Effects of High-Intensity Ultrasound Treatments on the Physicochemical and Structural Characteristics of Sodium Caseinate (SC) and the Stability of SC-Coated Oil-in-Water (O/W) Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
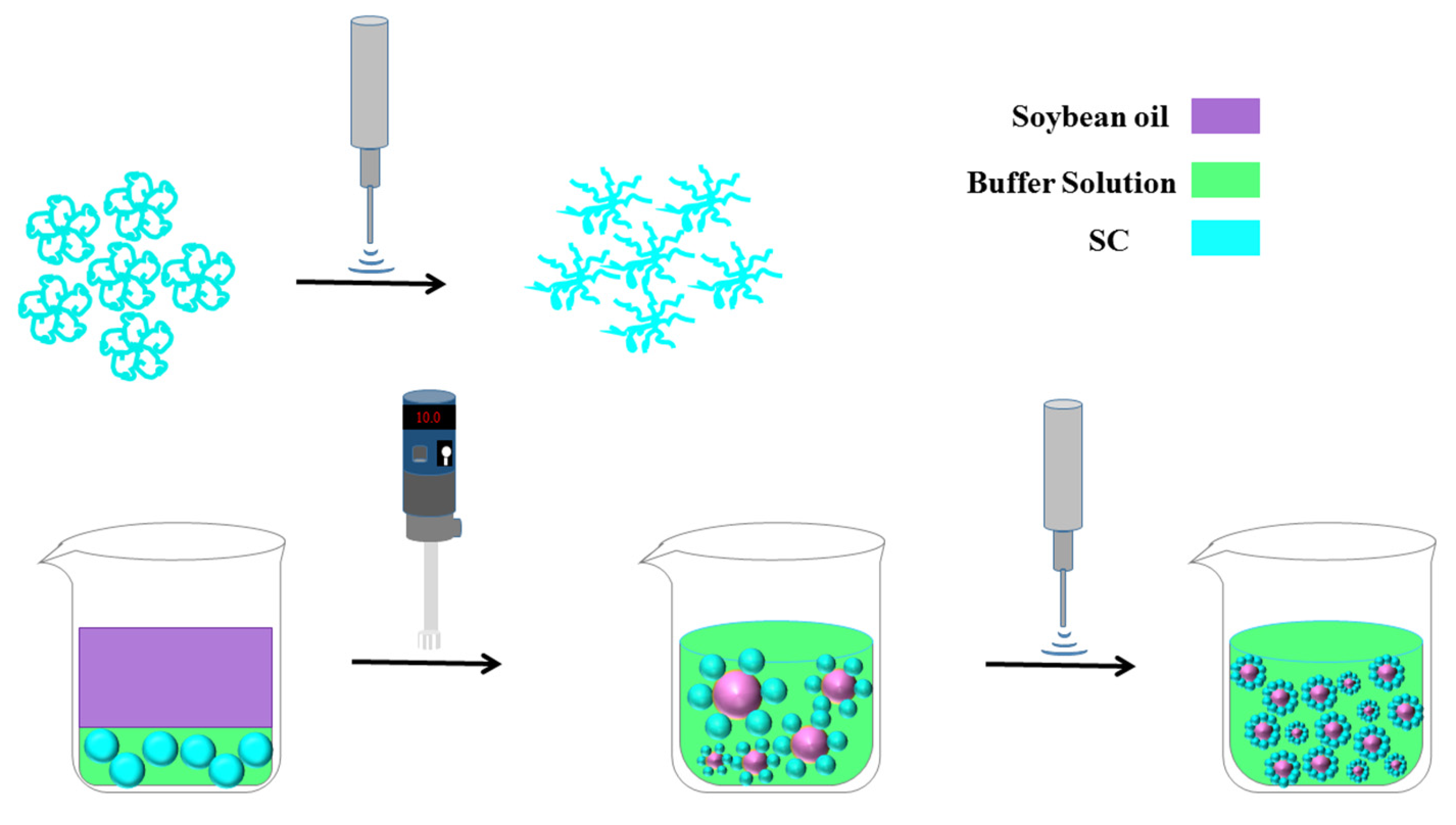
2.2. Preparing O/W Emulsions and the High-Intensity Ultrasound
2.3. Physicochemical and Structural Analysis
2.3.1. Particle Size and Zeta Potential Measurement
2.3.2. SDS-PAGE
2.3.3. FTIR
2.3.4. UV–Vis Spectroscopy
2.3.5. Intrinsic Fluorescence Spectroscopy
2.3.6. Surface Hydrophobicity
2.3.7. SEM
2.4. Emulsifying Properties
2.5. Zeta Potential
2.6. Influence of Environmental Stresses on Emulsions
2.6.1. Storage Stability
2.6.2. Thermal Stability
2.6.3. Freeze–Thaw Stability
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties and Structure
3.1.1. Particle Size and Zeta Potential
3.1.2. SDS-PAGE
3.1.3. FTIR
3.1.4. UV–Vis Spectroscopy
3.1.5. Intrinsic Fluorescence
3.1.6. Surface Hydrophobicity
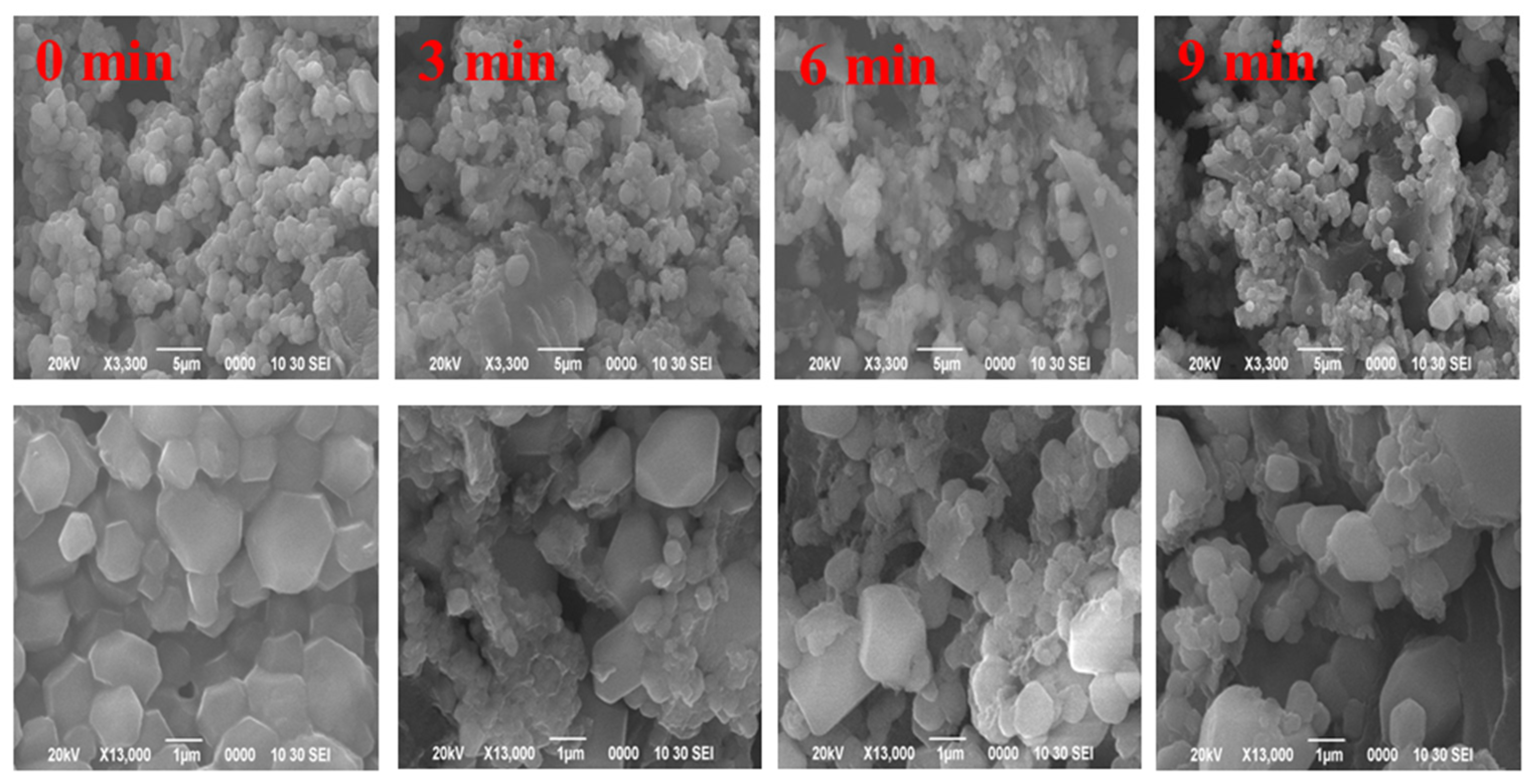
3.1.7. SEM
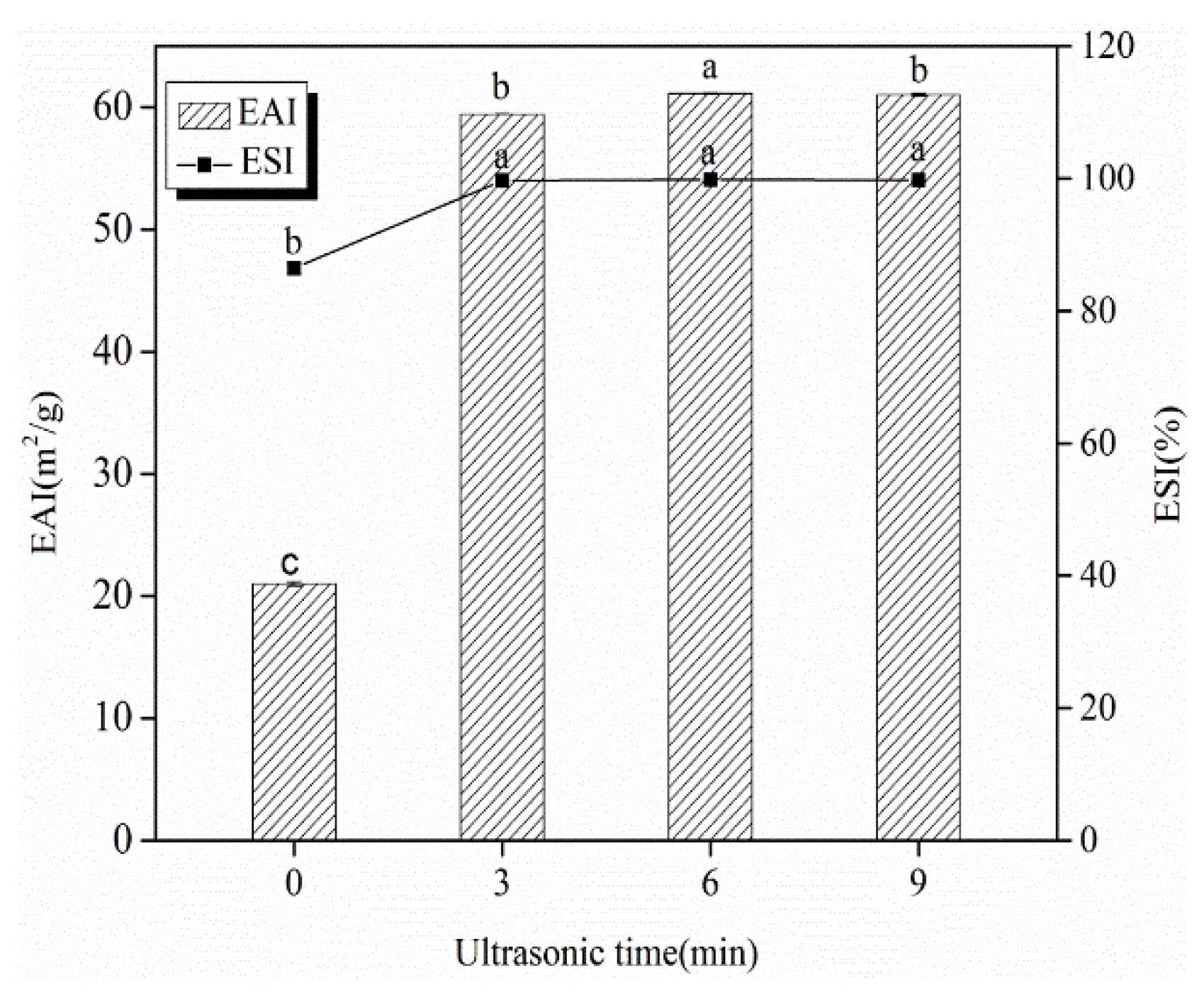
3.2. EAI and ESI
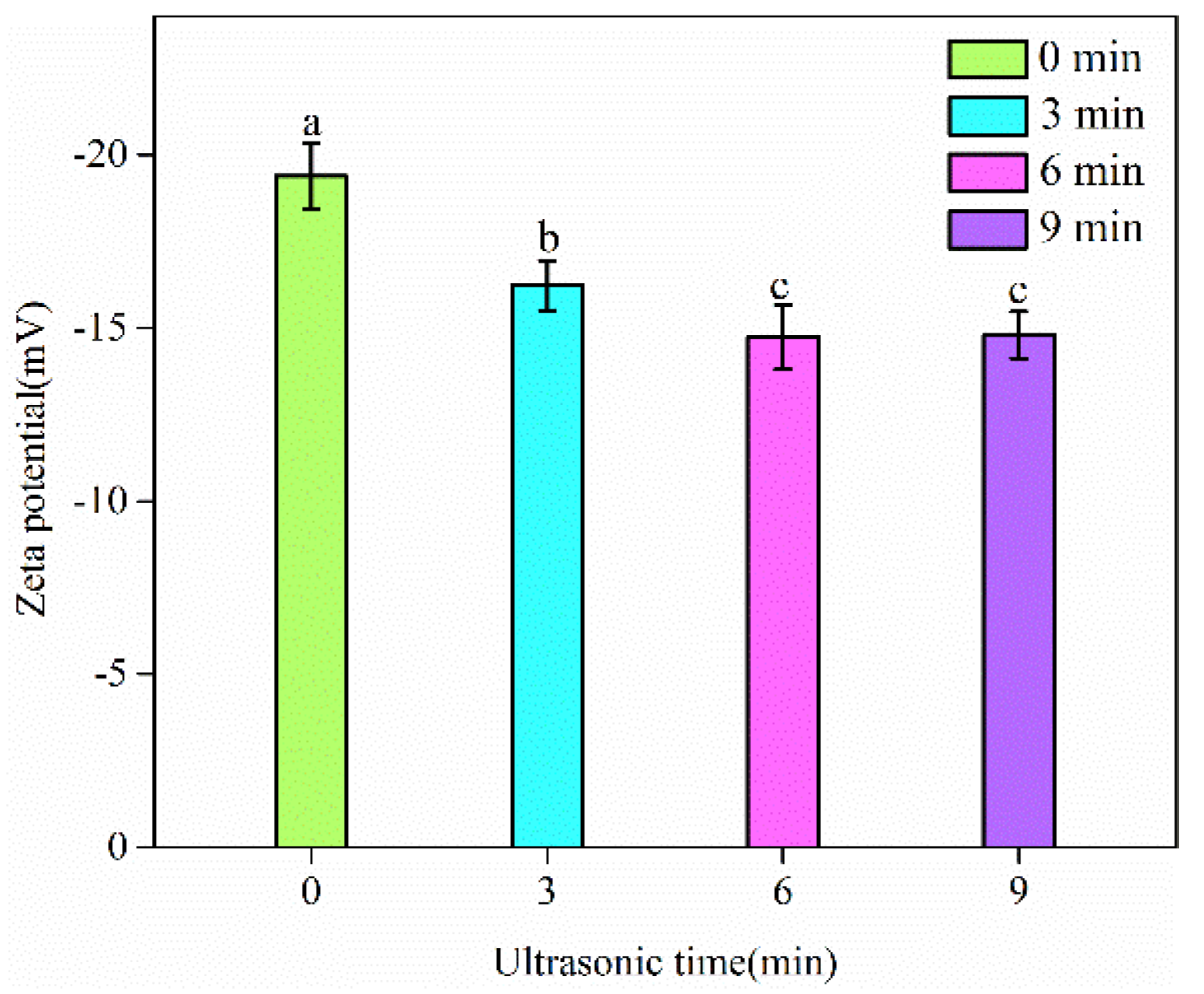
3.3. Zeta Potential of Emulsions
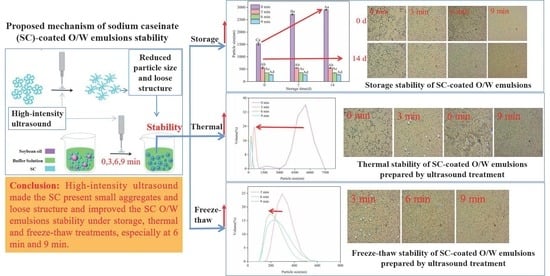
3.4. Impact of Environmental Stresses on Emulsion Stability
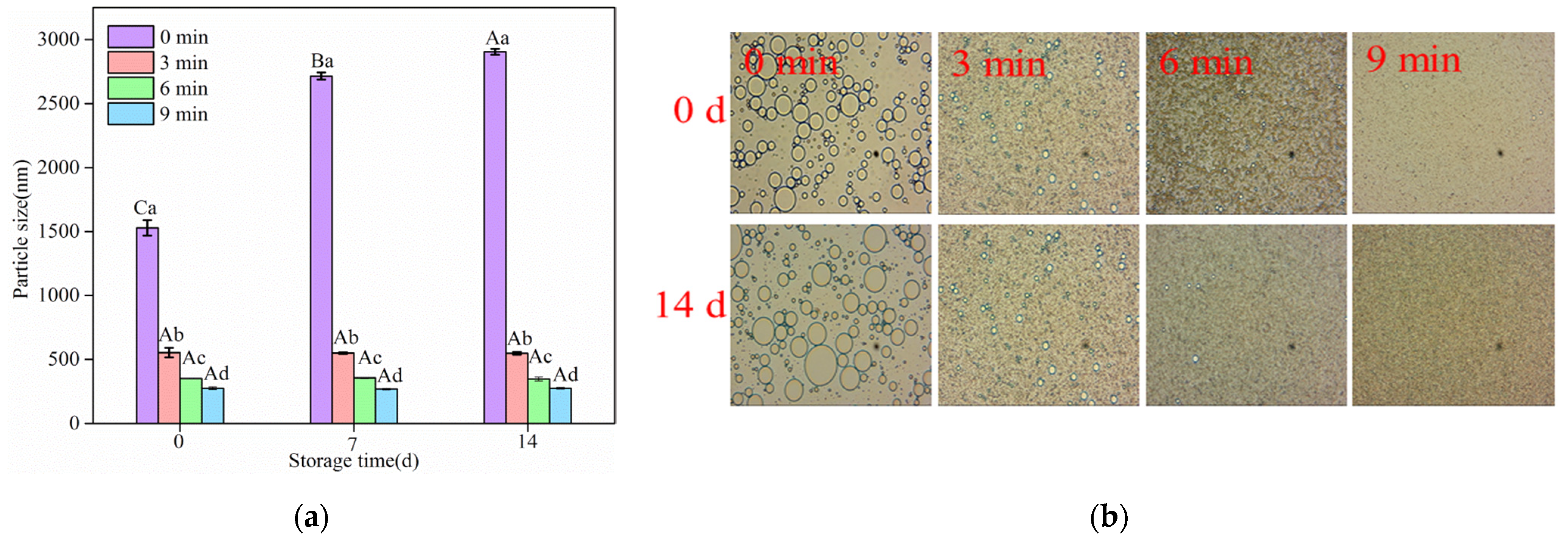
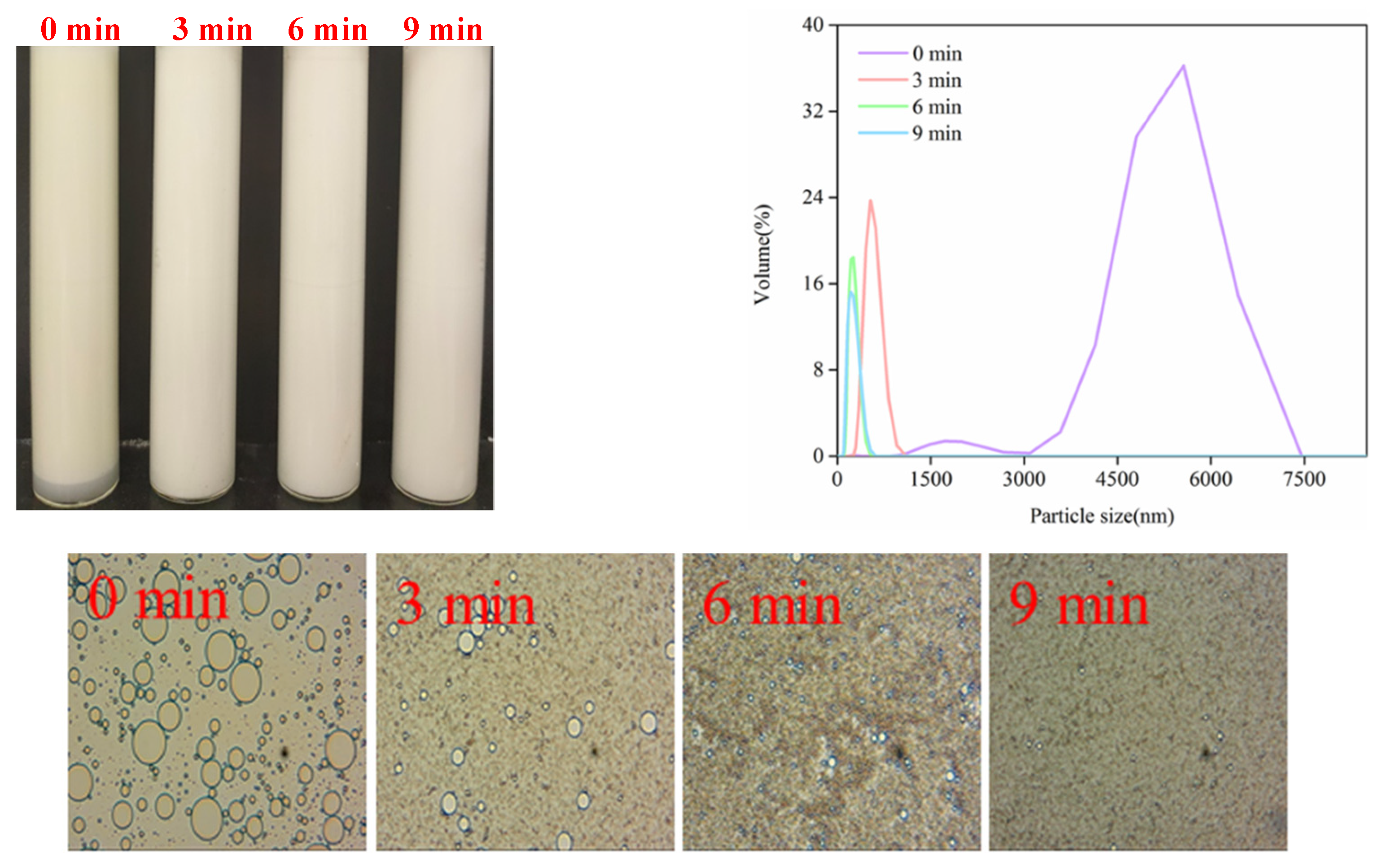
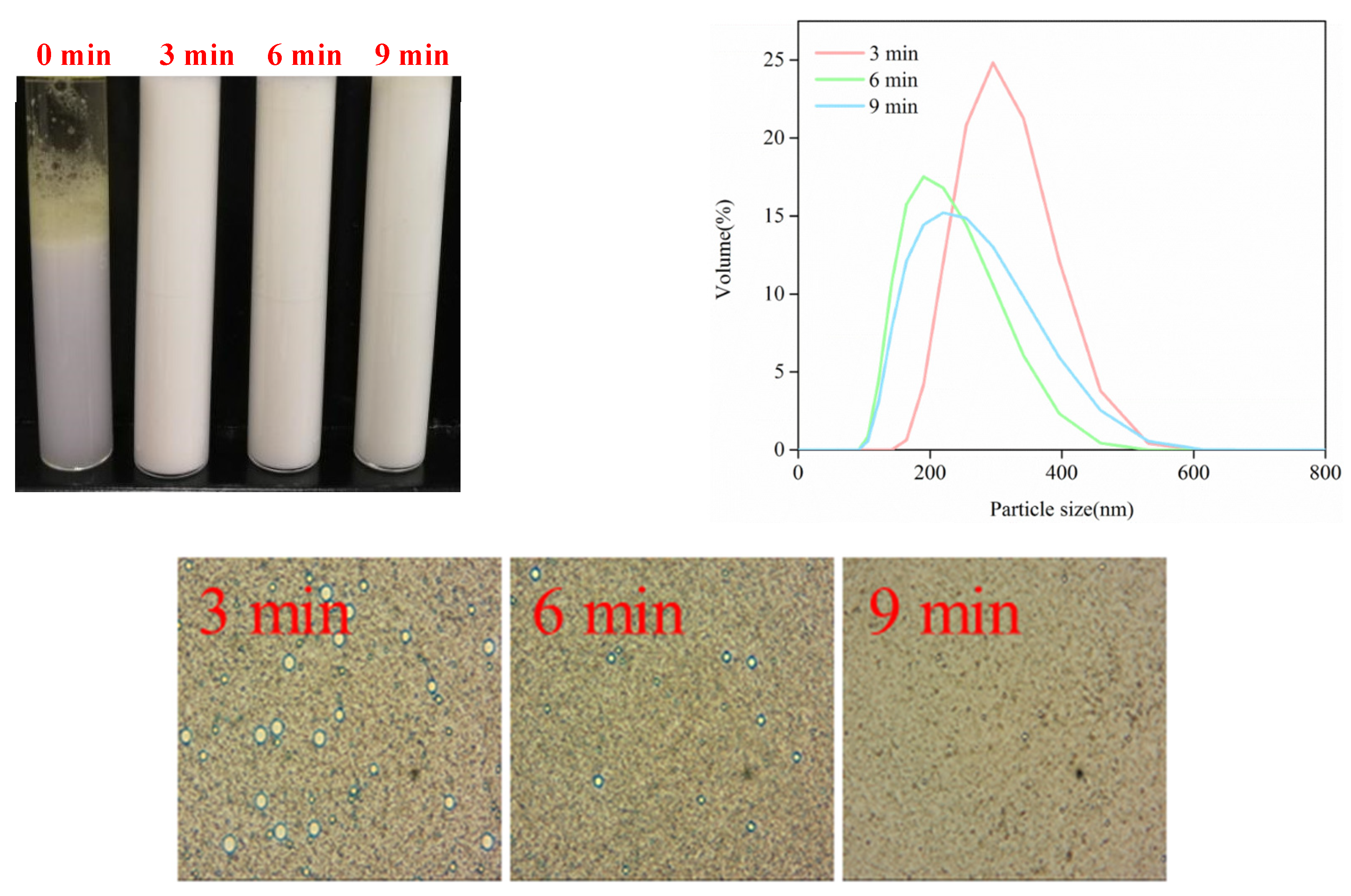
3.4.1. Storage Stability
3.4.2. Thermal Stability
3.4.3. Freeze–Thaw Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Xu, W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT-Food Sci. Technol. 2020, 129, 109563. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Liu, Q.; Kong, B.; Diao, X. Effects of zein hydrolysates coupled with sage (salvia officinalis) extract on the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions. Food Hydrocoll. 2019, 87, 149–157. [Google Scholar] [CrossRef]
