Abstract
The development of early civilizations was greatly associated with populations’ ability to exploit natural resources. The development of methods for food preservation was one of the pillars for the economy of early societies. In Ecuador, food fermentation significantly contributed to social advances and fermented foods were considered exclusive to the elite or for religious ceremonies. With the advancement of the scientific research on bioprocesses, together with the implementation of novel sequencing tools for the accurate identification of microorganisms, potential health benefits and the formation of flavor and aroma compounds in fermented foods are progressively being described. This review focuses on describing traditional fermented foods from Ecuador, including cacao and coffee as well as less popular fermented foods. It is important to provide new knowledge associated with nutritional and health benefits of the traditional fermented foods.
Keywords:
traditional foods; microbial fermentation; cacao; coffee; chicha; champús; starter culture 1. Introduction
Food fermentation is being used in different parts of the world as a method for food preservation and to improve the sensory attributes of food. Early civilizations used fermentation as a method for food preservation of vegetables, fruits, and diverse types of meat. Food fermentation has prompted the development of new products, which in many cases contributed to the advancement of the social, political, economic, and cultural aspects of society [1]. The food fermentation process consists of a series of biochemical reactions that—when occurring correctly—can improve the food digestibility and bioavailability of nutrients [2], enhance the organoleptic characteristics [3] and increase the quality of foods [4,5]. In addition, fermented foods can improve gastrointestinal health [6,7], lower blood cholesterol and pressure levels, and have shown anti-carcinogenic and anti-inflammatory activity [8,9,10]. Furthermore, some fermented-food microorganisms are known to also promote gastrointestinal health and inhibit intestinal pathogens [11].
Food fermentation is a complex biochemical process, in which microorganisms play an important role for the development of sensorial attributes [12]. In general, microorganisms break down different substrates present in the food matrix into important compounds that add value to food products using a wide range of different enzymes [13]. Indeed, during the fermentation process, carbohydrates present in the raw food matrices are usually converted into ethanol and carbon dioxide by yeasts as well as some heterofermentative lactic acid bacteria, whereas acetic acid bacteria convert ethanol mainly into organic acids, such as acetic acid. All these chemical conversions usually cause a decrease in the pH of the matrix, which benefits food preservation [14].
There is a growing demand for healthy food products, including those resulting from fermentation processes [15]. In addition, the number of scientific, peer-reviewed papers about fermented foods has increased in 5.99% since 2014 (PubMed, 2021). Additionally, the interest in research about the benefits, production, and prospects of fermented foods is increasing over the years. Regarding market share, in 2018, the annual sales of yogurt was USD 110 million in the United Stated, representing a compound annual growth rate (CARG) of 6.3 % during 2022–2027 [16]. Similarly, the value of the global kombucha market was approximately USD 1.5 billion in 2018 [17] and the annual sales of the global vinegar market reached USD 2.27 billion in 2021 [18]. Similarly, the global kefir market size was 1.23 billion in 2019 and could increase to 1.84 billion by 2027 [19]. Therefore, the fermented foods market has been proposed as a tool to target sustainable development by the year 2030 and to fight hunger and malnutrition. To achieve this, legislation and research-based policies have been suggested as a mechanism to support the implementation of microbial biotechnology in industrial set-ups [20].
2. Fermented Foods from Ecuador
The spontaneous fermentation of food and beverages has traditionally been applied by many cultures in South America. In Ecuador, fermentation processes have influenced the local gastronomy with a significant number of fermented foods consumed for generations, including cacao, coffee, and fermented beverages, such as chicha, chaguarmishqui and champús. This review focuses on the microbiological composition of the most important Ecuadorian fermented foods.
2.1. Cacao
Fermented cacao beans are the main raw material used to produce chocolate. The high worldwide demand for chocolate contributed to the fact that 1.8 million hectares were dedicated to cacao cultivation in Latin America in 2019 [21], with Ecuador being the top fine-flavor cacao-producing country worldwide. Only in the first five months of 2021, Ecuadorian exports of fermented cacao reached USD 266.4 million. Recent genomic research and archeological evidence showed that the domestication of the Theobroma cacao plant potentially occurred in the humid Amazon forest [22].
Chocolate production starts with the cacao fermentation process. Cacao fermentation is mostly carried out by different types of microbial communities, including yeast, lactic acid bacteria (LAB), and acetic acid bacteria (AAB) in cacao beans [23]. Overall, yeast usually act at the beginning of the fermentation process to degrade carbohydrates, depolymerize pectin, and produce ethanol [24]. At these early stages, pH and temperature changes occur [25], so ethanol production is usually accompanied by an increase in temperature from 35 to 40 °C within 48 h from the beginning of the fermentation [26]. Saccharomyces, Hanseniaspora, and Pichia are usually the most abundant yeasts found at the beginning of the fermentation process, although they are found at a lower abundances at later stages of the cacao fermentation process [27,28,29,30].
During the initial phases of cacao fermentation, colonization by Enterobacteriaceae, such as Tatumella spp., allows the assimilation of citric acid and production of gluconic acid [31]. Then, LAB perform heterolactic or homolactic fermentation processes to produce lactic acid, acetic acid, ethanol, and mannitol [32,33]. The most common genera of LAB found during the cacao fermentation process are Lactiplantibacillus, Limosilactobacillus, Bacillus, Fructobacillus, Enterococcus, Leuconostoc, Streptococcus, and Weissella [27]. LAB are Gram-positive and anaerobic bacteria that play an important role in carbohydrate and ethanol degradation. Additionally, LABs are responsible for the production of certain secondary metabolites, such as diacetyl and acetoin. These compounds possess a butter-like and butter scotch flavor, and are widely used as safe flavoring agents and potentially antifungal compounds [34,35]. Additionally, phenolic acid and flavonoids could be converted into other aromatic precursors with synergistic and/or additive effects that might contribute to the overall inhibition of molds [36].
In the final stage of the fermentation process, AAB oxidize ethanol to acetic acid and eventually overoxidize the acetic acid into carbon dioxide. Although, the presence of Acetobacter spp. in the first stages of fermentation it is not very high, the process allows them to develop and then become very abundant later on [37]. AAB are Gram-negative and aerobic bacteria that can also promote the taste, texture, and smell of fermented products, such as cacao [38,39].
