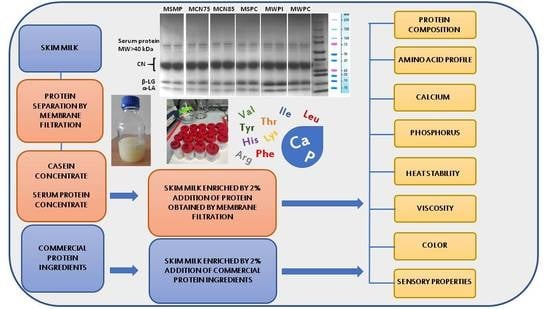
Protein Preparations as Ingredients for the Enrichment of Non-Fermented Milks
Abstract
1. Introduction
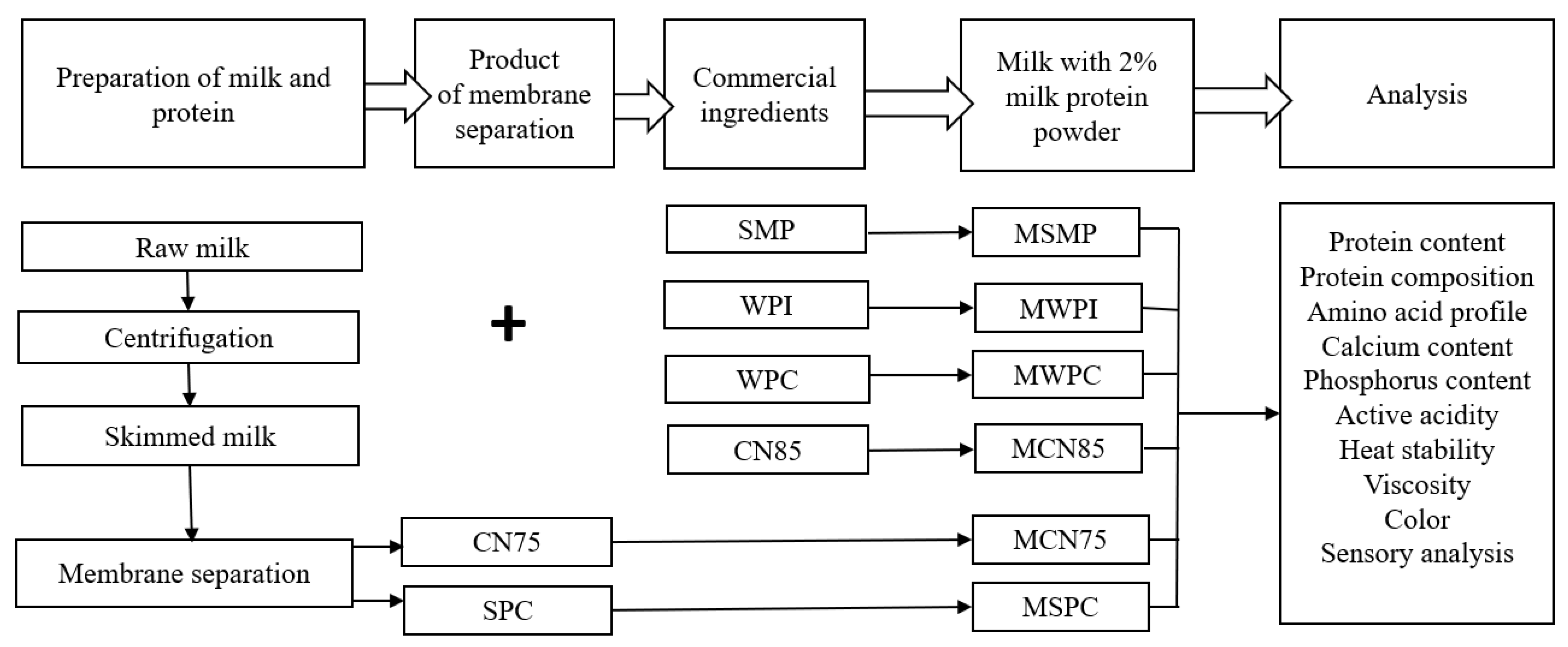
2. Materials and Methods
2.1. Materials
2.2. Separation of Casein and Serum Protein Preparations from Skim Milk
2.2.1. Production of Micellar Casein Concentrate
2.2.2. Microfiltration Process
2.2.3. Diafiltration Processes
2.2.4. SPC Produced in the Pilot Plant
2.2.5. Spray Drying
2.2.6. Protein Content
2.2.7. Reducing-Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.2.8. Amino Acid Profile
2.2.9. Calcium and Phosphorus Content
2.2.10. Active Acidity and Heat Stability
2.2.11. Viscosity
2.2.12. Color
2.2.13. Sensory Analysis
2.3. Statistical Analysis
3. Results and Discussion
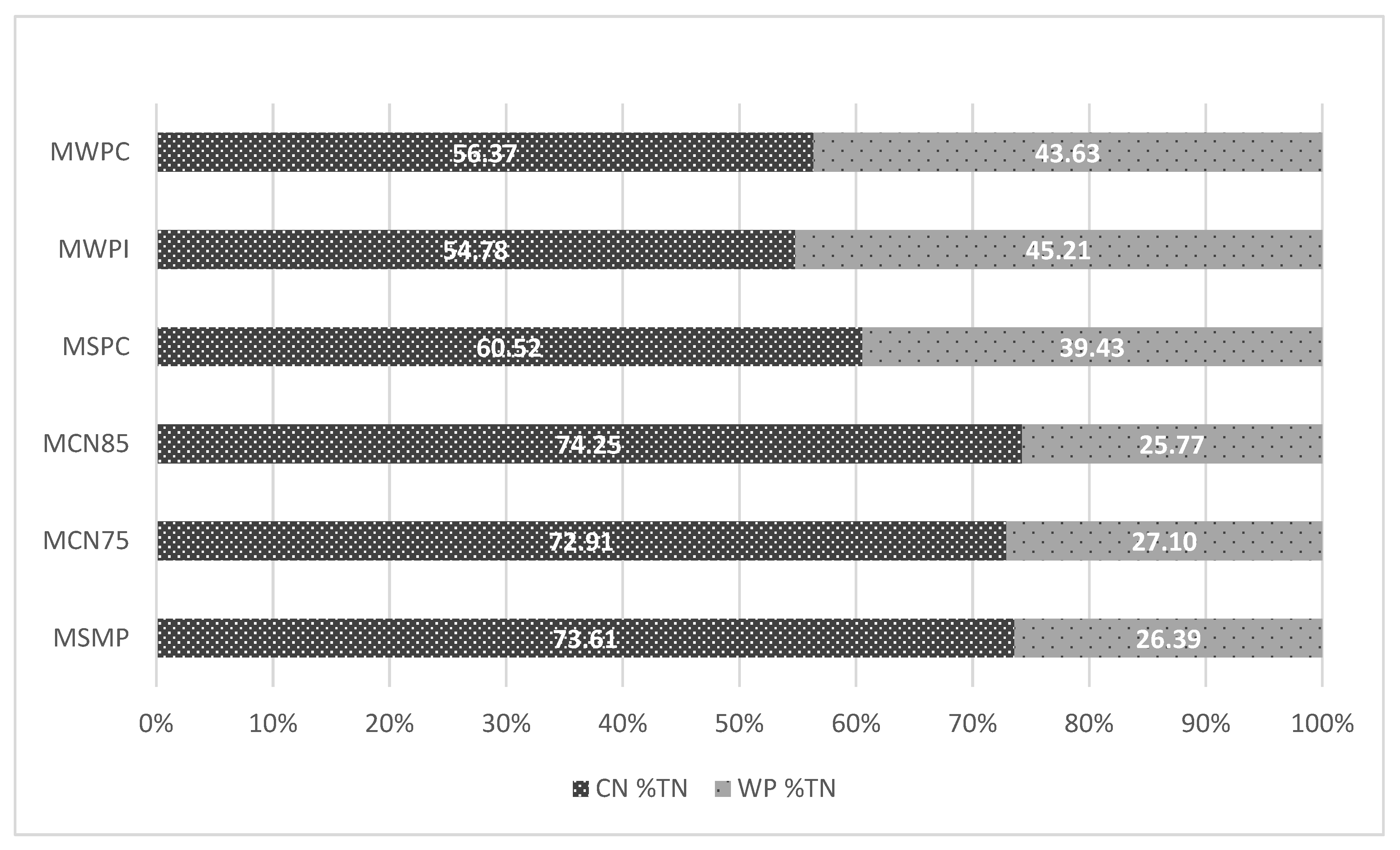
3.1. Proximate Composition
3.2. Amino Acid Profile
3.3. Calcium and Phosphorus
3.4. Heat Stability and Viscosity
3.5. Color
3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korhonen, H.J. Bioactive components in bovine milk. In Bioactive Components in Milk and Dairy Products, 1st ed.; Park, Y.W., Ed.; Wiley-Blackwell: Ames, IA, USA; Oxford, UK, 2009; pp. 15–42. [Google Scholar]
- Park, Y.W. Overview of Bioactive Components in Milk and Dairy Products. In Bioactive Components in Milk and Dairy Products, 1st ed.; Park, Y.W., Ed.; Wiley-Blackwell: Ames, IA, USA; Oxford, UK, 2009; pp. 1–12. [Google Scholar] [CrossRef]
- Przybyłowicz, K.E.; Morze, J.; Danielewicz, A.; Staniewska, K.; Dąbrowska, A.; Baranowska, M.; Darewicz, M.; Żulewska, J.; Staniewski, B. Association between Intake of Fermented Dairy Products and Diet Quality, Health Beliefs in a Representative Sample of Polish Population. Proceedings 2020, 61, 26. [Google Scholar] [CrossRef]