- Silva, K.C.; Sato, A.C. Sonication technique to produce emulsions: The impact of ultrasonic power and gelatin concentration. Ultrason. Sonochem. 2019, 52, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zheng, F.; Ma, F.; Kang, H.; Ren, H. The physical and oxidative stabilities of Pickering emulsion stabilized by starch particle and small molecular surfactant. Food Chem. 2020, 303, 125391. [Google Scholar] [CrossRef]
- Wu, N.N.; Huang, X.; Yang, X.Q.; Guo, J.; Zheng, E.L.; Yin, S.W.; Zhu, J.H.; Qi, J.R.; He, X.T.; Zhang, J.B. Stabilization of soybean oil body emulsions using ι-carrageenan: Effects of salt, thermal treatment and freeze-thaw cycling. Food Hydrocoll. 2012, 28, 110–120. [Google Scholar] [CrossRef]
- Zhao, Q.; Long, Z.; Kong, J.; Liu, T.; Sunwaterhouse, D.; Zhao, M. Sodium caseinate/flaxseed gum interactions at oil–water interface: Effect on protein adsorption and functions in oil-in-water emulsion. Food Hydrocoll. 2015, 43, 137–145. [Google Scholar] [CrossRef]
- Su, D.; Zhong, Q. Lemon oil nanoemulsions fabricated with sodium caseinate and Tween 20 using phase inversion temperature method. J. Food Eng. 2016, 171, 214–221. [Google Scholar] [CrossRef]
- Jahaniaval, F.; Kakuda, Y.; Abraham, V.; Marcone, M.F. Soluble protein fractions from pH and heat treated sodium caseinate: Physicochemical and functional properties. Food Res. Int. 2000, 33, 637–647. [Google Scholar] [CrossRef]
- de Figueiredo Furtado, G.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and emulsifying properties of sodium caseinate and lactoferrin influenced by ultrasound process. Food Hydrocoll. 2017, 63, 178–188. [Google Scholar] [CrossRef]
- Bi, A.; Xu, X.; Guo, Y.; Du, M.; Yu, C.; Wu, C. Ultrasound pre-fractured casein and in-situ formation of high internal phase emulsions. Ultrason. Sonochem. 2019, 64, 104916. [Google Scholar] [CrossRef]
- Han, T.; Wang, M.; Wang, Y.; Tang, L. Effects of high-pressure homogenization and ultrasonic treatment on the structure and characteristics of casein. LWT-Food Sci. Technol. 2020, 130, 109560. [Google Scholar] [CrossRef]