The quality of the fermented cacao beans depends on the cacao variety and various post-harvest processes. Additionally, cacao fermentation may yield batches with different quality as the microbial diversity of cacao can be influenced by the climatic region and farm practices [25,26]. Some studies have estimated various diversity indexes, such as Ace, Chao, Simpson, and Shannon, during cacao fermentation [40].
The diversity indexes have contributed to the analysis of fermented foods and have allowed an understanding of microbial ecosystems in the foods. Table 1 shows a variation in the species richness from different research on cacao and coffee bean fermentation [40]. Cacao and coffee are the most important fermented foods in Ecuador. For both products, microbial fermentation contributes to the hydrolyzation and removal of the pulp. The draining of the pulp facilitates the subsequent drying process. In addition, fermentation in both cacao and coffee contribute to the formation of precursors of aroma compounds.

Table 1.
Diversity indices of fermented cacao and coffee.
The values as Shannon’s index can be affected by initial microbiota, chemical composition of the beans, temperature, and interactions among microorganisms [41]. Shannon values for spontaneous fermentation between 2 and 3 are considered normal.
2.2. Coffee
The demand for coffee is also increasing worldwide. In 2018, global purchases of imported coffee increased to USD 32.9 billion, which represents a 9.4% increase when compared to the values of 2016 [43]. Coffee cultivation migrated through various parts of the world before reaching Latin America, where Brazil is currently the most important coffee exporting country. Although the benefits of coffee consumption are still under scrutiny, the energetic and therapeutic effects of coffee have been highlighted [44]. The different sensorial characteristics of coffee are largely dependent on the plant genotype, cultivation region, and the post-harvest processing methods used, including the fermentation process [45].
In general, coffee fermentation starts with the harvest of ripe coffee cherries. Immediately after harvesting, the cherries are de-pulped and fermented in sealed tanks before drying on handmade structures and greenhouses. However, the post-harvest and fermentation processes of coffee are usually specific to each farm, and can be classified as wet, dry, or semi-dry, each one triggering the development of unique characteristics [46]. In the wet method, the cherries are selected and submitted to submerged fermentation for 12 to 36 h to remove the mucilaginous layer. In the dry method, the cherries are dried together with the shell and fermented simultaneously, yielding an early perception of caramel sensation followed by chocolate and fruit-like aromas [47]. In the semi-dry method, the cherries are first de-pulped and then collected to ferment and dry in the open air, typically for 10 to 15 days and the result is usually a specialty coffee with citric and herbaceous flavors, as well as in some cases fruity notes [4]. In all cases, obtaining a high cup quality is influenced by the yeast richness and the abundance of Lactobacillales [42]. The genera Saccharomyces, Pichia, and Hanseniaspora are amongst the predominant yeasts, while Enterobacter, Pantoea, Enterobacteriaceae, and Rahnella have been reported as the predominant Gram-negative bacteria. Bacillus has been reported as the predominant Gram-positive bacterial genus [48].
The microbial species richness of fermented coffee from different Ecuadorian farms [49], as well as the impact of the microbial communities and their enzymes in coffee fermentation processes has been assessed [50]. Various microorganisms and a variety of extracellular enzymes contribute to the production of ethanol, acetic acid, and lactic acid. The most important enzymes acting in the coffee fermentation process are pectinase, polygalacturonase, and pectin methyl esterase, all of them using pectin as substrate [50,51]. The microbial diversity found during the coffee fermentation process has been associated with the altitude of the process above sea level (asl). In a study carried out in Brazil, at 800 m asl (meters above sea level), Gluconobacter (19.8%), Novosphingobium (18.9%), and Sphingomonas (12.2%) showed the highest abundances. However, Weissella (32.7%), Sphingomonas (36.2%), and Methylobacterium (39.4%) were the most abundant species at 1000 m asl, 1200 m asl, and 1400 m asl, respectively, with the highest yeast abundance found at 1000 m asl [52,53]. Other environmental and process conditions also influence the microbial community composition and activity [54]. Two strains of S. cerevisiae, obtained from different locations, showed clear differences in development and growth [4].
Among the different types of coffee, Luwak coffee is listed as the best in the world and is produced by a natural fermentation method in many countries of Southeast Asia, including Philippines, Malaysia, and Indonesia. This type of coffee is fermented in the intestinal tract of wild civet cats where various enzymes and intestinal microorganisms cause changes in the chemical composition of the beans. However, the high price has caused an increased hunting of Luwak animals. For this reason, the use of starter cultures with species of Gluconobacter, Lactobacillus, Leuconostoc, and Streptococcus isolated from the intestinal tract of Luwaks, have been proposed [55,56].
2.3. Traditional Fermented Beverages
Fermented beverages, such as chicha and champús, have traditionally been used for religious ceremonies and produced at small scales during specific festivities. During the fermentation process of these beverages, different microbial changes occur according to the geographical region and the methods used at home, village, or community in charge of the preparation. To date, there have been few reports on the microbiota of both drinks, as discussed in the sections below.
2.3.1. Chicha
Chicha is an Andean ancestral beverage that has remained perennial in different culinary rituals for more than 3000 years. Chicha is considered a traditional beverage representing brotherhood and reciprocity. Manufacturing chicha is a domestic and communal activity from Colombia, Ecuador, Peru, Brazil, and Bolivia. In Ecuador, different kinds of chicha can be found, including chicha de jora, chicha de cassava, and chicha de Yamor (also known as seven-grains chicha) [57]. Within ancestral cultures, such as the Incas, chicha consumption represented a high lineage and prestige. Today, chicha is used in ceremonial activities, for instance, to welcome an important person, as an accompanying drink of traditional dishes, and as a refreshing drink during community jobs (also known as mingas) [58,59].
