Bioprocessing of Wheat and Amaranth Bran for the Reduction of Fructan Levels and Application in 3D-Printed Snacks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
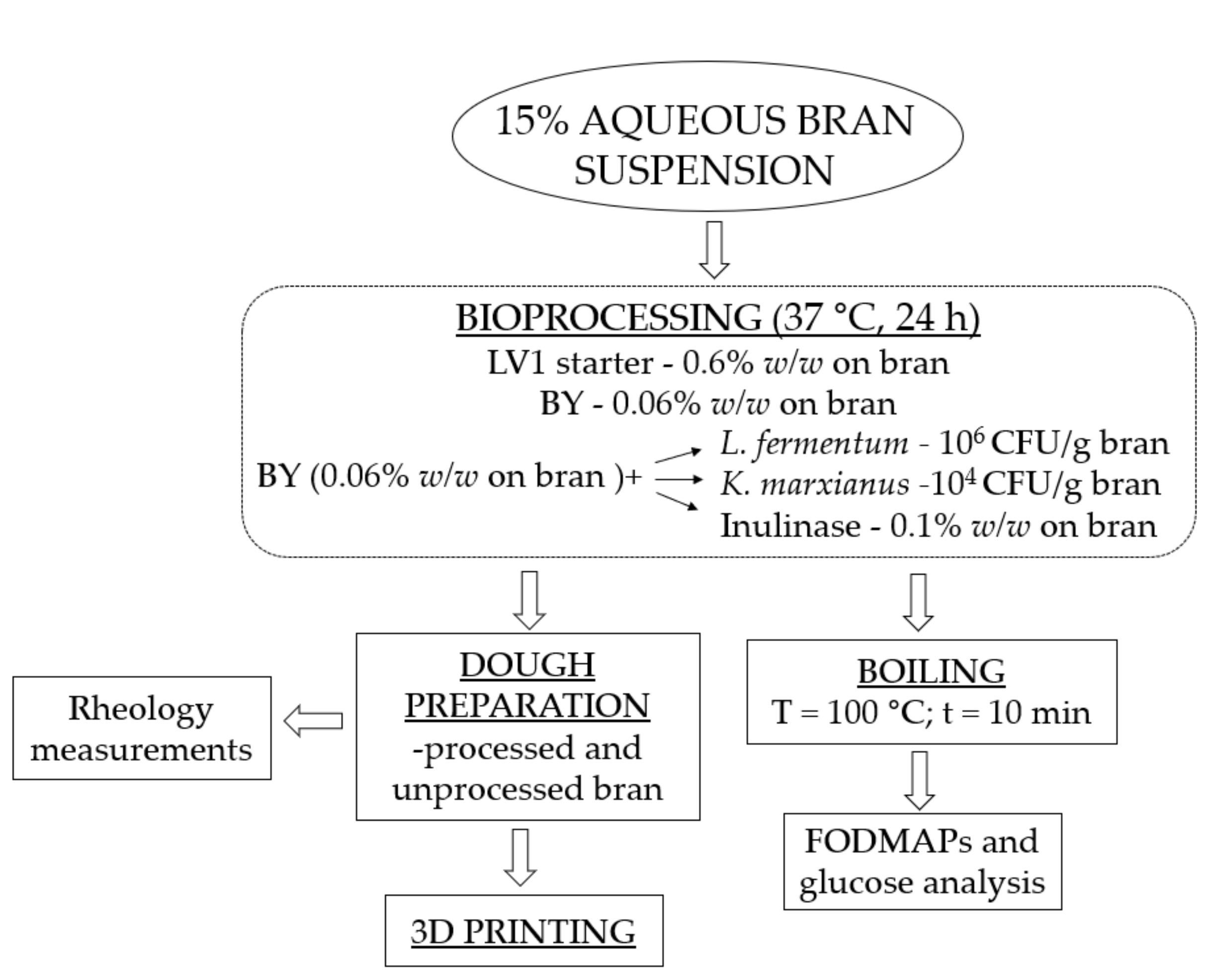
2.2. Bioprocessing of Bran
2.3. FODMAP Content
2.4. Preparation of Dough
2.5. Rheological Properties
2.6. Three-dimensional Printing and Post-Process Baking
2.7. Three-dimensional Printability and Physical Properties of Baked Snacks
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. FODMAP Content
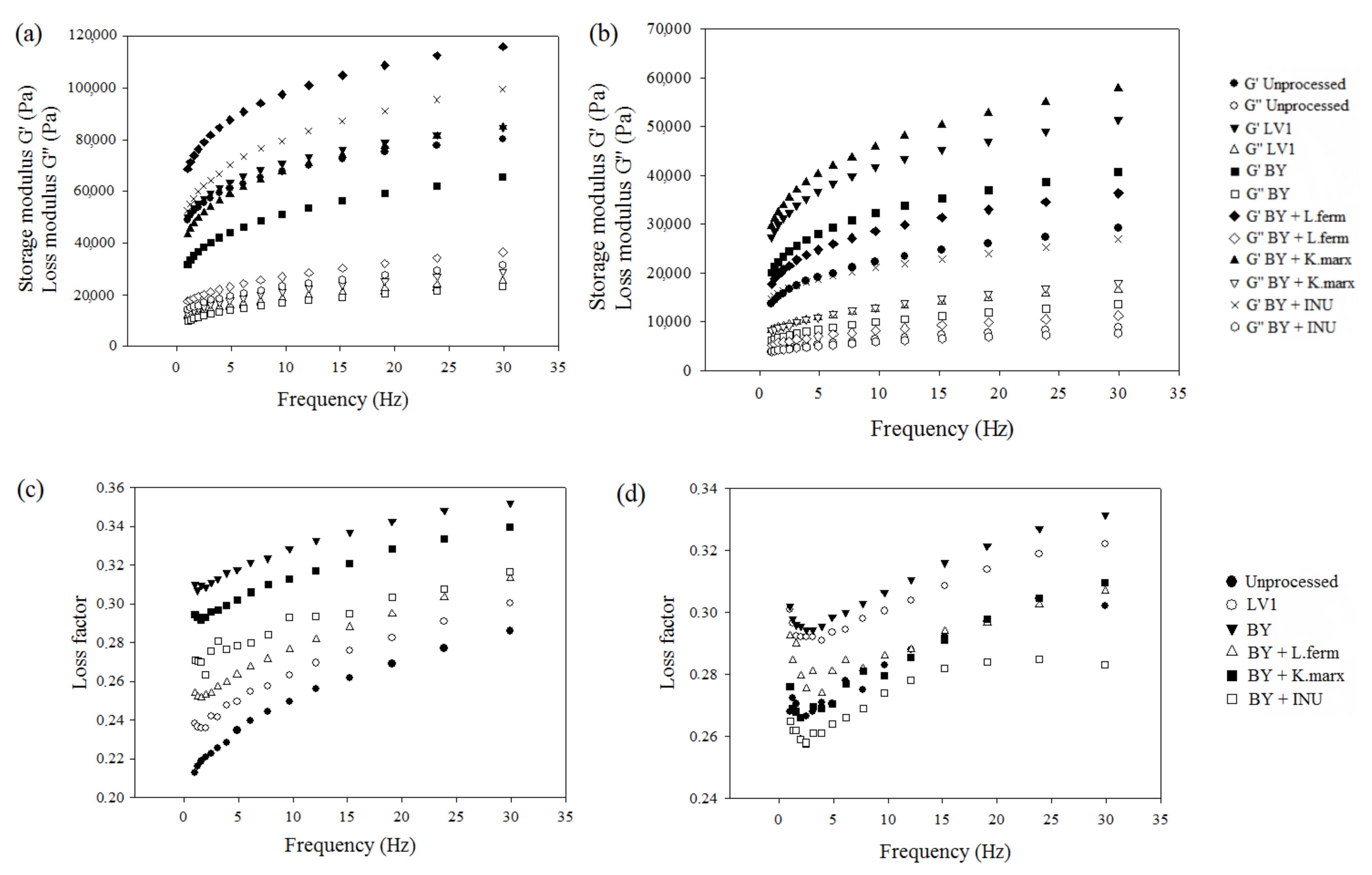
3.2. Effect of Bran Bioprocessing on the Rheology and 3D-Printability of the Dough
3.3. Physical and Sensorial Attributes of Snack
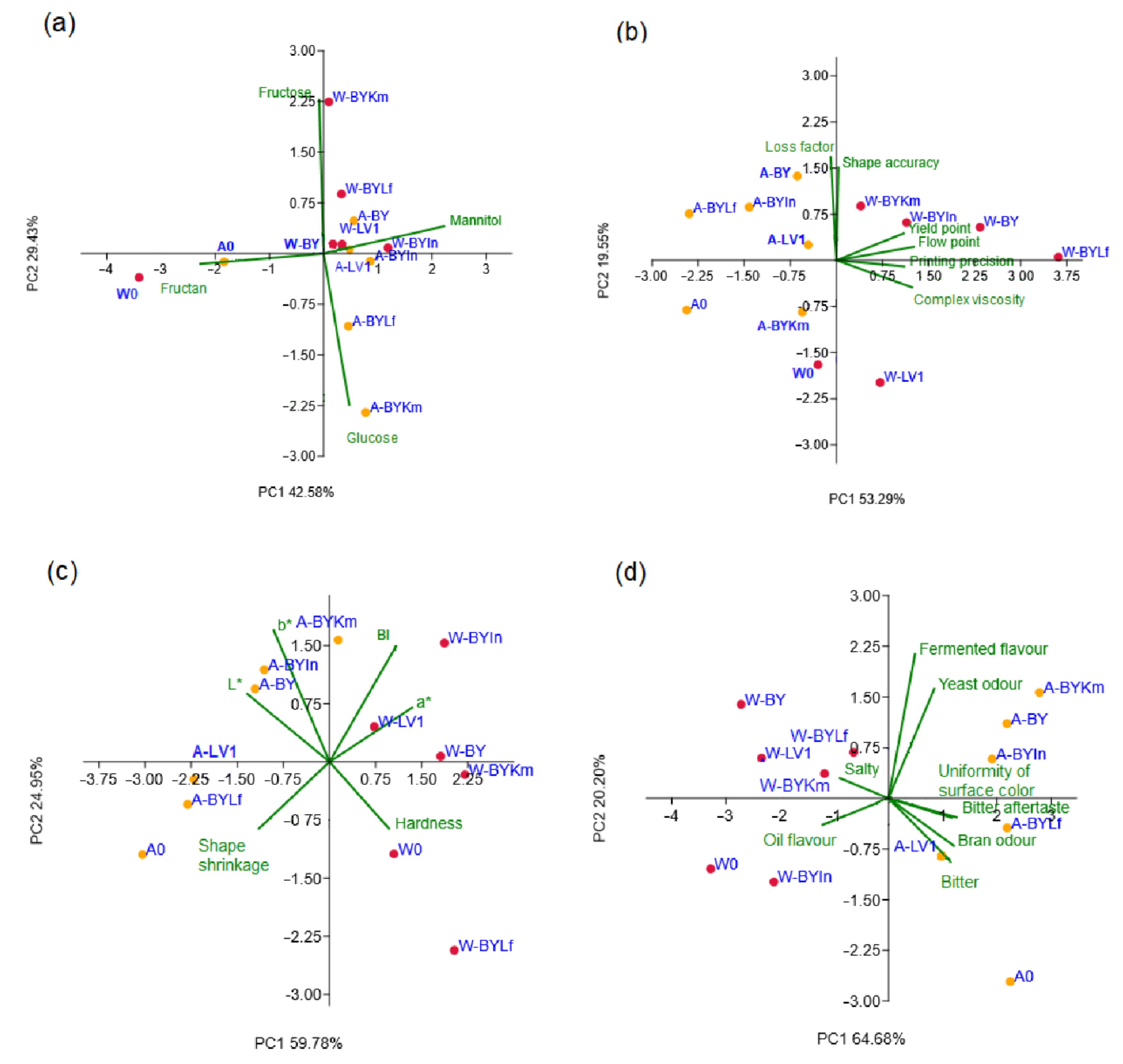
3.4. PCA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atzler, J.J.; Ispiryan, L.; Gallagher, E.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Enzymatic degradation of FODMAPS via application of β-fructofuranosidases and α-galactosidases-A fundamental study. J. Cereal Sci. 2020, 95, 102993. [Google Scholar] [CrossRef]
- Fang, S.; Yan, B.; Tian, F.; Lian, H.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. β-fructosidase FosE activity in Lactobacillus paracasei regulates fructan degradation during sourdough fermentation and total FODMAP levels in steamed bread. LWT 2021, 145, 111294. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Ellilä, S.; Nordlund, E.; Poutanen, K. Reduction of FODMAP content by bioprocessing. Trends Food Sci. Technol. 2020, 99, 257–272. [Google Scholar] [CrossRef]
