 1—What Is a Vitamin? In The Vitamins, 5th ed.; Combs, G.F., McClung, J.P., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 3–6. ISBN 978-0-12-802965-7. [Google Scholar]
- Perli, T.; Wronska, A.K.; Ortiz-Merino, R.A.; Pronk, J.T.; Daran, J.-M. Vitamin Requirements and Biosynthesis in Saccharomyces Cerevisiae. Yeast 2020, 37, 283–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña-Neira, A.; Hernández, T.; García-Vallejo, C.; Estrella, I.; Suarez, J.A. A Survey of Phenolic Compounds in Spanish Wines of Different Geographical Origin. Eur. Food Res. Technol. 2000, 210, 445–448. [Google Scholar] [CrossRef]
- Jackson, R.S. 6—Chemical Constituents of Grapes and Wine. In Wine Science, 5th ed.; Jackson, R.S., Ed.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2008; pp. 270–331. ISBN 978-0-12-373646-8. [Google Scholar]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of Winemaking Treatment and Wine Aging on Phenolic Content in Vranec Wines. J. Food Sci. Technol. 2012, 49, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.C.; Jackson, L.S.M.; Berke-Schlessel, D.W.; Sumpio, B.E. The Cardiovascular Protective Effect of Red Wine. J. Am. Coll. Surg. 2005, 200, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Piljac, J.; Martinez, S.; Valek, L.; Stipčević, T.; Kovačević Ganić, K. A Comparison of Methods Used to Define the Phenolic Content and Antioxidant Activity of Croatian Wines. Food Technol. Biotechnol. 2005, 43, 271–276. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant Capacity of Vegetables, Spices and Dressings Relevant to Nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Mazor Jolić, S.; Radojčić Redovniković, I.; Marković, K.; Ivanec Šipušić, Đ.; Delonga, K. Changes of Phenolic Compounds and Antioxidant Capacity in Cocoa Beans Processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD–MS/MS Characterization of Phenolic Compounds in White Wine Stored without Added Sulfite. Food Res. Int. 2014, 66, 207–215. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulic, E.; Horvat, I.; Plavsa, T.; Lukic, I.; Bubola, M.; Ganic, K.K.; Curko, N.; Korenika, A.-M.J.; Radeka, S. Comparison of Different Winemaking Processes for Improvement of Phenolic Composition, Macro- and Microelemental Content, and Taste Sensory Attributes of Teran (Vitis Vinifera L.) Red Wines. LWT Food Sci. Technol. 2022, 154, 112619. [Google Scholar] [CrossRef]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of Seed and Skin Polyphenolic Extracts of Two Red Grape Cultivars Grown in Croatia and Their Sensory Perception in a Wine Model Medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
- Mark, L.; Nikfardjam, M.S.P.; Avar, P.; Ohmacht, R. A Validated HPLC Method for the Quantitative Analysis of Trans-Resveratrol and Trans-Piceid in Hungarian Wines. J. Chromatogr. Sci. 2005, 43, 445–449. [Google Scholar] [CrossRef]
- OIV Compendium of International Methods of Wine and Must Analysis, Vol. 1., Paris: International Organisation of Vine and Wine—Determination of Nine Major Anthocyanins in Red and Rosé Wines Using HPLC (Oeno 22/2003, Oeno 12/2007) (OIV). 2018. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts (accessed on 29 April 2022).