The most popular chicha is chicha de jora, which is made from yellow maize as the main ingredient. The making of chicha de jora starts when maize grains are germinated inside containers with water for 13 days (Figure 1). During this time, enzymes inside the grains break down the starch into simple sugars. Then, grains are separated from the water with a mesh and sun-dried to stop biochemical reactions. The dried grains are ground to obtain a flour that is subsequently mixed with water and transferred into special vessels, known as Pondos, for spontaneous fermentation. Some producers add other ingredients, such as a brown sugar loaf known as panela, herbs and spices [59,60]. Other types of chicha, including “chicha de cassava”, are made in the Amazon region of Ecuador using cassava (Manihot esculenta) or chonta (Bactris gasipaes). This type of chicha is usually first chewed by the indigenous women and children to mash the cassava and break down the starch by the amylases present in the saliva, while providing saliva microorganisms for fermentation [61]. Similarly, “chicha de Yamor” is prepared with seven different types of maize, and is mostly consumed in the town of Otavalo in Ecuador [59]. Few studies have reported the interactions, relationships, and development of microorganisms during chicha fermentation. Yeasts from the genera Saccharomyces, Torulaspora, Pichia, Candida, and others have been reported to consume carbohydrates present and produce ethanol during the chicha fermentation [62]. Among the yeasts, Saccharomyces cerevisiae and Torulaspora delbrueckii have been the most frequently isolated from chicha samples. Restriction polymorphism mitochondrial DNA (mtDNA) analyses revealed a high diversity of S. cerevisiae from chicha, as 68 different mtDNA molecular profiles have been identified among 121 yeast isolates [59].
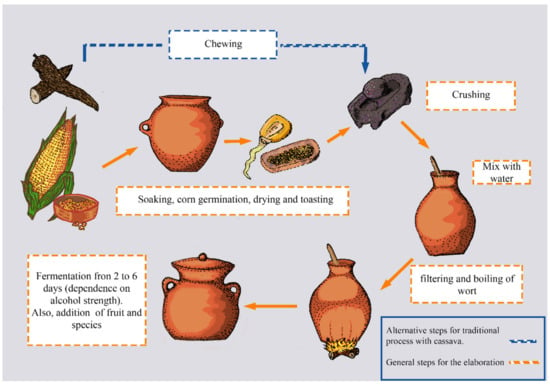
Figure 1.
Flow diagram depicting the production process of chicha. Depending on the geographical location, cassava or corn is used as the main ingredient.
Various LAB, such as Lactiplantibacillus plantarum (previously known as Lactobacillus plantarum), Leuconostoc, and Streptococcus, have contributed to an increased acidification, viscosity, and aroma formation in chicha [11,61,63]. Additionally, species of Klebsiella, Bacillus, Staphylococcus, Micrococcus, Enterobacter, and Weissella were detected in chicha samples from Brazil [64]. However, Acetobacter spp. was the only AAB found in chicha de jora from Peru, whereas the genera of potential foodborne pathogens and spoilage microorganisms have been seldomly found in the chicha samples [65]. Currently, using molecular microbiological methods, microorganisms involved in chicha fermentation are investigated to apply in novel fermentation strategies in the food industry [60].
2.3.2. Champús
Champús is a traditional beverage from Colombia, Ecuador, and Peru. The production of champús usually starts with grinding different cereals, such as wheat, rye, and maize, to obtain a flour that is then mixed with water. The flour–water mixture is placed in vessels (pondos) for approximately three days to allow microbial fermentation. Additionally, other non-flour ingredients are usually added, such as panela, pineapple, naranjilla (Solanum quitoense Lam), chamburo (Vasconcellea pubescens), syrup, clove, cinnamon, and orange tree leaves [66]. Finally, a low-alcohol beverage with a sweet-acid taste and a particular aroma is obtained (Figure 2). During the fermentation of the cereals, the presence of yeasts, such as S. cerevisiae, Issatchenkia orientalis, Pichia fermentans, P. kluyveri var. kluyveri, Zygosaccharomyces fermentati, Torulospora delbrueckii, Galactomyces geotrichum, and Hanseniaspora spp., have been reported [67]. Even though it is known that there are different bacterial groups within the fermentation process, there are no reports to date on the identification and isolation of microorganisms from champús.
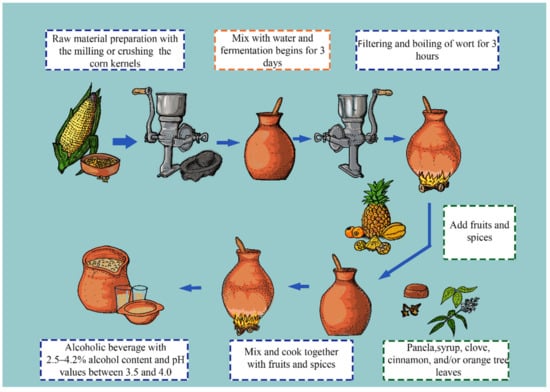
Figure 2.
Flow diagram depicting the champús brewing process, considered as the indigenous Andean beer.
3. Functionality of Microorganisms
In general, the fermentation of traditional foods is performed by yeast, LAB, AAB, among others (Table 2). Yeasts are one of the main microbial groups responsible for food fermentation, and mainly perform fermentation to obtain energy from carbohydrates, such as maltose, sucrose, glucose, and fructose, to generate ATP [68]. During this biochemical process, the yeasts transform carbohydrates into alcohol. Traditionally, fermenting yeasts can be classified as Saccharomyces or non-Saccharomyces. Saccharomyces cerevisiae has commonly been found in the fermentation processes of various foods, and is the most used in industrial processes because of its fermentative capacity, rapid growth, and easy adaptation [69]. At the same time, the diastatic properties of S. cerevisiae have offered insight into mechanisms used for adaptation to fermentation environments and formation of flavor-active esters [70]. Additionally, the interaction between Saccharomyces and other yeasts, such as Starmerella, Torulaspora, Hanseniaspora, and Metschnikowia, has shown a strong effect in the final carbohydrate and nitrogen contents of the fermenting matrix. This fact could be related to the nature and diversity of secreted proteins during the fermentation process [71] and a faster consumption of glucose, ammonium, and arginine [72].
LAB are Gram-positive cocci or bacillus-shaped, catalase-negative, and aerotolerant microorganisms. LAB can be classified as homofermentative and heterofermentative depending on the metabolic pathways that each species uses for carbohydrate consumption and metabolite production [73,74]. In general, LAB have the ability to metabolize carbohydrates mainly into lactic acid. The most common LAB associated with foods are Lactococcus, Streptococcus, Enterococcus, Pediococcus, Leuconostoc, Oenococcus, Tetragenococcus, Carnobacterium, Weissella, and Lactobacillus, although the genus Lactobacillus has recently been reclassified into 23 novel genera [74,75]. Some LAB strains, such as Lactiplantibacillus plantarum HEAL9, Lacticaseibacillus rhamnosus 271, Weissella confusa MD1, and Weissella cibaria MD2, have shown probiotic properties [76,77].