- Zhang, R.; Pang, X.; Lu, J.; Liu, L.; Zhang, S.; Lv, J. Effect of high intensity ultrasound pretreatment on functional and structural properties of micellar casein concentrates. Ultrason. Sonochem. 2018, 47, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Liu, C.L.; Fu, L.; Zhao, Y.Y.; Zhang, Y.Y.; Wang, Y.T.; Bai, Y.H. Improving interfacial properties, structure and oxidative stability by ultrasound application to sodium caseinate prepared pre-emulsified soybean oil. LWT-Food Sci. Technol. 2020, 131, 109775. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Role of interfacial protein membrane in oxidative stability of vegetable oil substitution emulsions applicable to nutritionally modified sausage. Meat Sci. 2015, 109, 56–65. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Villalobos-Carvajal, R.; Herrera-Lavados, C.; Moreno-Osorio, L.; Jarpa-Parra, M.; Pérez-Won, M. Physicochemical properties of high-pressure treated lentil protein-based nanoemulsions. LWT-Food Sci. Technol. 2019, 101, 590–598. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Ning, C.; Bao, P.; Fang, H.; Zhou, C. L-Arginine and L-Lysine improve the physical stability of soybean oil-myosin emulsions by changing penetration and unfolding behaviors of interfacial myosin. Food Hydrocoll. 2020, 98, 105265. [Google Scholar] [CrossRef]
- Li, K.; Fu, L.; Zhao, Y.Y.; Xue, S.W.; Wang, P.; Xu, X.L.; Bai, Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Sui, X.; Bi, S.; Qi, B.; Wang, Z.; Zhang, M.; Li, Y.; Jiang, L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar] [CrossRef]
- Shi, R.; Liu, Y.; Hu, J.; Gao, H.; Qayum, A.; Bilawal, A. Munkh-Amgalan G. Combination of high-pressure homogenization and ultrasound improves physiochemical, interfacial and gelation properties of whey protein isolate. Innov. Food Sci. Emerg. 2020, 65, 102450. [Google Scholar] [CrossRef]