- Larcher, R.; Nicolini, G. Survey of 22 Mineral Elements in Wines from Trentino (Italy) Using ICP-OES [Inductively Coupled Plasma—Optical Emission Spectrometry]. Ital. J. Food Sci. 2001, 13, 233–241. [Google Scholar]
- Leder, R. Chemometric Prediction of the Geographical Origin of Croatian Wines through Their Elemental Profiles. J. Food Nutr. Res. 2015, 54, 229–238. [Google Scholar]
- Trang, H.K. Development of HPLC Methods for the Determination of Water-Soluble Vitamins in Pharmaceuticals and Fortified Food Products. Master’s Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- Tomašević, M.; Gracin, L.; Ćurko, N.; Kovačević Ganić, K. Impact of Pre-Fermentative Maceration and Yeast Strain along with Glutathione and SO2 Additions on the Aroma of Vitis Vinifera L. Pošip Wine and Its Evaluation during Bottle Aging. LWT Food Sci. Technol. 2017, 81, 67–76. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between Antioxidant Capacity and Total Phenolic Content of Red, Rosé and White Wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Rastija, V.; Srečnik, G.; Medić-Šarić, M. Polyphenolic Composition of Croatian Wines with Different Geographical Origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Saliba, A.J.; Prenzler, P.D.; Ryan, D. Total Phenolic Content, Antioxidant Activity, and Cross-Cultural Consumer Rejection Threshold in White and Red Wines Functionally Enhanced with Catechin-Rich Extracts. J. Agric. Food Chem. 2012, 60, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Budić-Leto, I.; Gracin, L.; Lovrić, T.; Vrhovsek, U. Effects of Maceration Conditions on the Polyphenolic Composition of Red Wine Plavac Mali. VITIS 2008, 47, 245–250. [Google Scholar]
- Plavša, T.; Jurinjak, N.; Antunović, D.; Peršurić, Đ.; Kovačević Ganić, K. The Influence of Skin Maceration Time on the Phenolic Composition and Antioxidant Activity of Red Wine Teran (Vitis Vinifera L.). Food Technol. Biotechnol. 2012, 50, 152–158. [Google Scholar]
- Lukic, I.; Radeka, S.; Budic-Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS Profiling of Phenolic Compounds for Differentiation of Monovarietal Wines and Corroboration of Particular Varietal Typicity Concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, A.; Majek, P.; Lynen, F.; Crouch, A.; Lauer, H.; Sandra, P. Classification of South African Red and White Wines According to Grape Variety Based on the Non-Coloured Phenolic Content. Eur. Food Res. Technol. 2005, 221, 520–528. [Google Scholar] [CrossRef]
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant Capacity and Phenolic Composition of Red Wines from Various Grape Varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Ilak Peršurić, A.S.; Jeromel, A.; Radeka, S. Comparison of Different Maceration and Non-Maceration Treatments for Enhancement of Phenolic Composition, Colour Intensity, and Taste Attributes of Malvazija Istarska (Vitis Vinifera L.) White Wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Pérez-Trujillo, J.P.; Hernández, Z.; López-Bellido, F.J.; Hermosín-Gutiérrez, I. Characteristic Phenolic Composition of Single-Cultivar Red Wines of the Canary Islands (Spain). J. Agric. Food Chem. 2011, 59, 6150–6164. [Google Scholar] [CrossRef]