- Laurent, J.; Timmermans, E.; Struyf, N.; Verstrepen, K.J.; Courtin, C.M. Variability in yeast invertase activity determines the extent of fructan hydrolysis during wheat dough fermentation and final FODMAP levels in bread. Int. J. Food Microbiol. 2020, 326, 108648. [Google Scholar] [CrossRef]
- Schmidt, M.; Sciurba, E. Determination of FODMAP contents of common wheat and rye breads and the effects of processing on the final contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]
- Knez, M.; Abbott, C.; Stangoulis, J.C.R. Changes in the content of fructans and arabinoxylans during baking processes of leavened and unleavened breads. Eur. Food Res. Technol. 2014, 239, 803–811. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-profile in cereal-product ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Haskå, L.; Nyman, M.; Andersson, R. Distribution and characterisation of fructan in wheat milling fractions. J. Cereal Sci. 2008, 48, 768–774. [Google Scholar] [CrossRef]
- Ispiryan, L.; Kuktaite, R.; Zannini, E.; Arendt, E.K. Fundamental study on changes in the FODMAP profile of cereals, pseudo-cereals, and pulses during the malting process. Food Chem. 2021, 343, 128549. [Google Scholar] [CrossRef]
- Verspreet, J.; Dornez, E.; van den Ende, W.; Delcour, J.A.; Courtin, C.M. Cereal grain fructans: Structure, variability and potential health effects. Trends Food Sci. Technol. 2015, 43, 32–42. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steiner, D.; Longin, C.F.H.; Würschum, T.; Schweiggert, R.M.; Carle, R. Wheat and the irritable bowel syndrome—FODMAP levels of modern and ancient species and their retention during bread