- Taha, A.; Hu, T.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S.; Hu, H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, T.; Hou, F.; Chen, W.; Miao, S.; Liu, D. Formation of soy protein isolate (SPI)-citrus pectin (CP) electrostatic complexes under a high-intensity ultrasonic field: Linking the enhanced emulsifying properties to physicochemical and structural properties. Ultrason. Sonochem. 2019, 59, 104748. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, P.; Li, P.; Zhang, K.; Zhang, M.; Sun, Z.; Sun, C.; Wang, D.; Cao, J.; Xu, W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018, 113, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, N.A.; Kelly, P.M.; Maher, P.G.; Fenelon, M.A. Dissolution of milk protein concentrate (MPC) powders by ultrasonication. J. Food Eng. 2014, 126, 142–148. [Google Scholar] [CrossRef]
- Ma, S.; Yang, X.; Zhao, C.; Guo, M. Ultrasound-induced changes in structural and physicochemical properties of β-lactoglobulin. Food Sci. Nutr. 2018, 6, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Gorczyca, E.; Kasapis, S.; Zisu, B. Effect of low-frequency ultrasound on the particle size, solubility and surface charge of reconstituted sodium caseinate. Ultrason. Sonochem. 2019, 58, 104525. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Li, P.; Cai, P.; Zhang, M.; Sun, Z.; Sun, C.; Geng, Z.; Xu, W.; Xu, X.; et al. Effects of ultrasound assisted extraction on the physiochemical, structural and functional characteristics of duck liver protein isolate. Process Biochem. 2016, 52, 174–182. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, R. Effect of ultrasonication on secondary structure and heat induced gelation of chicken myofibrils. J. Food Sci. Tech. 2016, 53, 3340. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Paniwnyk, L.; Herceg, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- Tian, R.; Zhu, G.; Feng, J.; Tian, B.; Sui, X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020, 68, 105202. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Kamińska-Dwórznicka, A.; Antczak, A.; Jakubczyk, E.; Matwijczuk, A. Effect of ι-carrageenan and its acidic and enzymatic hydrolysates on ice crystal structure changes in model sucrose solution. Colloid. Surface A. 2022, 643, 128744. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, Y.; Zhang, C.; Wan, J.; Shah, B.R.; Pei, Y.; Li, B. High intensity ultrasound modified ovalbumin: Structure, interface and gelation properties. Ultrason. Sonochem. 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, S.; Oladejo, A.O.; Ruan, S.; Wang, Y.; Ma, H. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason. Sonochem. 2017, 38, 19–28. [Google Scholar] [CrossRef]
- Mach, H.; Volkin, D.B.; Burke, C.J.; Middaugh, C.R. Ultraviolet Absorption Spectroscopy. Methods Mol. Biol. 1995, 40, 91–114. [Google Scholar]
- Wang, K.; Sun, D.; Pu, H.; Wei, Q. Principles and applications of spectroscopic techniques for evaluating food protein conformational changes: A review. Trends Food Sci. Tech. 2017, 67, 207–219. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, X.X.; Ma, F.; Xu, B.C.; Li, P.J.; Chen, C.G. Origin of high-pressure induced changes in the properties of reduced-sodium chicken myofibrillar protein gels containing CaCl2: Physicochemical and molecular modification perspectives. Food Chem. 2020, 319, 126535. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus Major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Mcclements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Vera, A.; Valenzuela, M.A.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Conformational and physicochemical properties of quinoa proteins affected by different conditions of high-intensity ultrasound treatments. Ultrason. Sonochem. 2019, 51, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Flores-Jimenez, N.T.; Ulloa, J.A.; Silvas, J.E.U.; Ramirez, J.C.R.; Ulloa, P.R.; Rosales, P.U.B.; Carrillo, Y.S.; Leyva, R.G. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019, 121, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Resendiz-Vazquez, J.A.; Ulloa, J.A.; Urias-Silvas, J.E.; Bautista-Rosales, P.U.; Ramirez-Ramirez, J.C.; Rosas-Ulloa, P.; Gonzalez-Torres, L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017, 37, 436–444. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, Y.; Chen, J.; Zou, J.; Wang, Q.; Ma, M. Influence of high-intensity ultrasound on foaming and structural properties of egg white. Food Res. Int. 2018, 108, 604–610. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, J.; Wang, L.; Lu, F.; Wei, B.; Azam, R.S.; Bhandari, B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020, 63, 104930. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Saini, C.S. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: Effect of high intensity ultrasound. Food Hydrocoll. 2018, 81, 229–241. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidity technique. J. Agr. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Jiang, W.; Zhao, H.; Fu, J. Ability of casein hydrolysate-carboxymethyl chitosan conjugates to stabilize a nanoemulsion: Improved freeze-thaw and pH stability. Food Hydrocoll. 2020, 101, 105452. [Google Scholar] [CrossRef]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018, 41, 382–388. [Google Scholar] [CrossRef]
- Silva, E.K.; Gomes, M.T.M.; Hubinger, M.D.; Cunha, R.L.; Meireles, M.A.A. Ultrasound-assisted formation of annatto seed oil emulsions stabilized by biopolymers. Food Hydrocoll. 2015, 47, 1–13. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Li, B. Novel food-grade Pickering emulsions stabilized by tea water-insoluble protein nanoparticles from tea residues. Food Hydrocoll. 2019, 96, 322–330. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, D. Stability of oil-in-water emulsions performed by ultrasound power or high-pressure homogenization. PLoS ONE 2019, 14, e0213189. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An overview of ultrasound-assisted food-grade nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Leong, T.S.; Martin, G.J.; Ashokkumar, M. Ultrasonic encapsulation—A review. Ultrason. Sonochem. 2017, 35, 605–614. [Google Scholar] [CrossRef]