- Karataş, D.; AydinF; Aydin, I.; Karataş, H. Elemental Composition of Red Wines in Southeast Turkey. Czech J. Food Sci. 2015, 33, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 978-1-119-58776-7. [Google Scholar]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The Roles of Iron in Health and Disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Velić, D.; Velić, N.; Amidžić Klarić, D.; Klarić, I.; Petravić Tominac, V.; Košmerl, T.; Vidrih, R. The Production of Fruit Wines—A Review. Croat. J. Food Sci. Technol. 2018, 10, 279–290. [Google Scholar] [CrossRef]
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Alexandre, H. Vitamins in Wine: Which, What for, and How Much? Compr. Rev. Food Sci. Food Saf. 2021, 20, 2991–3035. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Peinado, R. (Eds.) Chapter 4—Composition of Wine. In Enological Chemistry; Academic Press: San Diego, CA, USA, 2012; pp. 41–52. ISBN 978-0-12-388438-1. [Google Scholar]
- Hall, A.P.; Brinner, L.; Amerine, M.A.; Morgan, A.F. The B Vitamin Content of Grapes, Musts, and Wines. J. Food Sci. 1956, 21, 362–371. [Google Scholar] [CrossRef]
- Moreno, P.; Salvadó, V. Determination of Eight Water- and Fat-Soluble Vitamins in Multi-Vitamin Pharmaceutical Formulations by High-Performance Liquid Chromatography. J. Chromatogr. A 2000, 870, 207–215. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martínez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized Red Wine Decreases Systolic and Diastolic Blood Pressure and Increases Plasma Nitric Oxide. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilford, J.M.; Pezzuto, J.M. Wine and Health: A Review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, G.N.; Dominitz, J.A.; Weiss, N.S.; Heagerty, P.J.; Kowdley, K.V. The Effect of Alcohol Consumption on the Prevalence of Iron Overload, Iron Deficiency, and Iron Deficiency Anemia. Gastroenterology 2004, 126, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.T. Managing Genetic Hemochromatosis: An Overview of Dietary Measures, Which May Reduce Intestinal Iron Absorption in Persons With Iron Overload. Gastroenterol. Res. 2021, 14, 66–80. [Google Scholar] [CrossRef]
- Shirreffs, S.M.; Maughan, R.J. Restoration of Fluid Balance after Exercise-Induced Dehydration: Effects of Alcohol Consumption. J. Appl. Physiol. 1997, 83, 1152–1158. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective Effect of Red Wine and Grape Pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef] [PubMed]
- Dfarhud, D.; Malmir, M.; Khanahmadi, M. Happiness & Health: The Biological Factors- Systematic Review Article. Iran. J. Public Health 2014, 43, 1468–1477. [Google Scholar] [PubMed]
- Ashby, F.G.; Isen, A.M.; Turken, A.U. A Neuropsychological Theory of Positive Affect and Its Influence on Cognition. Psychol. Rev. 1999, 106, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.L.C.; Phillips, L.H. The Psychological, Neurochemical and Functional Neuroanatomical Mediators of the Effects of Positive and Negative Mood on Executive Functions. Neuropsychologia 2007, 45, 617–629. [Google Scholar] [CrossRef]
- Hashemi, P.; Dankoski, E.C.; Lama, R.; Wood, K.M.; Takmakov, P.; Wightman, R.M. Brain Dopamine and Serotonin Differ in Regulation and Its Consequences. Proc. Natl. Acad. Sci. USA 2012, 109, 11510–11515. [Google Scholar] [CrossRef] [Green Version]