Similarly, AAB are another bacterial group commonly associated with food fermentation. AAB, such as Acetobacter and Gluconobacter spp., usually carry out acetic acid fermentation by which acetic acid and other volatiles with aroma descriptors of fruits, trees, and chocolate are produced [78].

Table 2.
Microbial species reported in fermented foods.
Table 2.
Microbial species reported in fermented foods.
| Community | Species | Fermented Food * | Study Observations | Reference |
|---|---|---|---|---|
| Yeast | Candida californica | C, CJ | The genus Candida is frequently found in spontaneous fermentation processes and has been assessed as a starter culture for alcohol production. | [67] |
| Candida humilis | CJ, Cf | |||
| Candida quercitrusa | Cf | |||
| Candida sake | CJ, CM | |||
| Candida solani | C, CJ | |||
| Candida sorbosivorans | C | |||
| Candida sorboxylosa | CJ | |||
| Candida zeylanoides | CJ | |||
| Candida vinaria | CJ | |||
| Candida tropicalis | C, CY | Used as a starter culture in sorghum beer and barley malt medium. | [79,80] | |
| Dekkera anomala | CJ | These species were related to the production of unpleasant aromas and were not recommended as starter culture. | [81] | |
| Dekkera bruxellensis | CJ, SC | |||
| Hanseniaspora opuntiae | C, CY | Was used as a possible starter culture in cacao fermentation. | [82] | |
| Hanseniaspora uvarum | Cf | Starter culture for the production of volatile compounds in fermented foods and beverages. | [71,83] | |
| Hanseniaspora spp. | CJ, CH, Cf | |||
| Issatchenkia orientalis | CH | Malic acid reduction and interaction mechanisms with S. cerevisiae. | [84] | |
| Kazachstania exigua | CJ | Starter culture for cacao fermentation. | [85] | |
| Kodamaea ohmeri | CY | |||
| Kluyveromyces marxianus | C | |||
| Pichia fermentans | CJ, CM, CH | Starter culture for wine to stabilize color and increase fruit and floral aromas. | [86] | |
| Pichia kluyveri | C, CJ, CH | Starter culture to increases volatile thiols (3-mercaptohexanol and its acetylated derivative 3-mercaptohexyl acetate) with fruity aroma as passion fruit and grapefruit. | [87] | |
| Pichia kudriavzevii | C, | Starter culture for cacao fermentation. | [85] | |
| Pichia manshurica | SC | |||
| Rhodotorula minuta | C | [60] | ||
| Rhodotorula mucilaginosa | CJ, Cf | |||
| Saccharomyces cerevisiae | C, Cf, CJ, SC, CY, CH | Starter culture for different types of beer. | [88] | |
| Saccharomycodes ludwigii | CJ, CM | |||
| Torulospora delbrueckii | C, CJ, CM, CY, CH | Alcohol production to improve flavor diversity. | [60,89] | |
| Zygoascus hellenicus | CJ | |||
| Zygosaccharomyces fermentati | CH | |||
| LAB | Enterococcus casseliflavus | C | Food bio-preservative. | [90] |
| Enterococcus saccharolyticus | C | |||
| Enterococcus sp. | C | |||
| Fructobacillus durionis | C | Some strains with probiotic potential. | [49] | |
| Fructobacillus ficulneus | C | |||
| Fructobacillus tropaeoli | C | |||
| Lactobacillus acidophilus | C, CY | Starter cultures for steering food fermentation processes. Some specific strains have probiotic potential. | [91,92]. | |
| Lactobacillus amylovorus | C | |||
| Levilactobacillus brevis | C, Cf | |||
| Liquorilactobacillus cacaonum | C | |||
| Lacticaseibacillus casei | C, CJ | |||
| Loigolactobacillus coryniformis | C | |||
| Lactobacillus delbrueckii | C, CY | |||
| Lactiplantibacillus fabifermentans | C | |||
| Lentilactobacilluss farraginis | C | |||
| Licmosilactobacillus fermentum | C, CY | |||
| Lactobacillus garvieae | C | |||
| Liquorilactobacillus nagelii | C | |||
| Lactiplantibacillus plantarum | C, O | Starter culture in different fermented foods and beverages. | [31,93] | |
| Limosilactobacillus reuteri | CY | Starter culture can produce antimicrobial molecules, such as organic acids, ethanol, and reuterin. | [94] | |
| Lactobacillus delbruckii subsp. Lactis | C, CY, Cf | Starter culture in yoghurt. | [95] | |
| Lactococcus hircilactis | Cf | |||
| Leuconostoc fallax | C, Cf | Starter culture for the production of butyric acid. | [96] | |
| Leuconostoc mesenteroides | C, CY, O | |||
| Leuconostoc pseudomesenteroides | C, Cf | |||
| Streptococcus thermopjhilus | CY | Starter culture in yoghurt and cheese by its rapidly growing in low pH conditions. | [97] | |
| Streptococcus salivarius | O | |||
| Weissella cibaria | C | High presence in different fermented foods; its redox potential influences the aromatic profile. Qualified Presumption of Safety (QPS) for food applications in in process. | [77] | |
| Weissella fabaria | C | |||
| Weissella confusa | O | |||
| AAB | Acetobacter cibinongensis | C, Cf | Starter culture for food fermentation processes to favor the production of volatile compounds. | [98] |
| Acetobacter lovaniensis | C | |||
| Acetobacter malorum/cerevisiae | C | |||
| Acetobacter malorum/indonesiensis | C, Cf | |||
| Acetobacter fabarum | C, Cf | |||
| Acetobacter ghanensis | C | |||
| Acetobacter orientalis | C, Cf | |||
| Acetobacter okinawensis | Cf | |||
| Acetobacter pasteurianus | C | |||
| Acetobacter peroxydans | C | |||
| Acetobacter pomorum | C | |||
| Acetobacter senegalensis | C, Cf | |||
| Acetobacter syzygii | C | |||
| Acetobacter thaillandicus | Cf | |||
| Frateuria aurantia | C | Starter culture potential when high concentrations of glucose are present. | [78] | |
| Microbacterium lacticum | C | |||
| Gluconobacter cerevisiae | Cf | |||
| Gluconobacter oxydans | C | |||
| Gluconobacter sp. | C |
* CJ = Chicha de Jora; Cf = Coffee; CM = Chicha de Morocho; C = Cacao; CH = Champús; CY = Chicha cassava; O = others; SC = Seven-grain Chicha.