- Almeida, C.C.; Álvares, T.S.; Costa, M.P.; Conte-Junior, C.A. Protein and Amino Acid Profiles of Different Whey Protein Supplements. J. Diet. Suppl. 2016, 13, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Darewicz, M.; Iwaniak, A.; Minkiewicz, P. Biologically active peptides derived from milk proteins. Med. Wet. 2014, 70, 348–352. [Google Scholar]
- Park, Y.W.; Nam, M.S. Bioactive peptides in milk and dairy products: A review. Korean J. Food Sci. Anim. Recour. 2015, 35, 831–840. [Google Scholar] [CrossRef]
- Özer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Egger, L. Validation of an In Vitro Digestive System for Studying Macronutrient Decomposition in Humans. J. Nutr. 2012, 142, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy żywienia dla populacji Polski i ich zastosowanie. Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny. 2020. Available online: https://ncez.pzh.gov.pl/wp-content/uploads/2021/03/normy_zywienia_2020web.pdf (accessed on 15 May 2022).
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (Text with EEA Relevance); European Union: Luxembourg, 2008.
- Evans, J.; Zulewska, J.; Newbold, M.; Drake, M.; Barbano, D. Comparison of composition and sensory properties of 80% whey protein and milk serum protein concentrates. J. Dairy Sci. 2010, 93, 1824–1843. [Google Scholar] [CrossRef]
- Suthar, J.; Jana, A.; Balakrishnan, S. High protein milk ingredients-A tool for value-addition to dairy and food products. J. Dairy Vet. Anim. Res. 2017, 6, 00171. [Google Scholar] [CrossRef]
- Singh, H. Heat stability of milk. Int. J. Dairy Technol. 2004, 57, 111–119. [Google Scholar] [CrossRef]