making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Cyrkot, S.; Marcon, M.; Brill, H.; Mileski, H.; Dowhaniuk, J.; Frankish, A.; Carroll, M.W.; Persad, R.; Turner, J.M.; Mager, D.R. FODMAP intake in children with coeliac disease influences diet quality and health-related quality of life and has no impact on gastrointestinal symptoms. Int. J. Food Sci. Nutr. 2021, 72, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Loponen, J.; Gänzle, M.G. Use of sourdough in low FODMAP baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Magueyal, F.J.; Bautista-Méndez, A.; Villanueva-Tierrablanca, H.D.; García-Ruíz, J.L.; Jiménez-Islas, H.; Navarrete-Bolaños, J.L. Novel process to obtain agave sap (aguamiel) by directed enzymatic hydrolysis of agave juice fructans. LWT 2020, 127, 109387. [Google Scholar] [CrossRef]
- Fraberger, V.; Call, L.M.; Domig, K.J.; D’Amico, S. Applicability of yeast fermentation to reduce fructans and other FODMAPs. Nutrients 2018, 10, 1247. [Google Scholar] [CrossRef] [Green Version]
- Struyf, N.; Vandewiele, H.; Herrera-Malaver, B.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Kluyveromyces marxianus yeast enables the production of low FODMAP whole wheat breads. Food Microbiol. 2018, 76, 135–145. [Google Scholar] [CrossRef]
- Wang, S.A.; Li, F.L. Invertase SUC2 is the key hydrolase for inulin degradation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 403–406. [Google Scholar] [CrossRef] [Green Version]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces cerevisiae and Kluyveromyces marxianus cocultures allow reduction of fermentable oligo-, di-, and monosaccharides and polyols levels in whole wheat bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar] [CrossRef] [Green Version]