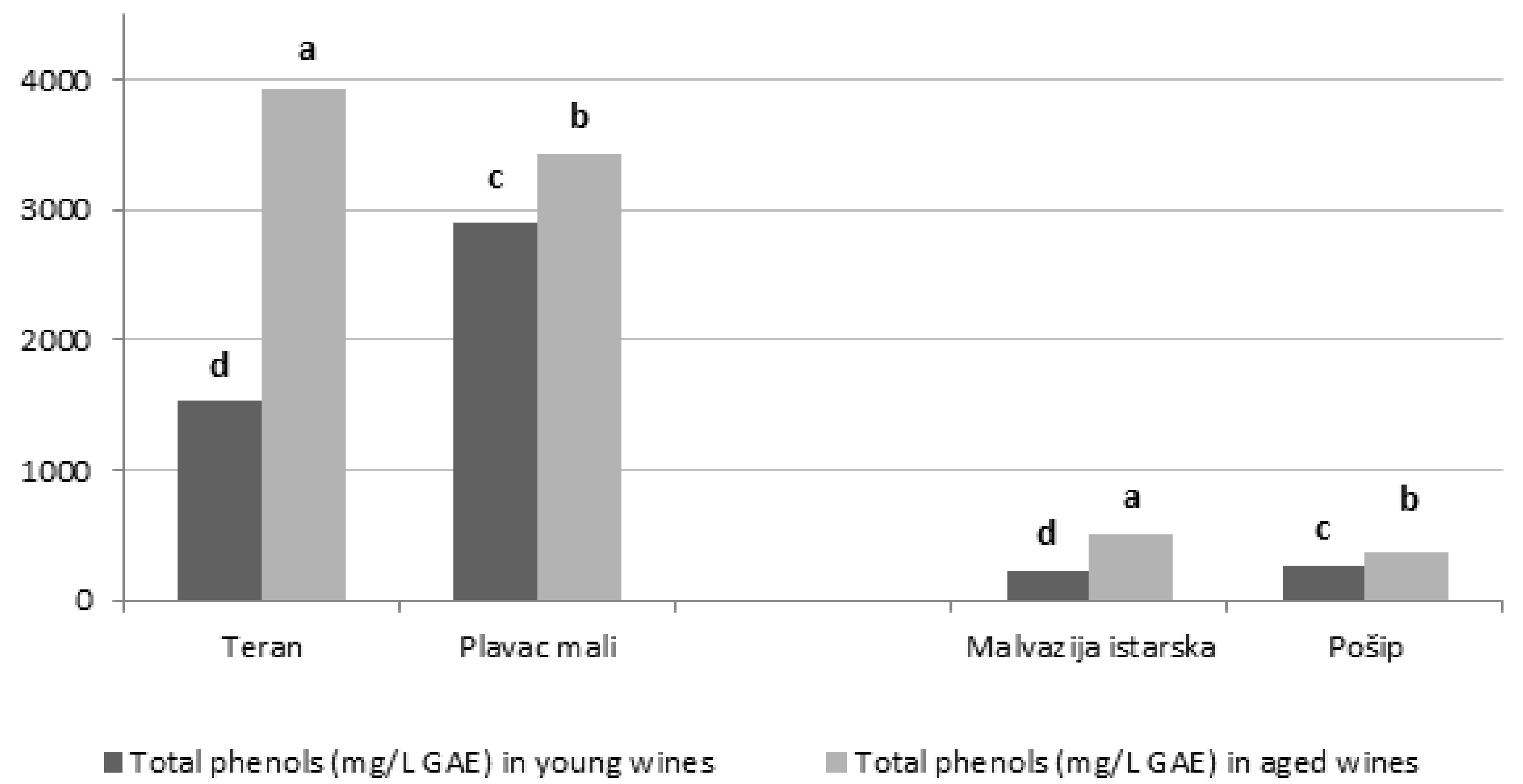
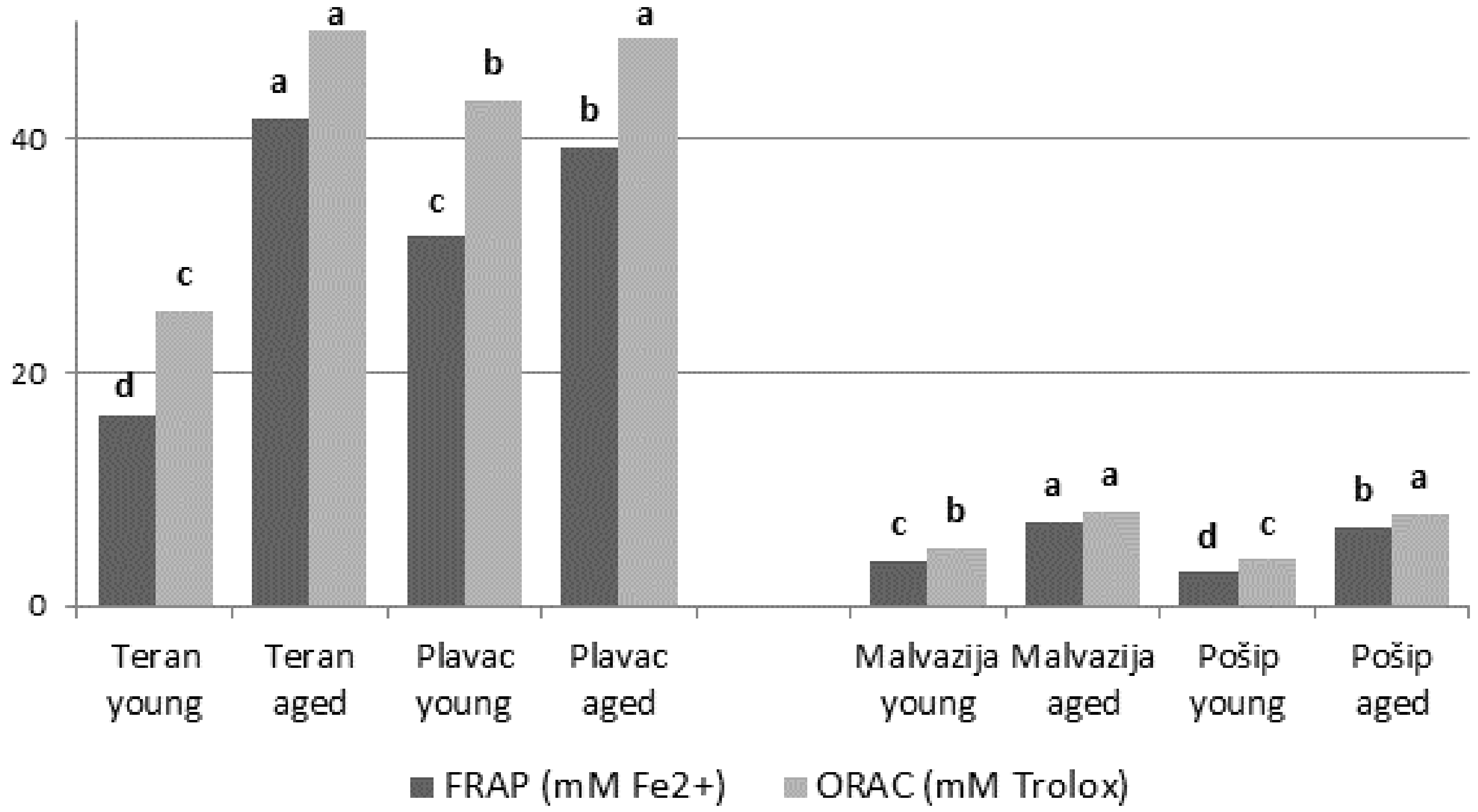
| Phenols | Malvazija Istarska | Pošip | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| Phenolic acids, flavonols, and flavanonols | ||||
| Gallic acid | 28.53 ± 0.01 b | 4.64 ± 0.02 c | 3.08 ± 0.01 d | 30.93 ± 0.03 a |
| Protocatechuic acid | 0.64 ± 0.02 c | 4.56 ± 0.05 a | 1.02 ± 0.17 b | 1.03 ± 0.04 b |
| p-Hydroxybenzoic acid | 0.67 ± 0.01 b | 1.48 ± 0.05 a | 0.29 ± 0.01 c | 0.23 ± 0.01 d |
| Syringic acid | 0.43 ± 0.00 a | 0.16 ± 0.00 c | 0.14 ± 0.01 d | 0.28 ± 0.00 b |
| cis-Caftaric acid | 0.71 ± 0.00 a | 0.35 ± 0.00 c | 0.50 ± 0.00 b | 0.32 ± 0.00 d |
| trans-Caftaric acid | 7.17 ± 0.00 d | 45.92 ± 0.12 b | 21.07 ± 0.01 c | 46.70 ± 0.04 a |
| Caffeic acid | 11.71 ± 0.00 a | 8.13 ± 0.04 c | 1.22 ± 0.01 d | 10.61 ± 0.01 b |
| p-Coumaric acid | 1.51 ± 0.00 d | 2.79 ± 0.00 b | 1.79 ± 0.00 c | 3.91 ± 0.02 a |
| Ferulic acid | 0.95 ± 0.00 a | n.d. | 0.54 ± 0.00 c | 0.79 ± 0.01 b |
| Taxifolin | 0.38 ± 0.00 d | 0.50 ± 0.00 c | 0.90 ± 0.01 a | 0.70 ± 0.01 b |
| Quercetin | 0.17 ± 0.00 d | 0.78 ± 0.04 c | 2.37 ± 0.01 a | 1.21 ± 0.00 b |
| Total phenolic acids, flavonols, and flavanonols | 52.88 ± 0.03 c | 69.33 ± 0.17 b | 32.92 ± 0.17 d | 96.71 ± 0.11 a |
| Flavan-3-ols | ||||
| Procyanidin B1 | 0.56 ± 0.07 c | 0.98 ± 0.09 b | 1.89 ± 0.19 a | 0.94 ± 0.27 b |
| Procyanidin B3 | 3.60 ± 0.01 b | 1.14 ± 0.07 d | 4.68 ± 0.03 a | 1.58 ± 0.07 c |
| (+)-Catechin | 2.96 ± 0.32 d | 5.06 ± 0.10 c | 8.08 ± 0.30 a | 6.16 ± 0.79 b |
| Procyanidin B2 | 0.38 ± 0.05 c | 0.53 ± 0.06 b | 0.96 ± 0.08 a | 0.50 ± 0.08 bc |
| (−)-Epicatechin | 3.40 ± 0.26 a | 0.99 ± 0.01 c | 3.61 ± 0.09 a | 2.64 ± 0.21 b |
| Procyanidin C1 | 0.36 ± 0.01 b | 0.35 ± 0.01 b | 0.29 ± 0.01 c | 0.42 ± 0.01 a |
| Total flavan-3-ols | 11.26 ± 0.66 b | 9.05 ± 0.32 c | 19.52 ± 0.69 a | 12.24 ± 1.38 b |
| Stilbenes | ||||
| trans-Piceid | n.d. | n.d. | 0.35 ± 0.08 b | 0.85 ± 0.08 a |
| Piceatannol | 0.09 ± 0.01 ab | 0.08 ± 0.00 b | 0.10 ± 0.02 a | 0.06 ± 0.00 c |
| Resveratrol | 0.12 ± 0.00 b | 0.12 ± 0.00 b | 0.12 ± 0.01 b | 0.17 ± 0.00 a |
| cis-Piceid | 0.18 ± 0.00 c | 0.30 ± 0.02 b | 0.39 ± 0.03 a | 0.40 ± 0.01 a |
| Total stilbenes | 0.39 ± 0.01 c | 0.51 ± 0.02 c | 0.96 ± 0.14 b | 1.48 ± 0.08 a |
| Total HPLC phenols | 64.54 ± 0.63 c | 78.89 ± 0.22 b | 53.4 ± 0.75 d | 110.42 ± 1.44 a |
| Phenols | Teran | Plavac Mali | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| Phenolic acids, flavonols, and flavanonols | ||||
| Gallic acid | 42.69 ± 0.05 d | 157.92 ± 0.31 a | 52.58 ± 0.07 c | 94.44 ± 0.11 b |
| Protocatechuic acid | 5.16 ± 0.09 c | 7.37 ± 0.07 b | 5.27 ± 0.18 c | 7.75 ± 0.29 a |
| p-Hydroxybenzoic acid | 1.26 ± 0.02 c | 1.68 ± 0.05 b | 1.28 ± 0.01 c | 2.05 ± 0.04 a |
| Syringic acid | 10.83 ± 0.02 a | 9.16 ± 0.01 b | 6.00 ± 0.05 d | 7.43 ± 0.05 c |
| cis-Caftaric acid | 0.46 ± 0.00 a | 0.29 ± 0.01 c | 0.41 ± 0.00 b | n.d. |
| trans-Caftaric acid | 55.95 ± 0.04 b | 61.79 ± 0.10 a | 8.13 ± 0.01 d | 26.18 ± 0.03 c |
| Caffeic acid | 3.29 ± 0.00 d | 4.24 ± 0.06 c | 8.80 ± 0.00 a | 6.72 ± 0.01 b |
| p-Coumaric acid | 3.33 ± 0.06 b | 2.04 ± 0.03 d | 2.81 ± 0.02 c | 5.17 ± 0.04 a |
| Ferulic acid | 0.39 ± 0.00 c | 0.37 ± 0.01 c | 1.01 ± 0.05 a | 0.54 ± 0.03 b |