- Crowley, S.; Megemont, M.; Gazi, I.; Kelly, A.; Huppertz, T.; O’Mahony, J.A. Heat stability of reconstituted milk protein concentrate powders. Int. Dairy J. 2014, 37, 104–110. [Google Scholar] [CrossRef]
- Pandalaneni, K.; Amamcharla, J.K.; Marella, C.; Metzger, L.E. Influence of milk protein concentrates with modified calcium content on enteral dairy beverage formulations: Physicochemical properties. J. Dairy Sci. 2018, 101, 9714–9724. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Beausire, R.L.W.; Patel, S.; Patel, H. Innovative Uses of Milk Protein Concentrates in Product Development. J. Food Sci. 2015, 80, A23–A29. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.; Żulewska, J.; Newbold, M.; Barbano, D.M. Micellar casein concentrate production with a 3X, 3-stage, uniform transmembrane pressure ceramic membrane process at 50 °C. J. Dairy Sci. 2010, 93, 12, 5588–5600. [Google Scholar] [CrossRef]
- Verdi, R.; Barbano, D.; Dellavalle, M.; Senyk, G. Variability in True Protein, Casein, Nonprotein Nitrogen, and Proteolysis in High and Low Somatic Cell Milks. J. Dairy Sci. 1987, 70, 230–242. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists; Official Methods of Analysis; AOAC: Rockville, MD, USA, 1990. [Google Scholar]
- Moreno-Torres, R.; Navarro, M.; Ruóz-Lípez, M.D.; Artacho, R.; López, C. A mineralization procedure for determining magnesium in milk. LWT 2000, 33, 397–400. [Google Scholar] [CrossRef][Green Version]
- ISO 8070:2007, IDF 119:2007; Milk and Milk Products—Determination of Calcium, Sodium, Potassium and Magnesium Contents. Atomic Absorption Spectrometric Method. International Dairy Federation (IDF). International Dairy Federation AISBL: Brussels, Belgium, 2007.