- Verspreet, J.; Hemdane, S.; Dornez, E.; Cuyvers, S.; Delcour, J.A.; Courtin, C.M. Maximizing the concentrations of wheat grain fructans in bread by exploring strategies to prevent their yeast (Saccharomyces cerevisiae)-mediated degradation. J. Agric. Food Chem. 2013, 61, 1397–1404. [Google Scholar] [CrossRef]
- Prückler, M.; Lorenz, C.; Endo, A.; Kraler, M.; Dürrschmid, K.; Hendriks, K.; Soares da Silva, F.; Auterith, E.; Kneifel, W.; Michlmayr, H. Comparison of homo- and heterofermentative lactic acid bacteria for implementation of fermented wheat bran in bread. Food Microbiol. 2015, 49, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.; Sahin, A.W.; Lynch, K.M.; Arendt, E.K.; Coffey, A. Isolation, characterisation and exploitation of lactic acid bacteria capable of efficient conversion of sugars to mannitol. Int. J. Food Microbiol. 2020, 321, 108546. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, S.; Xiao, Z. Technologies for enhancement of bioactive components and potential health benefits of cereal and cereal-based foods: Research advances and application challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Habuš, M.; Golubić, P.; Vukušić Pavičić, T.; Čukelj Mustač, N.; Voučko, B.; Herceg, Z.; Ćurić, D.; Novotni, D. Influence of flour type, dough acidity, printing temperature and bran pre-processing on browning and 3D printing performance of snacks. Food Bioprocess Technol. 2021, 14, 2365–2379. [Google Scholar] [CrossRef]