| Taxifolin | 0.56 ± 0.01 c | 0.38 ± 0.01 d | 1.74 ± 0.01 b | 3.15 ± 0.02 a |
| Quercetin 3-glucoside + Quercetin 3-glucuronide | n.d. | 0.39 ± 0.01 b | 7.02 ± 0.24 a | 0.52 ± 0.01 b |
| Myricetin | 4.35 ± 0.09 b | 2.67 ± 0.03 d | 2.86 ± 0.00 c | 5.36 ± 0.00 a |
| Quercetin | 19.74 ± 0.04 c | 35.21 ± 0.45 a | 20.81 ± 0.17 b | 19.11 ± 0.13 d |
| Total phenolic acids, flavonols, and flavanonols | 148.00 ± 0.29 c | 283.53 ± 0.75 a | 118.72 ± 0.37 d | 178.41 ± 0.26 b |
| Flavan-3-ols | ||||
| Procyanidin B1 | 10.68 ± 0.01 d | 40.49 ± 0.35 a | 29.41 ± 0.01 b | 27.70 ± 0.06 c |
| Procyanidin B3 | 4.53 ± 0.01 c | 14.51 ± 0.23 a | 7.99 ± 0.01 b | 7.77 ± 0.08 b |
| (+)-Catechin | 23.52 ± 0.01 d | 44.30 ± 0.32 a | 26.80 ± 0.05 c | 32.18 ± 0.12 b |
| Procyanidin B2 | 11.91 ± 0.01 d | 34.32 ± 0.32 a | 18.03 ± 0.11 b | 17.39 ± 0.05 c |
| (−)-Epicatechin | 18.40 ± 0.02 b | 27.7 ± 0.29 a | 13.83 ± 0.05 d | 14.69 ± 0.04 c |
| Procyanidin C1 | 1.37 ± 0.03 d | 7.44 ± 0.14 a | 4.60 ± 0.02 b | 3.03 ± 0.04 c |
| Total flavan-3-ols | 70.41 ± 0.01 d | 168.75 ± 1.38 a | 100.66 ± 0.17 c | 102.76 ± 1.22 b |
| Stilbenes | ||||
| trans-Piceid | 4.42 ± 0.02 d | 8.98 ± 0.02 b | 5.68 ± 0.03 c | 9.05 ± 0.03 a |
| Piceatannol | 0.17 ± 0.00 b | 0.14 ± 0.01 c | 0.06 ± 0.00 d | 0.19 ± 0.00 a |
| Resveratrol | 0.72 ± 0.00 b | 0.45 ± 0.01 c | 0.69 ± 0.02 b | 0.82 ± 0.00 a |
| cis-Piceid | 2.97 ± 0.01 d | 5.58 ± 0.04 a | 4.23 ± 0.01 b | 3.46 ± 0.04 c |
| Total stilbenes | 8.28 ± 0.04 d | 15.15 ± 0.06 a | 10.66 ± 0.06 c | 13.52 ± 0.07 b |
| Anthocyanins | ||||
| Delphinidin 3-glucoside | 0.52 ± 0.01 d | 1.15 ± 0.02 b | 1.55 ± 0.02 a | 1.03 ± 0.00 c |
| Cyanidin 3-glucoside | 0.08 ± 0.00 d | 0.21 ± 0.01 a | 0.17 ± 0.00 b | 0.11 ± 0.00 c |
| Petunidin 3-glucoside | 0.54 ± 0.01 d | 0.93 ± 0.02 b | 1.63 ± 0.02 a | 0.79 ± 0.00 c |
| Pelargonidin 3-glucoside | 0.15 ± 0.01 a | 0.09 ± 0.00 b | 0.16 ± 0.00 a | 0.07 ± 0.00 c |
| Peonidin 3-glucoside | 0.50 ± 0.01 d | 0.84 ± 0.02 b | 1.70 ± 0.01 a | 0.58 ± 0.00 c |
| Malvidin 3-glucoside | 9.09 ± 0.14 c | 6.54 ± 0.15 d | 26.30 ± 0.14 a | 10.26 ± 0.04 b |
| Peonidin 3-acetylglucoside | 0.17 ± 0.01 c | 0.37 ± 0.03 a | 0.36 ± 0.01 a | 0.25 ± 0.00 b |
| Malvidin 3-acetylglucoside | 1.26 ± 0.02 c | 1.10 ± 0.02 d | 1.75 ± 0.01 a | 1.30 ± 0.01 b |
| Peonidin 3-cumarylglucoside | 0.07 ± 0.01 c | 0.11 ± 0.01 b | 0.20 ± 0.01 a | 0.11 ± 0.00 b |
| Malvidin 3-cumarylglucoside | 0.93 ± 0.04 c | 0.82 ± 0.03 d | 1.82 ± 0.05 a | 1.65 ± 0.01 b |
| Total anthocyanins | 13.31 ± 0.24 c | 12.15 ± 0.30 d | 35.64 ± 0.25 a | 16.15 ± 0.05 b |
| Total HPLC phenols | 240.00 ± 0.42 d | 479.58 ± 0.67 a | 265.67 ± 0.53 c | 310.84 ± 0.39 b |
| Macro- and Microelements (mg/L) | Malvazija Istarska | Pošip | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| K | 888.0 ± 1.73 b | 778.37 ± 1.66 d | 894.1 ± 2.70 a | 785.87 ± 1.86 c |
| Ca | 71.28 ± 0.84 b | 62.76 ± 0.58 d | 82.42 ± 0.43 a | 70.11 ± 0.1 c |
| Mg | 82.4 ± 0.20 d | 93.6 ± 0.20 c | 95.27 ± 0.35 b | 98.47 ± 0.25 a |
| Na | 39.68 ± 0.05 a | 37.56 ± 0.05 c | 38.32 ± 0.08 b | 36.62 ± 0.51 d |
| Total macroelements (mg/L) | 1081.36 ± 2.14 b | 972.29 ± 2.37 d | 1110.1 ± 2.85 a | 991.07 ± 1.22 c |
| Al | 1.27 ± 0.03 a | 1.04 ± 0.01 c | 1.30 ± 0.02 a | 1.11 ± 0.02 b |