4. Benefits and Risk
The increased interest in fermented food products is mainly due to the benefits that some of the microorganisms provide to human health, including their contribution to a healthy gut microbiome and their potential role as probiotics, as well as the improved organoleptic characteristics of the fermented food products. Additionally, the enzymes produced by fermenting microorganisms, such as phytases, amilases, proteases, mannase, catalase, cellulose, pullulanase and lipases, help with the release of polyphenol compounds from vegetal matrixes [7,13,93,94,99,100].
However, the spontaneous nature of various fermentation processes may result in the presence of undesired fungi, such as Aspergillus, Fusarium and Penicillium, which are capable of producing toxic secondary metabolites, such as mycotoxins [101]. Approximately 400 mycotoxins have been reported in different cereals, grains, and other food, with aflatoxin, ochratoxin A, fumonisins, zearaleone, and patulin being amongst the most significant ones. Spontaneous fermented foods may carry traces of mycotoxins. Some researchers have reported aflatoxin and ochratoxin on fermented cacao bean clones [102], arabica and robusta coffee [103], and in the different grains used for the production of traditional fermented beverages [104]. Similarly, the corn and rice used for the production of different types of chicha can carry fungal species that may produce toxic metabolites [105,106]. The development of mycotoxins has been associated to the length of storage, excessive moisture, and unsanitary handling of certain fermented foods [107]. For instance, a recent study carried out in Brazil detected aflatoxin contamination in 38% of the analyzed cacao samples, while ochratoxin A was detected in 18% of the samples [103]. Similarly, high levels of ochratoxin A were detected in about 25% of the coffee samples [104]. In Ecuador, mycotoxins have also been detected in 23% of paddy rice, 33% of white wheat noodles, and 17% of oat flakes [104].
5. Future Perspectives
Nowadays, there is a great variety of fermented foods produced spontaneously. However, with an increasing body of knowledge regarding the microbial community involved in the fermentation processes and technological advancements regarding large-scale fermentations, there is a need to select proper microbial strains that can steer the fermentation process, select quality raw materials, and apply proper process control. Additional research is needed for reducing the risk of contamination and negative changes in the sensory properties.
6. Development of Starter Cultures
As microorganisms play an important role in food fermentation, there is a growing interest in the development of starter cultures. Commercial starter cultures of yeasts and bacteria are used for the production of bread, beer, wine, and cheese [108,109,110]. The commercially available starter cultures have been selected considering the ability of the strains to withstand strong stress conditions during the fermentation process, the identification of the key metabolites produced, and the evaluation of the necessary technological parameter needed for a proper fermentation process [94,110,111]. With the purpose of controlling and even steering the fermentation process, many studies have considered various combinations of microorganisms, among which are Saccharomyces cerevisiae in conjunction with other yeasts, such as Pichia and Hanseniaspora, for cacao and wine fermentation [84,85]. Also, different strains of Lactobacillus can be used as starter culture, due to their probiotic properties, lactic acid production, and biological reduction of mycotoxin-producing mold-fermented drinks [91,92].
7. Concluding Remarks
With the increasing demand of fermented foods, it becomes necessary to improve the productivity, food safety, and efficiency of the manufacturing processes, while decreasing production costs. Further research on innovative fermentation processes should include the bioprospection of microbial ecosystems for starter culture selection and development, as well as the natural inclusion of new flavors. Additionally, there is a need to control the intrinsic and extrinsic factors that may affect the fermentation processes. In general, the use of fermentors that allow monitoring the fermentation conditions, as well as the use of biosensors or similar devices for the control and discrimination of undesired microorganisms and sensitive detection of pathogenic bacteria, could allow to obtain a stable and safe final product [81,112].
Amongst the fermented foods mentioned in this review, cacao and coffee have been the most investigated products, given their economic importance. Further research is needed to better characterize the fermentation processes of other traditional fermented foods, such as chicha and champús. However, it is possible to apply the knowledge obtained from cacao and coffee research to understand the processes of other fermented foods.
Author Contributions
L.S.G., J.M.C.-C., S.W. and J.R. were responsible for visualization, writing—original draft preparation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed within the framework of the VLIR-UOS TEAM project EC2018TEA461A105. Funding was provided by VLIR-UOS and DGD through grant VLIR Network Ecuador.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ojha, K.S.; Tiwari, B.K. Novel Food Fermentation Technologies BT—Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–5. [Google Scholar]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Montanari, C.; Levante, A.; Alinovi, M.; Neviani, E.; Gardini, F.; Gatti, M. Lactobacillus paracasei 4341 as adjunct culture to enhance flavor in short ripened Caciotta-type cheese. Food Res. Int. 2020, 135, 109284. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.; da Cruz PedrozoMiguel, M.G.; Evangelista, S.R.; Martins, P.M.M.; van Mullem, J.; Belizario, M.H.; Schwan, R.F. Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res. Int. 2017, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020, 128, 108773. [Google Scholar] [CrossRef]
- Toktaş, B.; Bildik, F.; Özçelik, B. Effect of fermentation on anthocyanin stability and in vitro bioaccessibility during shalgam (şalgam) beverage production. J. Sci. Food Agric. 2018, 98, 3066–3075. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented Foods and Beverages in Human Diet and Their Influence on Gut Microbiota and Health. Fermentation 2018, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Aslam, H.; Green, J.; Jacka, F.N.; Collier, F.; Berk, M.; Pasco, J.; Dawson, S.L. Fermented foods, the gut and mental health: A mechanistic overview with implications for depression and anxiety. Nutr. Neurosci. 2020, 23, 659–671. [Google Scholar] [CrossRef]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Tempère, S.; Marchal, A.; Barbe, J.-C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The complexity of wine: Clarifying the role of microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Pérez-Díaz, I.M.; Altuntas, E.G.; Juneja, V.K. Microbial Fermentation in Food Preservation BT—Microbial Control and Food Preservation: Theory and Practice; Juneja, V.K., Dwivedi, H.P., Sofos, J.N., Eds.; Springer: New York, NY, USA, 2017; pp. 281–298. [Google Scholar]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food. Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef] [PubMed]
- IMARC. Global Yogurt Market Strengthened by Growing Consumers’ Health Awareness. 2022. Available online: https://www.imarcgroup.com/global-yogurt-market-strengthened-growing (accessed on 28 April 2022).