- Pulliainen, T.K.; Wallin, H.C. Determination of Total Phosphorus in Foods by Colorimetric Measurement of Phosphorus as Molybdenum Blue after Dry-Ashing: NMKL1 Interlaboratory Study. J. AOAC Int. 1994, 77, 1557–1561. [Google Scholar] [CrossRef]
- Dumpler, J.; Huppertz, T.; Kulozik, U. Invited review: Heat stability of milk and concentrated milk: Past, present, and future research objectives. J. Dairy Sci. 2020, 103, 10986–11007. [Google Scholar] [CrossRef]
- Bielecka, M.; Cichosz, G. The effect of milk fat replacement and the addition of Lactobacillus paracasei LPC-37 on the sensory properties of cheeses. Mljekarstvo 2020, 70, 28–39. [Google Scholar] [CrossRef]
- Dobrzańska, A.; Cais-Sokolińska, D. Ocena przydatności systemów pomiaru barwy do badań preparatów białek mleka i serwatki. Apar. Badaw. Dydakt. 2014, 19, 267–272. [Google Scholar]
- EN ISO 13299:2016-05E; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. International Organization for Standardization: Geneva, Switzerland, 2016.
- EN ISO 8586:2014–03; Sensory Analysis. General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2014.
- Barłowska, J.; Brodziak, A.; Król, J.; Kędzierska-Matysek, M.; Litwińczuk, Z. Zawartość kazeiny w mleku krowim z regionu wschodniej Polski i jej zmiany w okresie 5 lat. Rocz. Nauk. Pol. Tow. Zootech. 2014, 10, 37–44. [Google Scholar]
- James, B.J.; Jing, Y.; Chen, X.D. Membrane fouling during filtration of milk—A microstructural study. J. Food Eng. 2003, 60, 431–437. [Google Scholar] [CrossRef]
- Zulewska, J.; Barbano, D.M. Influence of casein on flux and passage of serum proteins during microfiltration using polymeric spiral-wound membranes at 50 °C. J. Dairy Sci. 2013, 96, 2048–2060. [Google Scholar] [CrossRef]
- Tan, T.J.; Wang, D.; Moraru, C.I. A physicochemical investigation of membrane fouling in cold microfiltration of skim milk. J. Dairy Sci. 2014, 97, 4759–4771. [Google Scholar] [CrossRef]
- France, T.C.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. Cold Microfiltration as an Enabler of Sustainable Dairy Protein Ingredient Innovation. Foods 2021, 10, 2091. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Pasha, I.; Sameen, A.; Mukhtar, O.; Khan, M.I. Chemical Composition, Nitrogen Fractions and Amino Acids Profile of Milk from Different Animal Species. Asian-Australas. J. Anim. Sci. 2016, 29, 1022–1028. [Google Scholar] [CrossRef]
- Misawa, N.; Barbano, D.M.; Drake, M. Influence of casein as a percentage of true protein and protein level on color and texture of milks containing 1 and 2% fat. J. Dairy Sci. 2016, 99, 5284–5304. [Google Scholar] [CrossRef]
- Wojtasik, A.; Woźniak, A.; Stoś, K.; Jarosz, M. Składniki mineralne. In Normy Żywienia dla Populacji Polski i Ich Zastosowanie; Jarosz, M., Rychlik, E., Stoś, K., Charzewska, J., Eds.; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warsaw, Poland, 2020; pp. 273–315. [Google Scholar]