- Pulatsu, E.; Su, J.W.; Lin, J.; Lin, M. Factors affecting 3D printing and post-processing capacity of cookie dough. Innov. Food Sci. Emerg. Technol. 2020, 61, 102316. [Google Scholar] [CrossRef]
- Keerthana, K.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Development of fiber-enriched 3D printed snacks from alternative foods: A study on button mushroom. J. Food Eng. 2020, 287, 110116. [Google Scholar] [CrossRef]
- Vukušić Pavičić, T.; Grgić, T.; Ivanov, M.; Novotni, D.; Herceg, Z. Influence of flour and fat type on dough rheology and technological characteristics of 3D-printed cookies. Foods 2021, 10, 193. [Google Scholar] [CrossRef]
- Jagadiswaran, B.; Alagarasan, V.; Anandharamakrishnan, C. Valorization of food industry waste and by-products using 3D printing: A study on the development of value-added functional cookies. Futur. Foods 2021, 4, 100036. [Google Scholar] [CrossRef]
- Lille, M.; Kortekangas, A.; Heiniö, R.L.; Sozer, N. 2020. Structural and textural characteristics of 3D-printed protein- and dietary fibre-rich snacks made of milk powder and wholegrain rye flour. Foods 2020, 9, 1527. [Google Scholar] [CrossRef]
- Habuš, M.; Novotni, D.; Gregov, M.; Štifter, S.; Čukelj Mustač, N.; Voučko, B.; Ćurić, D. Influence of particle size reduction and high-intensity ultrasound on polyphenol oxidase, phenolics, and technological properties of wheat bran. J. Food Process. Preserv. 2021, 45, 15204. [Google Scholar] [CrossRef]
- ISO Standard No. 11035; Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. International Organization for Standardization: Geneva, Switzerland, 1994.