- Kim, J.; Adhikari, K. Current Trends in Kombucha: Marketing Perspectives and the Need for Improved Sensory Research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- IMARC. Vinegar Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. 2021. Available online: https://www.imarcgroup.com/vinegar-manufacturing-plant (accessed on 28 April 2022).
- Moretti, A.F.; Moure, M.C.; Quiñoy, F.; Esposito, F.; Simonelli, N.; Medrano, M.; León-Peláez, Á. Water kefir, a fermented beverage containing probiotic microorganisms: From ancient and artisanal manufacture to industrialized and regulated commercialization. Future Foods 2022, 5, 100123. [Google Scholar] [CrossRef]
- Akinsemolu, A.A. The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 2018, 182, 139–155. [Google Scholar] [CrossRef]
- Sánchez, V.H.; Iglesias, C.; Zambrano, J.L. Diagnóstico y prospectiva de la cadena de valor del cacao en América Latina y El Caribe. In La Cadena de Valor del Cacao en América Latina y El Caribe; Sánchez, V.H., Iglesias, C., Zambrano, J.L., Eds.; INIAP: Quito, Ecuador, 2019; p. 104. [Google Scholar]
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The use and domestication of Theobroma cacao during the mid-Holocene in the upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented food products in the era of globalization: Tradition meets biotechnology innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Camu, N.; Falony, G.; De Vuyst, L. Comparison of the bacterial species diversity of spontaneous cocoa bean fermentations carried out at selected farms in Ivory Coast and Brazil. Food Microbiol. 2011, 28, 964–973. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hamdouche, Y.; Guehi, T.; Durand, N.; Kedjebo, K.B.D.; Montet, D.; Meile, J.C. Dynamics of microbial ecology during cocoa fermentation and drying: Towards the identification of molecular markers. Food Control 2015, 48, 117–122. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and Biodiversity of Bacterial and Yeast Communities during Fermentation of Cocoa Beans. Appl. Environ. Microbiol. 2018, 84, e01164-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visintin, S.; Alessandria, V.; Valente, A.; Dolci, P.; Cocolin, L. Molecular identification and physiological characterization of yeasts, lactic acid bacteria and acetic acid bacteria isolated from heap and box cocoa bean fermentations in West Africa. Int. J. Food Microbiol. 2016, 216, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Alvareza, J.P.; de C. Netoa, D.P.; Soccol, V.T.; Tanobea, V.O.A.; Rogezb, H.; Góes-Netoc, A.; Soccola, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Falony, G.; Romanens, E.; Jimenez, J.C.; Amores, F.; Daniel, H.-M.; De Vuyst, L. Species Diversity, Community Dynamics, and Metabolite Kinetics of the Microbiota Associated with Traditional Ecuadorian Spontaneous Cocoa Bean Fermentations. Appl. Environ. Microbiol. 2011, 77, 7698–7714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, V.T.T.; Zhao, J.; Fleet, G. The effect of lactic acid bacteria on cocoa bean fermentation. Int. J. Food Microbiol. 2015, 205, 54–67. [Google Scholar] [CrossRef]
- Ouattara, H.D.; Ouattara, H.G.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S.L. Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: Species diversity and citrate lyase production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine—Diacetyl—Desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef]
- Shi, C.; Knøchel, S. Susceptibility of dairy associated molds towards microbial metabolites with focus on the response to diacetyl. Food Control 2021, 121, 107573. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016, 236, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Börner, R.A.; Kandasamy, V.; Axelsen, A.M.; Nielsen, A.T.; Bosma, E.F. High-throughput Genome Editing Tools for Lactic Acid Bacteria: Opportunities for Food, Feed, Pharma and Biotech. FEMS Microbiol. Lett. 2018, 366, 291. [Google Scholar] [CrossRef]
- Illeghems, K.; Weckx, S.; De Vuyst, L. Applying meta-pathway analyses through metagenomics to identify the functional properties of the major bacterial communities of a single spontaneous cocoa bean fermentation process sample. Food Microbiol. 2015, 50, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.L.; Moura, F.G.; de Melo Pereira, G.V.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-based metagenomic sequencing. LWT 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Triboletti, S.; Alessandria, V.; Serio, A.; Sergi, M.; Paparella, A.; Rantsiou, K.; Chaves-López, C. Functional Biodiversity of Yeasts Isolated from Colombian Fermented and Dry Cocoa Beans. Microorganisms 2020, 8, 1086. [Google Scholar] [CrossRef]
- Cruz-O’Byrne, R.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Microbial diversity associated with spontaneous coffee bean fermentation process and specialty coffee production in northern Colombia. Int. J. Food Microbiol. 2021, 354, 109282. [Google Scholar] [CrossRef]
- Slavova, G.; Georgieva, V. World production of coffee imports and exportsin Europe, Bulgaria and USA. Trakia J. Sci. 2019, 17, 619–626. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Consumers’ Perceptions of Coffee Health Benefits and Motives for Coffee Consumption and Purchasing. Nutrients 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Bytof, G.; Knopp, S.-E.; Breitenstein, B. Germination of Coffee Seeds and its Significance for Coffee Quality. Plant Biol. 2006, 8, 260–264. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2016, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, S.R.; Silva, C.F.; da CruzMiguel, M.G.P.; de SouzaCordeiro, C.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Dong, H.; Yang, P.; Yang, R.; Lu, J.; Lv, J.; Sheng, J. Culture-Dependent and -Independent Methods to Investigate the Predominant Microorganisms Associated with Wet Processed Coffee. Curr. Microbiol. 