- Cheng, N.; Barbano, D.M.; Drake, M.A. Effect of pasteurization and fat, protein, casein to serum protein ratio, and milk temperature on milk beverage color and viscosity. J. Dairy Sci. 2019, 102, 2022–2043. [Google Scholar] [CrossRef]
- Popov-Raljić, J.V.; Lakić, N.S.; Laličić-Petronijević, J.G.; Barać, M.B.; Sikimić, V.M. Color changes of UHT milk during storage. Sensors 2008, 8, 5961–5974. [Google Scholar] [CrossRef]
- Magan, J.B.; O’Callaghan, T.F.; Zheng, J.; Zhang, L.; Mandal, R.; Hennessy, D.; Fenelon, M.A.; Wishart, D.S.; Kelly, A.L.; McCarthy, N.A. Effect of Diet on the Vitamin B Profile of Bovine Milk-Based Protein Ingredients. Foods 2020, 9, 578. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What Is the Color of Milk and Dairy Products and How Is It Measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef]
- Chudy, S.; Bilska, A.; Kowalski, R.; Teichert, J. Colour of milk and milk products in CIE L*a*b* space. Med. Weter. 2020, 76, 77–81. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef]
- Jeličić, I.; Božanić, R.; Tratnik, L. Whey-based beverages-a new generation of dairy products. Mljekarstvo 2008, 58, 257–274. [Google Scholar]
- Królczyk, J.B.; Dawidziuk, T.; Janiszewska-Turak, E.; Sołowiej, B. Use of Whey and Whey Preparations in the Food Industry—A Review. Pol. J. Food Nutr. Sci. 2016, 66, 157–165. [Google Scholar] [CrossRef]

| Amino Acid | MSMP | MCN75 | MCN85 | MSPC | MWPI | MWPC |
|---|---|---|---|---|---|---|
| Threonine | 8.33 ± 0.16 cd | 6.84 ± 0.55 b | 8.75 ± 0.01 cd | 4.81 ± 0.04 a | 7.93 ± 0.12 c | 8.98 ± 0.30 d |
| Valine | 11.96 ± 0.08 bc | 11.61 ± 0.28 bc | 12.43 ± 0.02 c | 10.03 ± 0.45 a | 11.30 ± 0.18 b | 11.45 ± 0.24 b |
| Isoleucine | 9.98 ± 0.07 bc | 9.47 ± 0.21 ab | 12.57 ± 0.01 c | 9.04 ± 0.51 a | 10.22 ± 0.13 bc | 10.06 ± 0.02 bc |
| Leucine | 24.27 ± 0.08 a | 24.40 ± 0.14 a | 23.92 ± 0.01 a | 26.08 ± 0.21 bc | 27.25 ± 0.53 c | 25.81 ± 0.63 b |
| Phenylalanine | 8.33 ± 0.15 b | 7.90 ± 0.04 ab | 8.18 ± 0.02 b | 7.17 ± 0.47 a | 7.16 ± 0.36 a | 7.56 ± 0.08 ab |
| Histidine | 4. 96 ± 0.01 ab | 5.27 ± 0.07 cd | 5.49 ± 0.01 d | 5.10 ± 0.12 bc | 4.82 ± 0.08 ab | 4.79 ± 0.13 a |
| Lysine | 16.88 ± 0.49 ab | 20.09 ± 0.99 b | 14.93 ± 0.02 a | 25.10±2.42 c | 17.85 ± 0.07 ab | 16.45 ± 0.13 ab |
| Arginine | 5.91 ± 0.04 bc | 5.76 ± 0.16 b | 6.85 ± 0.01 d | 5.32 ± 0.05 a | 5.78 ± 0.09 b | 6.09 ± 0.12 c |
| Tyrosine | 9.38 ± 0.20 b | 8.66 ± 0.44 ab | 9.13 ± 0.01 b | 7.35 ± 0.81 a | 7.71 ± 0.85 ab | 8.81 ± 0.12 ab |
| Sample | Calcium, mg 100 g−1 | Phosphorus, mg 100 g−1 | Ca:P Ratio |
|---|---|---|---|
| MSMP | 164.24 ± 0.41 c | 112.81 ± 0.36 f | 1.46 ± 0.02 a |
| MCN75 | 178.85 ± 0.58 f | 90.63 ± 0.38 e | 1.97 ± 0.01 c |
| MCN85 | 166.60 ± 0.14 d | 83.11 ± 1.05 d | 2.00 ± 0.03 d |
| MSPC | 142.38 ± 0.17 a | 66.32 ± 0.24 a | 2.15 ± 0.01 e |
| MWPI | 149.85 ± 0.43 b | 79.72 ± 0.25 c | 1.88 ± 0.01 b |
| MWPC | 167.54 ± 0.50 e | 72.08 ± 0.24 b | 2.32 ± 0.01 f |