- Desrumaux, A.; Marcand, J. Formation of sunflower oil emulsions stabilized by whey proteins with high-pressure homogenization (up to 350 MPa): Effect of pressure on emulsion characteristics. Int. J. Food Sci. Tech. 2002, 37, 263–269. [Google Scholar] [CrossRef]
- Lad, V.N.; Murthy, Z.V. Enhancing the stability of oil-in-water emulsions emulsified by coconut milk protein with the application of acoustic cavitation. Ind. Eng. Chem. Res. 2012, 51, 4222–4229. [Google Scholar] [CrossRef]
- Kaltsa, O.; Michon, C.; Yanniotis, S.; Mandala, I. Ultrasonic energy input influence on the production of sub-micron O/W emulsions containing whey protein and common stabilizers. Ultrason. Sonochem. 2013, 20, 881–891. [Google Scholar] [CrossRef]
- Troncoso, E.; Aguilera, J.M.; Mcclements, D.J. Fabrication, characterization and lipase digestibility of food-grade nanoemulsions. Food Hydrocoll. 2012, 27, 355–363. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Chen, Z.; Li, Y.; Liu, C.; Xu, J. Fabrication of β-conglycinin-stabilized nanoemulsions via ultrasound process and influence of SDS and PEG 10000 co-emulsifiers on the physicochemical properties of nanoemulsions. Food Res. Int. 2018, 106, 800–808. [Google Scholar] [CrossRef]
- Chu, B.S.; Ichikawa, S.; Kanafusa, S.; Nakajima, M. Stability of protein stabilised β-carotene nanodispersions against heating, salts and pH. J. Sci. Food Agr. 2008, 88, 1764–1769. [Google Scholar] [CrossRef]
- Teo, A.; Goh, K.K.; Wen, J.; Oey, I.; Ko, S.; Kwak, H.; Lee, S.J. Physicochemical properties of whey protein, lactoferrin and Tween 20 stabilised nanoemulsions: Effect of temperature, pH and salt. Food Chem. 2016, 197, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, N.; Lin, W.; Tang, C. Freeze-thaw stability of pickering emulsions stabilized by soy and whey protein particles. Food Hydrocoll. 2017, 69, 173–184. [Google Scholar] [CrossRef]