- Pastor, K.; Ačanski, M.; Vujić Kojić, P. A rapid dicrimination of wheat, walnut and hazelnut flour samples using chemometric algorithms on GC/MS data. J Food Meas Charact. 2019, 13, 2961–2969. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Pulatsu, E.; Su, J.W.; Kenderes, S.M.; Lin, J.; Vardhanabhuti, B.; Lin, M. Effects of ingredients and pre-heating on the printing quality and dimensional stability in 3D printing of cookie dough. J. Food Eng. 2021, 294, 110412. [Google Scholar] [CrossRef]
- Carrizo, S.L.; De Oca, C.E.M.; Hébert, M.E.; Saavedra, L.; Vignolo, G.; LeBlanc, J.G.; Rollán, G.C. Lactic acid bacteria from andean grain amaranth: A source of vitamins and functional value enzymes. J. Mol. Microbiol. Biotechnol. 2017, 27, 289–298. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Salmenkallio-Marttila, M.; Katina, K.; Autio, K. Effects of bran fermentation on quality and microstructure of high-fiber wheat bread. Cereal Chem. 2001, 78, 429–435. [Google Scholar] [CrossRef]
- Valerio, F.; Di Biase, M.; Caputo, L.; Creanza, T.M.; Ancona, N.; Visconti, A.; Lavermicocca, P. Effect of Lactobacillus brevis-based bioingredient and bran on microbiological, physico-chemical and textural quality of yeast-leavened bread during storage. Innov. Food Sci. Emerg. Technol. 2014, 25, 2–8. [Google Scholar] [CrossRef]
- Raya, A.; Rabiae, M.M.; Shazly, A.S.; Fadaly, E.S.E. Effect of adding barley and oat flour on the rheological properties of bread dough. J. Food Dairy Sci. 2014, 5, 641–652. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, A.; Purohit, S.R.; Rao, P.S. Extrusion of fermented rice-black gram flour for development of functional snacks: Characterization, optimization and sensory analysis. J. Food Sci. Technol. 2021, 58, 494–509. [Google Scholar] [CrossRef] [PubMed]

| Bioprocessing | Fructans | Fructose | Glucose | Mannitol | Total FODMAPs |
|---|---|---|---|---|---|
| Wheat bran | |||||
| None | 2.64 ± 0.04 a | 0.14 ± 0.05 c | 0.47 ± 0.00 efgh | n.d. | 0.12 |
| LV1 | 0.18 ± 0.01 de | 0.06 ± 0.00 c | 0.03 ± 0.00 i | 0.47 ± 0.01 fgh | 0.06 |
| BY | 0.96 ± 0.02 b | 0.09 ± 0.03 c | 0.24 ± 0.02 ghi | 0.65 ± 0.03 bc | 0.08 |
| BY + L. fermentum | 0.33 ± 0.02 c | 0.51 ± 0.06 b | 0.09 ± 0.04 i | 0.52 ± 0.01 def | 0.08 |
| BY + K. marxianus | 0.30 ± 0.01 c | 1.47 ± 0.16 a | 0.52 ± 0.08 cdef | 0.44 ± 0.01 gh | 0.12 |
| BY + inulinase | 0.19 ± 0.02 de | n.d. | 0.25 ± 0.02 fghi | 0.74 ± 0.02 a | 0.06 |
| Amaranth bran | |||||