2016, 73, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal shotgun metagenomics of an Ecuadorian coffee fermentation process highlights the predominance of lactic acid bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The Role of Microbes in Coffee Fermentation and Their Impact on Coffee Quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Isolation, Identification, and Characterization of Pectinolytic Yeasts for Starter Culture in Coffee Fermentation. Microorganisms 2019, 7, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, S.J.; Simão, J.B.P.; Pylro, V.S.; Schwan, R.F. The Altitude of Coffee Cultivation Causes Shifts in the Microbial Community Assembly and Biochemical Compounds in Natural Induced Anaerobic Fermentations. Front. Microbiol. 2021, 12, 671395. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Batista, N.N.; da Cruz Pedrozo Miguel, M.G.; Simão, J.B.P.; Soares, J.R.; Schwan, R.F. Coffee growing altitude influences the microbiota, chemical compounds and the quality of fermented coffees. Food Res. Int. 2020, 129, 108872. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; ten Caten, C.S. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef]
- Fitri; Tawali, A.B.; Laga, A. Luwak coffee in vitro fermentation: Literature review. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 12096. [Google Scholar] [CrossRef]
- Watanabe, H.; Ng, C.H.; Limviphuvadh, V.; Suzuki, S.; Yamada, T. Gluconobacter dominates the gut microbiome of the Asian palm civet Paradoxurus hermaphroditus that produces kopi luwak. PeerJ 2020, 8, e9579. [Google Scholar] [CrossRef] [PubMed]
- Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J.; Magalhães, F.; Aranda, A.; Matallana, E.; Gibson, B. Potential application of yeasts from Ecuadorian chichas in controlled beer and chicha production. Food Microbiol. 2021, 98, 103644. [Google Scholar] [CrossRef] [PubMed]
- Faria-Oliveira, F.; Diniz, R.; Godoy-Santos, F.; Piló, F.; Mezadri, H.; Castro, I.; Brandão, R. El papel de la levadura y las bacterias del ácido láctico en la producción de bebidas fermentadas en América del Sur. In Food Production and Industry; IntechOpen: London, UK, 2015; pp. 107–135. [Google Scholar]
- Piló, F.B.; Carbajal-Barriga, E.J.; Guamán-Burneo, M.C.; Portero-Barahona, P.; Morato-Dias, A.M.; Daher de Freitas, L.; Oliverira Gomes, F.; Rosa, C.A. Saccharomyces cerevisiae populations and other yeasts associated with indigenous beers (chicha) of Ecuador. Braz. J. Microbiol. 2018, 49, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, G.A.C.; Palma, G.B.A.; Sandoval-Cañas, G.J.; Ordoñez-Araque, R.H. Ancestral fermented indigenous beverages from South America made from cassava (Manihot esculenta). Food Sci. Technol. 2021, 41, 360–367. [Google Scholar] [CrossRef]
- Freire, A.L.; Zapata, S.; Mosquera, J.; Mejia, M.L.; Trueba, G. Bacteria associated with human saliva are major microbial components of Ecuadorian indigenous beers (chicha). PeerJ 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Grijalva-Vallejos, N.; Aranda, A.; Matallana, E. Evaluation of yeasts from Ecuadorian chicha by their performance as starters for alcoholic fermentations in the food industry. Int. J. Food Microbiol. 2020, 317, 108462. [Google Scholar] [CrossRef]
- Colehour, A.M.; Meadow, J.F.; Liebert, M.A.; Cepon-Robins, T.J.; Gildner, T.E.; Urlacher, S.S.; Bohannan, B.J.M.; Snodgrass, J.J.; Sugiyama, L.S. Local domestication of lactic acid bacteria via cassava beer fermentation. PeerJ 2014, 2, e479. [Google Scholar] [CrossRef] [Green Version]
- Resende, L.V.; Pinheiro, L.K.; da Cruz Pedroso Miguel, M.G.; Ramos, C.L.; Vilela, D.M.; Schwan, R.F. Microbial community and physicochemical dynamics during the production of ‘Chicha’, a traditional beverage of Indigenous people of Brazil. World J. Microbiol. Biotechnol. 2018, 34, 46. [Google Scholar] [CrossRef]
- Bassi, D.; Orrù, L.; Cabanillas Vasquez, J.; Cocconcelli, P.S.; Fontana, C. Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage. Microorganisms 2020, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.; Lacerda, I.; Libkind, D.; Lopes, C.; Carvajal Barriga, E.; Rosa, C. Traditional foods and beverages from South America: Microbial communities and production strategies. In Industrial Fermentation: Food Processes, Nutrient Sources and Production Strategies; Nova Science Pub Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Osorio-Cadavid, E.; Chaves-López, C.; Tofalo, R.; Paparella, A.; Suzzi, G. Detection and identification of wild yeasts in Champús, a fermented Colombian maize beverage. Food Microbiol. 2008, 25, 771–777. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Sirén, K.; Mak, S.S.T.; Melkonian, C.; Carøe, C.; Swiegers, J.H.; Molenaar, D.; Fischer, U.; Gilbert, M.T.P. Taxonomic and Functional Characterization of the Microbial Community During Spontaneous in vitro Fermentation of Riesling Must. Front. Microbiol. 2019, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Gibson, B. A re-evaluation of diastatic Saccharomyces cerevisiae strains and their role in brewing. Appl. Microbiol. Biotechnol. 2020, 104, 3745–3756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The Interaction between Saccharomyces cerevisiae and Non-Saccharomyces Yeast during Alcoholic Fermentation Is Species and Strain Specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Storti, L.V.; Glibota, N.A.; Parente, E. Aerobic and respirative growth of heterofermentative lactic acid bacteria: A screening study. Food Microbiol. 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2021, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Önning, G.; Palm, R.; Linninge, C.; Larsson, N. New Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus strains: Well tolerated and improve infant microbiota. Pediatr. Res. 2021. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 125, 109261. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Soccol, V.T.; Soccol, C.R. Current state of research on cocoa and coffee fermentations. Curr. Opin. Food Sci. 2016, 7, 50–57. [Google Scholar] [CrossRef]
- Alloue-Boraud, W.A.M.; N’Guessan, K.F.; Djeni, N.T.; Hiligsmann, S.; Djè, K.M.; Delvigne, F. Fermentation profile of Saccharomyces cerevisiae and Candida tropicalis as starter cultures on barley malt medium. J. Food Sci. Technol. 2015, 52, 5236–5242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N’Guessan, F.K.; N’Dri, D.Y.; Camara, F.; Djè, M.K. Saccharomyces cerevisiae and Candida tropicalis as starter cultures for the alcoholic fermentation of tchapalo, a traditional sorghum beer. World J. Microbiol. Biotechnol. 2010, 26, 693–699. [Google Scholar] [CrossRef]