| Ultrasonic Time (min) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|
| 0 | 274.9 ± 5.1 a | −11.73 ± 1.01 a |
| 3 | 241.0 ± 3.7 b | −13.07 ± 0.32 a |
| 6 | 208.3 ± 3.2 c | −12.98 ± 0.50 a |
| 9 | 205.1 ± 9.1 c | −12.76 ± 0.83 a |
| Ultrasonic Time (min) | α-Helix | β-Sheet | β-Turn | Random Coil |
|---|---|---|---|---|
| 0 | 41.21 ± 1.42 a | 24.12 ± 0.77 a | 17.38 ± 0.64 a | 18.72 ± 0.51 a |
| 3 | 39.07 ± 2.89 a | 25.20 ± 0.40 a | 17.71 ± 0.72 a | 19.25 ± 1.52 a |
| 6 | 41.86 ± 1.05 a | 24.34 ± 0.71 a | 17.63 ± 0.79 a | 20.56 ± 1.23 a |
| 9 | 40.85 ± 2.45 a | 24.32 ± 0.05 a | 18.57 ± 0.06 a | 19.65 ± 1.35 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Jia, S.; Wan, J.; Li, Y.; Zhang, Y.; Zhu, H.; Li, K. Effects of High-Intensity Ultrasound Treatments on the Physicochemical and Structural Characteristics of Sodium Caseinate (SC) and the Stability of SC-Coated Oil-in-Water (O/W) Emulsions. Foods 2022, 11, 2817. https://doi.org/10.3390/foods11182817
He X, Jia S, Wan J, Li Y, Zhang Y, Zhu H, Li K. Effects of High-Intensity Ultrasound Treatments on the Physicochemical and Structural Characteristics of Sodium Caseinate (SC) and the Stability of SC-Coated Oil-in-Water (O/W) Emulsions. Foods. 2022; 11(18):2817. https://doi.org/10.3390/foods11182817
Chicago/Turabian StyleHe, Xiangli, Shangxi Jia, Jiayun Wan, Yan Li, Yanyan Zhang, He Zhu, and Ke Li. 2022. "Effects of High-Intensity Ultrasound Treatments on the Physicochemical and Structural Characteristics of Sodium Caseinate (SC) and the Stability of SC-Coated Oil-in-Water (O/W) Emulsions" Foods 11, no. 18: 2817. https://doi.org/10.3390/foods11182817
APA StyleHe, X., Jia, S., Wan, J., Li, Y., Zhang, Y., Zhu, H., & Li, K. (2022). Effects of High-Intensity Ultrasound Treatments on the Physicochemical and Structural Characteristics of Sodium Caseinate (SC) and the Stability of SC-Coated Oil-in-Water (O/W) Emulsions. Foods, 11(18), 2817. https://doi.org/10.3390/foods11182817