| Mineral | Sex/Age (years) Group | RDA * (mg/day) | MSMP | MCN75 | MCN85 | MSPC | MWPI | MWPC |
|---|---|---|---|---|---|---|---|---|
| Calcium | Children aged 4–6 | 1000 | 16.4 | 17.9 | 16.7 | 14.2 | 15.0 | 16.8 |
| Boys aged 13–15 | 1300 | 12.6 | 13.8 | 12.8 | 11.0 | 11.5 | 12.9 | |
| Men aged 51–65 | 1000 | 16.4 | 17.9 | 16.7 | 14.2 | 15.0 | 16.8 | |
| Girls aged 13–15 | 1300 | 12.6 | 13.8 | 12.8 | 11.0 | 11.5 | 12.9 | |
| Women aged 51–65 | 1200 | 13.7 | 14.9 | 13,9 | 11.9 | 12.5 | 14.0 | |
| Phosphorus | Children aged 4–6 | 500 | 32.8 | 35.8 | 33.3 | 28.5 | 30.0 | 33.5 |
| Boys aged 13–15 | 1250 | 13.1 | 14.3 | 13.3 | 11.4 | 12.0 | 13.4 | |
| Men aged 51–65 | 700 | 23.5 | 25.6 | 23.8 | 20.3 | 21.4 | 23.9 | |
| Girls aged 13–15 | 1250 | 13.1 | 14.3 | 13.3 | 11.4 | 12.0 | 13.4 | |
| Women aged 51–65 | 700 | 23.5 | 25.6 | 23.8 | 20.3 | 21.4 | 23.9 |
| Sample | Viscosity mPa s | Heat Stability min |
|---|---|---|
| MSMP | 2.8 ± 0.1 a | 5.32 ± 0.08 a |
| MCN75 | 3.3 ± 0.1 b | 8.42 ± 0.03 c |
| MCN85 | 3.2 ± 0.1 b | 8.40 ± 0.13 c |
| MSPC | 4.1 ± 0.1 c | 7.49 ± 0.22 b |
| MWPI | 4.8 ± 0.1 d | 7.51 ± 0.12 b |
| MWPC | 3.2 ± 0.1 b | 7.53 ± 0.20 b |
| Sample | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| MSMP | 81.51 ± 0.13 a | −5.84 ± 0.01 a | 0.87 ± 0.08 b | 5.91 ± 0.02 b | 49.76 ± 0.03 b |
| MCN75 | 82.83 ± 0.13 c | −5.67 ± 0.01 c | 2.79 ± 0.04 c | 6.32 ± 0.02 d | 54.23 ± 0.13 e |
| MCN85 | 82.49± 0.08 b | −5.71 ± 0.02 b | 0.36 ± 0.01 a | 5.73 ± 0.01 a | 46.59 ± 0.13 a |
| MSPC | 83.18 ± 0.14 d | −5.03 ± 0.01 d | 3.63 ± 0.06 d | 6.20 ± 0.04 c | 52.64 ± 0.22 d |
| MWPI | 82.46 ± 0.06 b | −4.03 ± 0.02 e | 4.35 ± 0.03 e | 5.92 ± 0.03 b | 50.41 ± 0.33 c |
| MWPC | 82.76 ± 0.24 c | −5.65 ± 0.02 c | 2.77 ± 0.05 c | 6.29 ± 0.03 d | 53.92 ± 0.23 e |
| SAMPLE | MSMP | MCN75 | MCN85 | MSPC | MWPI | MWPC | p-Value | |
|---|---|---|---|---|---|---|---|---|
| APPEARANCE | Creamy color | 2.0 a | 2.0 a | 2.4 ab | 2.8 b | 3.0 b | 2.6 ab | 0.014 |
| Beige color | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | >0.05 | |
| Gray color | 1.9 | 1.9 | 1.8 | 1.9 | 2.0 | 1.9 | >0.05 | |
| Uniform color distribution | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | >0.05 | |
| Layer separation | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Adhesion to packaging | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| AROMA | Overall intensity | 2.6 abc | 2.5 ab | 2.3 a | 3.0 cd | 2.9 bcd | 3.3 d | 0.000 |
| Typical of additive | 1.3 | 1.3 | 1.3 | 1.4 | 1.3 | 1.4 | >0.05 | |
| Milky | 2.6 ab | 2.3 a | 2.3 a | 2.9 b | 2.9 b | 2.9 b | 0.003 | |
| Buttery | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Milk powder | 1.4 a | 1.4 a | 1.5 a | 2.6 b | 2.6 b | 2.6 b | 0.000 | |
| Sweet | 1.5 a | 1.5 a | 1.5 a | 2.0 ab | 2.3 b | 2.3 b | 0.016 | |
| Sour | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Whey | 1.0 a | 1.1 a | 1.1 a | 1.1 a | 1.9 b | 1.9 b | 0.000 | |