- Yokota, K.; Takeo, A.; Abe, H.; Kurokawa, Y.; Hashimoto, M.; Kajimoto, K.; Tanaka, M.; Murayama, S.; Nakajima, Y.; Taniguchi, M.; et al. Application of Micropore Device for Accurate, Easy, and Rapid Discrimination of Saccharomyces pastorianus from Dekkera spp. Biosensors 2021, 11, 272. [Google Scholar] [CrossRef]
- Sin, O.T. Effect of selected yeast starter in cocoa fermentation: A Study on Antioxidant Content, Volatile Organic Compounds and Sensory Profile of Malaysian Cocoa Beans and Chocolates Produced. Ph.D. Thesis, Monash University, Melbourne, Australia, 2021. [Google Scholar] [CrossRef]
- Moreira, N.; Pina, C.; Mendes, F.; Couto, J.A.; Hogg, T.; Vasconcelos, I. Volatile compounds contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during red wine vinifications. Food Control 2011, 22, 662–667. [Google Scholar] [CrossRef]
- Zhang, W.; Weng, P.; Wu, Z. Interaction profile of a mixed-culture fermentation of Issatchenkia orientalis and Saccharomyces cerevisiae by transcriptome sequencing. Br. Food J. 2020. ahead-of-print. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; da Silva Martins, L.H.; Lopes, A.S. Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef]
- Kong, C.-L.; Li, A.-H.; Su, J.; Wang, X.-C.; Chen, C.-Q.; Tao, Y.-S. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT 2019, 109, 83–92. [Google Scholar] [CrossRef]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Hutajulu, I.B.E.; Kulla, P.D.K.; Retnaningrum, E. Diversity of lactic acid bacteria isolated during fermentation of indigenous cassava obtained from Sumba, East Nusa Tenggara, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 2561–2570. [Google Scholar] [CrossRef]
- Munanga, B.d.J.C.; Loiseau, G.; Grabulos, J.; Mestres, C. Modeling Lactic Fermentation of Gowé Using Lactobacillus Starter Culture. Microorganisms 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosallaie, F.; Jooyandeh, H.; Hojjati, M.; Fazlara, A. Biological reduction of aflatoxin B1 in yogurt by probiotic strains of Lactobacillus acidophilus and Lactobacillus rhamnosus. Food Sci. Biotechnol. 2020, 29, 793–803. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why Are Weissella spp. Not Used as Commercial Starter Cultures for Food Fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Traisaeng, S.; Batsukh, A.; Chuang, T.-H.; Herr, D.R.; Huang, Y.-F.; Chimeddorj, B.; Huang, C.-M. Leuconostoc mesenteroides fermentation produces butyric acid and mediates Ffar2 to regulate blood glucose and insulin in type 1 diabetic mice. Sci. Rep. 2020, 10, 7928. [Google Scholar] [CrossRef]
- Tarrah, A.; Noal, V.; Giaretta, S.; Treu, L.; da Silva Duarte, V.; Corich, V.; Giacomini, A. Effect of different initial pH on the growth of Streptococcus macedonicus and Streptococcus thermophilus strains. Int. Dairy J. 2018, 86, 65–68. [Google Scholar] [CrossRef]
- Lee, A.H.; Neilson, A.P.; O’Keefe, S.F.; Ogejo, J.A.; Huang, H.; Ponder, M.; Chu, H.S.S.; Jin, Q.; Pilot, G.; Stewart, A.C. A laboratory-scale model cocoa fermentation using dried, unfermented beans and artificial pulp can simulate the microbial and chemical changes of on-farm cocoa fermentation. Eur. Food Res. Technol. 2019, 245, 511–519. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, C.; Guo, Y.; Wang, X.; Meng, Y. Polyphenols in fermented apple juice: Beneficial effects on human health. J. Funct. Foods 2021, 76, 104294. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Maciel, L.F.; de Souza Madureira Felício, A.L.; Miranda, L.C.R.; Pires, T.C.; da Silva Bispo, E.; Hirooka, E.Y. Aflatoxins and ochratoxin A in different cocoa clones (Theobroma cacao L.) developed in the southern region of Bahia, Brazil. Food Addit. Contam. Part A 2018, 35, 134–143. [Google Scholar] [CrossRef]
- dos Santos, D.G.; Coelho, C.C.; Ferreira, A.B.; Freitas-Silva, O. Brazilian Coffee Production and the Future Microbiome and Mycotoxin Profile Considering the Climate Change Scenario. Microorganisms 2021, 9, 858. [Google Scholar] [CrossRef]
- Ortiz, J.; Van Camp, J.; Mestdagh, F.; Donoso, S.; De Meulenaer, B. Mycotoxin co-occurrence in rice, oat flakes and wheat noodles used as staple foods in Ecuador. Food Addit. Contam. Part A 2013, 30, 2165–2176. [Google Scholar] [CrossRef]
- Ali, N. Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef]
- Ducos, C.; Pinson-Gadais, L.; Chereau, S.; Richard-Forget, F.; Vásquez-Ocmín, P.; Cerapio, J.P.; Casavilca-Zambrano, S.; Ruiz, E.; Pineau, P.; Bertani, S.; et al. Natural Occurrence of Mycotoxin-Producing Fusaria in Market-Bought Peruvian Cereals: A Food Safety Threat for Andean Populations. Toxins 2021, 13, 172. [Google Scholar] [CrossRef]
- Umesha, S.; Manukumar, H.M.G.; Chandrasekhar, B.; Shivakumara, P.; Shiva Kumar, J.; Raghava, S.; Avinash, P.; Shirin, M.; Bharathi, T.R.; Rajini, S.B.; et al. Aflatoxins and food pathogens: Impact of biologically active aflatoxins and their control strategies. J. Sci. Food Agric. 2017, 97, 1698–1707. [Google Scholar] [CrossRef]
- Guerrini, S.; Barbato, D.; Guerrini, L.; Mari, E.; Buscioni, G.; Mangani, S.; Romboli, Y.; Galli, V.; Parenti, A.; Granchi, L. Selection of Indigenous Saccharomyces cerevisiae Strains and Exploitation of a Pilot-Plant to Produce Fresh Yeast Starter Cultures in a Winery. Fermentation 2021, 7, 99. [Google Scholar] [CrossRef]
- Larroque, M.N.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef] [PubMed]
- Litwinek, D.; Boreczek, J.; Gambuś, H.; Buksa, K.; Berski, W.; Kowalczyk, M. Developing lactic acid bacteria starter cultures for wholemeal rye flour bread with improved functionality, nutritional value, taste, appearance and safety. PLoS ONE 2022, 17, e0261677. [Google Scholar] [CrossRef] [PubMed]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Y.; Lin, J.; Liu, Y. Biosensors Coupled with Signal Amplification Technology for the Detection of Pathogenic Bacteria: A Review. Biosensors 2021, 11, 190. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).