| Cu | 0.040 ± 0.001 b | 0.035 ± 0.001 c | 0.042 ± 0.002 a | 0.040 ± 0.001 b |
| Fe | 1.88 ± 0.02 a | 1.54 ± 0.01 c | 1.81 ± 0.01 b | 1.27 ± 0.02 d |
| Mn | 0.737 ± 0.002 b | 0.631 ± 0.001 c | 0.797 ± 0.001 a | 0.614 ± 0.001 d |
| Total microelements (mg/L) | 3.93 ± 0.01 a | 3.25 ± 0.02 b | 3.95 ± 0.03 a | 3.04 ± 0.03 c |
| Macro- and Microelements (mg/L) | Teran | Plavac Mali | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| K | 864.83 ± 0.8 b | 867.24 ± 1.61 ab | 869.73 ± 2.53 a | 869.03 ± 1.81 a |
| Ca | 165.23 ± 1.08 a | 160.4 ± 1.08 b | 142.3 ± 1.15 c | 142.4 ± 0.61 c |
| Mg | 119.07 ± 1.01 b | 121.33 ± 0.70 a | 114.67 ± 0.60 c | 115.07 ± 1.06 c |
| Na | 7.56 ± 0.01 b | 7.38 ± 0.01 a | 7.12 ± 0.01 c | 7.1 ± 0.01 d |
| Total macroelements (mg/L) | 1156.69 ± 2.87 a | 1156.35 ± 3.38 a | 1133.82 ± 4.25 b | 1133.61 ± 2.94 b |
| Al | 2.28 ± 0.02 a | 2.07 ± 0.01 b | 1.96 ± 0.01 c | 1.78 ± 0.01 d |
| Cu | 0.058 ± 0.002 d | 0.062 ± 0.001 c | 0.074 ± 0.002 a | 0.067 ± 0.001 b |
| Fe | 9.99 ± 0.01 a | 9.55 ± 0.01 b | 8.45 ± 0.01 c | 8.28 ± 0.01 d |
| Mn | 1.50 ± 0.01 a | 1.48 ± 0.01 b | 1.34 ± 0.00 c | 1.32 ± 0.02 c |
| Total microelements (mg/L) | 13.83 ± 0.03 a | 13.16 ± 0.02 b | 11.82 ± 0.02 c | 11.45 ± 0.02 d |
| Vitamins (μg/L) | Malvazija Istarska | Pošip | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| Vitamin C | n.d. | n.d. | n.d. | n.d. |
| Vitamin B1 | 4.23 ± 0.21 a | 2.10 ± 0.00 c | 3.80 ± 0.00 b | 1.90 ± 0.00 d |
| Vitamin B2 | 62.93 ± 0.25 a | 31.43 ± 0.49 c | 62.10 ± 0.10 b | 29.53 ± 0.45 d |
| Vitamin B3 | 501.43 ± 5.26 a | 383.57 ± 4.00 c | 485.23 ± 2.95 b | 277.73 ± 2.70 d |
| Vitamin B6 | 165.73 ± 2.55 a | 104.3 ± 2.23 c | 157.6 ± 2.51 b | 96.47 ± 1.50 d |
| Total vitamins (μg/L) | 734.33 ± 2.77 a | 521.4 ± 6.64 c | 708.74 ± 5.55 b | 405.63 ± 1.66 d |
| Vitamins (μg/L) | Teran | Plavac Mali | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| Vitamin C | n.d. | n.d. | n.d. | n.d. |
| Vitamin B1 | 25.47 ± 0.70 a | 16.00 ± 0.20 b | 13.98 ± 0.25 c | 10.15 ± 0.90 d |
| Vitamin B2 | 329.13 ± 1.95 a | 217.27 ± 4.25 b | 221.5 ± 1.67 b | 127.83 ± 2.52 c |
| Vitamin B3 | 807.73 ± 4.08 a | 643.37 ± 3.85 b | 597.2 ± 2.67 c | 306.2 ± 5.71 d |
| Vitamin B6 | 349.5 ± 2.05 a | 227.57 ± 2.73 c | 239.3 ± 0.95 b | 147.1 ± 3.68 d |
| Total vitamins (μg/L) | 1511.83 ± 8.76 a | 1071.98 ± 0.2 c | 1104.2 ± 10.9 b | 591.28 ± 7.77 d |
| Medical Examination Parameters | Non-Consumer Control Group | Consumer Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Malvazija Istarska | Pošip | Teran | Plavac Mali | |||||||
| Young | Aged | Young | Aged | Young | Aged | Young | Aged | |||
| Weight (kg) | −0.58 ± 1.67 bc | 0.66 ± 0.91 ab | −0.06 ± 0.76 abc | −1.03 ± 1.81 c | 1.0 ± 1.3 a | 0.01 ± 1.28 abc | 0.27 ± 1.92 abc | 0.01 ± 1.43 abc | −0.4 ± 0.64 abc | |
| Body mass index BMI (kg/m2) | −0.2 ± 0.6 ab | 0.31 ± 0.37 a | 0.15 ± 0.45 ab | −0.36 ± 0.72 ab | 0.32 ± 0.38 a | 0.07 ± 0.6 ab | 0.14 ± 0.62 ab | −0.06 ± 0.53 ab | −0.17 ± 0.37 ab | |
| Waistline (cm) | −2.6 ± 6.47 bd | 5.75 ± 5.6 ab | 4.0 ± 5.1 abc | 7.86 ± 4.14 a | 3.29 ± 3.64 abc | −0.57 ± 5.74 bc | 2.33 ± 6.44 abc | 0.71 ± 6.29 b | 1.29 ± 6.7 b | |
| Hip width (cm) | 1.3 ± 3.65 | 1.25 ± 2.76 | 0.71 ± 3.45 | 3.71 ± 5.38 | 1.14 ± 3.24 | 0.86 ± 2.19 | 2.0 ± 3.41 | 1.71 ± 1.8 | 0.57 ± 2.57 | n.s. |