| Pasteurization | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | >0.05 | |
| Metallic | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Foreign | 1.1 | 1.4 | 1.3 | 1.3 | 1.3 | 1.3 | >0.05 | |
| CONSISTENCY | Homogeneous | 4.9 | 4.9 | 4.9 | 4.9 | 4.9 | 4.9 | >0.05 |
| Watery | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | >0.05 | |
| Adhesive | 1.0 | 1.0 | 1.0 | 1.3 | 1.3 | 1.3 | >0.05 | |
| Grainy | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Smooth mouthfeel | 4.0 | 4.0 | 3.9 | 4.3 | 4.3 | 4.3 | >0.05 | |
| Mealy mouthfeel | 1.4 a | 1.4 a | 2.1 b | 1.3 a | 1.3 a | 1.3 a | 0.021 | |
| Fatty mouthfeel | 1.4 a | 1.5 ab | 2.0 c | 1.5 ab | 1.9 bc | 2.0 c | 0.005 | |
| Sticky mouthfeel | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | >0.05 | |
| Thickness | 1.0 | 1.0 | 1.3 | 1.3 | 1.3 | 1.3 | >0.05 | |
| TASTE | Milky | 2.1 | 2.0 | 2.0 | 2.0 | 2.1 | 2.0 | >0.05 |
| Typical of additive | 1.0 a | 1.1 a | 1.0 a | 1.9 b | 1.9 b | 2.3 c | 0.000 | |
| Milk powder | 2.8 ab | 2.3 a | 2.4 a | 3.1 b | 3.0 b | 3.9 c | 0.000 | |
| Sweet | 3.4 b | 2.5 a | 2.5 a | 2.9 ab | 3.6 b | 3.6 b | 0.002 | |
| Salty | 1.3 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | >0.05 | |
| Sour | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Bitter | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Whey | 1.1 a | 1.3 a | 1.3 a | 1.4 a | 2.4 b | 2.5 b | 0.004 | |
| Chalky | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.1 | >0.05 | |
| Cardboard | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 | 1.1 | >0.05 | |
| Astringent | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.05 | |
| Pasteurization | 1.6 | 1.6 | 1.5 | 1.6 | 1.6 | 1.6 | >0.05 | |
| Foreign | 1.4 | 1.4 | 1.3 | 1.3 | 1.3 | 1.4 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełczewska, K.; Dąbrowska, A.; Bielecka, M.M.; Dec, B.; Baranowska, M.; Ziajka, J.; Zhennai, Y.; Żulewska, J. Protein Preparations as Ingredients for the Enrichment of Non-Fermented Milks. Foods 2022, 11, 1817. https://doi.org/10.3390/foods11131817
Kiełczewska K, Dąbrowska A, Bielecka MM, Dec B, Baranowska M, Ziajka J, Zhennai Y, Żulewska J. Protein Preparations as Ingredients for the Enrichment of Non-Fermented Milks. Foods. 2022; 11(13):1817. https://doi.org/10.3390/foods11131817
Chicago/Turabian StyleKiełczewska, Katarzyna, Aneta Dąbrowska, Marika Magdalena Bielecka, Bogdan Dec, Maria Baranowska, Justyna Ziajka, Yang Zhennai, and Justyna Żulewska. 2022. "Protein Preparations as Ingredients for the Enrichment of Non-Fermented Milks" Foods 11, no. 13: 1817. https://doi.org/10.3390/foods11131817
APA StyleKiełczewska, K., Dąbrowska, A., Bielecka, M. M., Dec, B., Baranowska, M., Ziajka, J., Zhennai, Y., & Żulewska, J. (2022). Protein Preparations as Ingredients for the Enrichment of Non-Fermented Milks. Foods, 11(13), 1817. https://doi.org/10.3390/foods11131817