| Heart Rate/min | −3.1 ± 9.17 | −1.5 ± 8.26 | −1.43 ± 9.29 | 4.57 ± 12.26 | −1.43 ± 9.14 | −1.43 ± 10.05 | −2.0 ± 4.2 | 1.43 ± 4.58 | 0.29 ± 10.16 | n.s. |
| RR Systolic mmHg | −1.5 ± 14.35 ab | 6.25 ± 11.88 a | 2.14 ± 5.67 ab | 1.43 ± 9.88 ab | −2.86 ± 6.99 ab | −0.71 ± 10.58 ab | 8.33 ± 7.53 a | 2.14 ± 7.56 ab | −6.43 ± 14.06 b | |
| RR Diastolic mmHg | −2 ± 11.1 | 0.63 ± 7.76 | 0.71 ± 5.35 | −2.14 ± 4.88 | −1.43 ± 8.02 | −0.71 ± 4.5 | −0.83 ± 8.01 | 1.423 ± 6.27 | −3.57 ± 6.9 | n.s. |
| Blood Parameters | Non-Consumer Control Group | Consumer Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Malvazija Istarska | Pošip | Teran | Plavac Mali | ||||||
| Young | Aged | Young | Aged | Young | Aged | Young | Aged | ||
| Erythrocytes [1 × 1012]/L | −0.03 ± 0.22 | −0.13 ± 0.13 | 0.02 ± 0.23 | −0.01 ± 0.12 | 0.07 ± 0.13 | −0.08 ± 0.25 | 0.08 ± 0.17 | 0.02 ± 0.15 | −0.08 ± 0.25 |
| Hemoglobin (g/L) | −1.2 ± 5.22 ab | −3.13 ± 3.23 ab | 0.71 ± 7.61 ab | −1.57 ± 4.76 ab | 2.0 ± 4.16 a | −4.14 ± 7.29 b | 1.83 ± 4.26 ab | 0.14 ± 4.53 ab | −1.86 ± 7.1 ab |
| RDW (%) | 0.03 ± 0.3 b | 0.06 ± 0.23 b | 0.04 ± 0.37 b | −0.09 ± 0.28 b | −0.03 ± 0.26 b | 0.07 ± 0.53 b | 0.62 ± 1.16 a | 0.1 ± 0.31 ab | 0.09 ± 0.43 b |
| Thrombocytes ([1 × 109]/L) | −10.9 ± 13.2 bc | −4.6 ± 15.57 abc | 7.14 ± 18.76 ab | 5.86 ± 18.74 ab | −2.86 ± 22.56 abc | 12.0 ± 26.7 a | −17.83 ± 27.37 c | −3.0 ± 14.8 abc | 1.29 ± 16.45 abc |
| MPV (fL) | 0.08 ± 0.24 a | 0 ± 0.33 a | 0.07 ± 0.29 a | −0.36 ± 0.37 b | −0.04 ± 0.4 ab | −0.03 ± 0.33 ab | 0.22 ± 0.27 a | 0.19 ± 0.16 a | 0.2 ± 0.34 a |
| Leukocytes ([1 × 109]/L) | −0.22 ± 0.72 b | 0.48 ± 0.74 ab | 0.39 ± 1.32 ab | 0.63 ± 0.77 a | −0.07 ± 0.78 ab | −0.13 ± 0.75 ab | −0.13 ± 0.41 ab | 0.21 ± 0.4 ab | 0.06 ± 0.75 ab |
| Glucose (mmol/L) | −0.16 ± 0.33 | −0.29 ± 0.36 | −0.26 ± 0.61 | 0.1 ± 0.44 | 0.06 ± 0.4 | −0.3 ± 0.61 | −0.32 ± 0.38 | 0.13 ± 0.36 | −0.3 ± 0.8 |
| Urea (mmol/L) | −0.35 ± 1.05 b | 0.88 ± 1.06 a | 0.49 ± 0.53 ab | −0.19 ± 1.02 b | 0.56 ± 1.42 ab | −0.18 ± 0.71 b | −0.02 ± 1.07 ab | 1.02 ± 1.01 a | 0.35 ± 0.81 ab |
| Creatinine (µmol/L) | 3.3 ± 5.6 ab | 3.25 ± 4.65 ab | 8.14 ± 4.14 a | 4.29 ± 6.87 ab | 3.43 ± 5.32 ab | 3.23 ± 7.43 ab | 1.0 ± 8.3 b | 5.29 ± 3.40 ab | 2.57 ± 6.02 ab |
| eGFR CKD-EPI | −4.3 ± 8.06 | −4.25 ± 4.65 | −8.29 ± 5.38 | −5.43 ± 8.42 | −3.57 ± 6.24 | −4.43 ± 9.38 | −1.17 ± 11.48 | −5.0 ± 3.83 | −2.87 ± 6.47 |
| Na (mmol/L) | 0.0 ± 1.94 b | 0.75 ± 1.75 ab | 0.29 ± 1.98 ab | 1.0 ± 2.65 ab | 1.29 ± 2.29 ab | 1.71 ± 3.3 ab | −0.17 ± 1.94 b | 2.5 ± 1.81 a | 1.43 ± 1.62 ab |
| K (mmol/L) | 0.02 ± 0.31 | −0.05 ± 0.27 | 0.14 ± 0.53 | −0.03 ± 0.4 | 0.16 ± 0.17 | 0.19 ± 0.33 | 0.02 ± 0.19 | 0.2 ± 0.23 | −0.04 ± 0.29 |
| Bilirubin (µmol/L) | −0.5 ± 4.72 | 0.25 ± 5.68 | 0.86 ± 4.38 | 0.57 ± 2.37 | −1.86 ± 3.24 | −1.29 ± 5.41 | 0.33 ± 2.94 | 0.0 ± 2.3 | −3.29 ± 6.99 |
| Uric acid (µmol/L) | 8.9 ± 39.04 a | −25 ± 23.11 b | 0.43 ± 18.08 ab | −0.29 ± 39.85 ab | 10.57 ± 33.29 a | −7.43 ± 43.06 ab | −4.12 ± 43.28 ab | −0.86 ± 30.09 ab | 0.86 ± 27.53 ab |
| AST (U/L) | 0.8 ± 3.36 | 0.75 ± 2.82 | −0.14 ± 4.1 | 0.14 ± 4.14 | 0.14 ± 1.95 | 0.29 ± 3.99 | 1.16 ± 1.17 | 2.0 ± 2.45 | −0.43 ± 2.3 |
| ALT (U/L) | 1.3 ± 11.94 b | 3.75 ± 2.76 b | 1.29 ± 7.25 b | 1.14 ± 5.46 b | 5.57 ± 3.41 ab | 0.57 ± 8.66 b | 4.0 ± 1.79 ab | 16.0 ± 29.82 a | 0.57 ± 3.82 b |
| ALP (U/L) | 0.3 ± 5.44 ab | 0.87 ± 3.94 ab | −3.0 ± 5.92 ab | 1.29 ± 4.11 ab | −0.57 ± 3.36 ab | −4.29 ± 9.41 b | 0.16 ± 5.95 ab | 0.43 ± 8.1 ab | 3.0 ± 7.16 a |
| GGT (U/L) | −0.9 ± 9.76 b | −0.5 ± 4.14 b | 2.0 ± 2.94 b | 0.15 ± 6.91 b | 2.43 ± 1.72 b | −0.29 ± 3.9 b | 2.33 ± 2.8 ab | 18.0 ± 40.89 a | 0.86 ± 1.77 b |
| Iron (µmol/L) | −1.8 ± 7.74 | −0.63 ± 3.85 | −0.86 ± 8.17 | −0.29 ± 2.69 | 0.71 ± 7.16 | 0.29 ± 4.79 | 1.83 ± 8.04 | 0.14 ± 4.45 | −4.57 ± 10.26 |
| UIBC (µmol/L) | 1.2 ± 7.48 | 1.63 ± 6.39 | 2.93 ± 7.32 | 0.57 ± 3.1 | 0.861 ± 8.51 | 0.57 ± 5.62 | −1.5 ± 11.45 | 1.43 ± 4.86 | 4.29 ± 8.48 |
| Feritin (µg/L) | −0.9 ± 11.43 | −0.88 ± 28.26 | −9.86 ± 26.31 | −6.14 ± 18.95 | −9.57 ± 14.18 | −19.71 ± 24.07 | −3.83 ± 16.67 | −14.0 ± 19.02 | −14.43 ± 20.98 |
| Cholesterol (mmol/L) | −0.09 ± 0.59 ab | −0.04 ± 0.7 ab | −0.19 ± 0.51 ab | −0.04 ± 0.43 ab | 0.37 ± 0.61 a | 0.23 ± 0.5 a | 0.23 ± 0.4 a | −0.06 ± 0.6 ab | −0.56 ± 0.65 b |
| HDL (mmol/L) | 0.04 ± 0.15 | 0.15 ± 0.19 | 0.11 ± 0.22 | 0.07 ± 0.11 | 0.14 ± 0.1 | 0.16 ± 0.17 | 0.2 ± 0.14 | 0.07 ± 0.11 | 0.06 ± 0.17 |
| LDL (mmol/L) | −0.15 ± 0.61 abc | −0.23 ± 0.5 abc | −0.34 ± 0.51 bc | −0.90 ± 0.51 abc | 0.24 ± 0.52 a | 0.06 ± 0.54 ab | −0.08 ± 0.33 abc | −0.2 ± 0.4 abc | −0.6 ± 0.5 c |
| Triglycerides (mmol/L) | 0.16 ± 0.94 | 0.125 ± 0.25 | 0.1143 ± 0.47 | −0.03 ± 0.66 | −0.01 ± 0.26 | 0.01 ± 0.45 | 0.25 ± 0.38 | 0.16 ± 0.79 | −0.06 ± 0.19 |
| hs-CRP (mg/L) | −0.62 ± 1.57 b | 0 ± 0.71 ab | −0.07 ± 0.24 ab | 0.36 ± 1.03 ab | −0.16 ± 0.42 b | −0.4 ± 0.68 b | 0.23 ± 0.7 ab | 1.63 ± 4.45 a | 0.04 ± 0.26 ab |
| SEROTONIN (ng/mL) | 2.2 ± 44.16 ab | 21.36 ± 115.12 ab | 35.86 ± 36.34 a | 12.14 ± 64.67 ab | −11.85 ± 23.6 ab | −8.29 ± 50.95 ab | −24.7 ± 29.6 ab | 16.57 ± 36.13 ab | −33.14 ± 59.34 b |
| DOPAMINE (ng/mL) | 0.51 ± 2.01 ab | 6.05 ± 9.17 a | −0.95 ± 3.17 b | 0.17 ± 0.47 ab | −0.69 ± 2.59 b | 0.22 ± 5.87 ab | 1.79 ± 11.05 ab | −2.81 ± 9.04 b | −0.84 ± 5.35 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeka, S.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Lukić, I.; Orbanić, F.; Zaninović Jurjević, T.; Dvornik, Š. Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health. Foods 2022, 11, 1804. https://doi.org/10.3390/foods11121804
Radeka S, Rossi S, Bestulić E, Budić-Leto I, Kovačević Ganić K, Horvat I, Lukić I, Orbanić F, Zaninović Jurjević T, Dvornik Š. Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health. Foods. 2022; 11(12):1804. https://doi.org/10.3390/foods11121804
Chicago/Turabian StyleRadeka, Sanja, Sara Rossi, Ena Bestulić, Irena Budić-Leto, Karin Kovačević Ganić, Ivana Horvat, Igor Lukić, Fumica Orbanić, Teodora Zaninović Jurjević, and Štefica Dvornik. 2022. "Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health" Foods 11, no. 12: 1804. https://doi.org/10.3390/foods11121804
APA StyleRadeka, S., Rossi, S., Bestulić, E., Budić-Leto, I., Kovačević Ganić, K., Horvat, I., Lukić, I., Orbanić, F., Zaninović Jurjević, T., & Dvornik, Š. (2022). Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health. Foods, 11(12), 1804. https://doi.org/10.3390/foods11121804