| None | 0.96 ± 0.00 b | 0.13 ± 0.02 c | 0.17 ± 0.00 i | n.d. | 0.07 |
| LV1 | 0.11 ± 0.01 ef | n.d. | 0.02 ± 0.00 i | 0.49 ± 0.02 efg | 0.05 |
| BY | 0.05 ± 0.00 f | 0.30 ± 0.02 c | 0.20 ± 0.01 hi | 0.50 ± 0.01 efg | 0.05 |
| BY + L. fermentum | 0.11 ± 0.00 ef | n.d. | 2.34 ± 0.10 b | 0.41 ± 0.00 h | 0.05 |
| BY + K. marxianus | 0.19 ± 0.01 de | n.d. | 5.10 ± 0.09 a | 0.45 ± 0.01 fgh | 0.06 |
| BY + inulinase | 0.08 ± 0.00 f | n.d. | 0.05 ± 0.02 defg | 0.58 ± 0.02 c | 0.05 |
| Treatment | Complex Viscosity (Pa s) | Yield Stress (Pa) | Flow Point (Pa) | Printing Precision (%) |
|---|---|---|---|---|
| Wheat bran | ||||
| None | 7893.2 ± 131.2 de | 18.9 ± 0.3 f | 254.8 ± 0.6 ef | 84.3 ± 2.6 defg |
| LV1 | 8184.1 ± 185.0 cd | 25.0 ± 1.0 ef | 447.2 ± 0.8 c | 86.3 ± 3.1 cdef |
| BY | 10,466.2 ± 182.2 b | 34.0 ± 1.0 cde | 533.8 ± 0.5 b | 93.1 ± 2.8 a |
| BY + L. fermentum | 11,275.5 ± 27.5 a | 51.4 ± 1.0 a | 612.0 ± 1.6 a | 94.9 ± 2.4 a |
| BY + K. marxianus | 7182.2 ± 11.8 e | 27.0 ± 1.0 def | 467.4 ± 0.6 c | 84.0 ± 2.7 efg |
| BY + inulinase | 8632.3 ± 15.9 cd | 26.4 ± 0.7 def | 472.5 ± 8.9 c | 88.4 ± 4.0 b |
| Amaranth bran | ||||
| None | 2237.6 ± 24.1 i | 5.1 ± 0.7 g | 126.9 ± 1.0 g | 83.3 ± 2.6 fg |
| LV1 | 4540.0 ± 139.5 f | 37.4 ± 3.2 bcd | 301.3 ± 4.4 de | 82.8 ± 2.7 fg |
| BY | 3326.3 ± 73.1 gh | 27.9 ± 4.7 def | 290.9 ± 2.2 de | 86.1 ± 2.6 cdef |
| BY + L. fermentum | 2931.7 ± 182.5 hi | 2.2 ± 0.4 g | 229.3 ± 2.4 f | 80.1 ± 1.2 g |
| BY + K. marxianus | 4879.6 ± 209.2 f | 7.3 ± 0.1 g | 290.9 ± 6.7 de | 90.8 ± 2.0 a |
| BY + inulinase | 2503.4 ± 125.3 i | 20.8 ± 3.1 f | 221.3 ± 2.5 f | 84.0 ± 3.4 efg |
| Bioprocessing | Shape Shrinkage (%) | Lightness L* | Redness a* | Yellowness b* | BI | Hardness (N) |
|---|---|---|---|---|---|---|
| Wheat bran | ||||||
| None | 28.6 ± 4.1 de | 57.8 ± 2.0 cd | 5.8 ± 1.3 efg | 17.2 ± 0.4 c | 42.3 ± 3.6 ef | 12.0 ± 1.6 bcd |
| LV1 | 22.6 ± 5.3 fgh | 60.3 ± 0.9 b | 6.1 ± 0.4 def | 18.8 ± 0.7 defg | 44.2 ± 1.5 cde | 11.1 ± 1.3 defg |
| BY | 22.1 ± 3.4 gh | 56.7 ± 1.0 de | 7.6 ± 0.2 bc | 17.0 ± 0.8 bc | 44.8 ± 1.2 cde | 10.2 ± 0.7 efgh |
| BY + L. fermentum | 22.2 ± 2.9 gh | 53.7 ± 1.8 f | 5.6 ± 1.0 fgh | 15.3 ± 1.1 a | 40.6 ± 1.7 fg | 12.3 ± 1.4 abcd |
| BY + K. marxianus | 12.3 ± 2.3 j | 55.0 ± 0.4 ef | 6.9 ± 0.3 cd | 16.4 ± 0.3 bc | 44.0 ± 0.7 cde | 10.1 ± 0.6 efgh |
| BY + inulinase | 20.8 ± 3.2 hi | 60.1 ± 0.3 b | 7.7 ± 0.1 ab | 20.0 ± 0.2 fghi | 48.2 ± 0.3 a | 11.7 ± 2.0 cdef |
| Amaranth bran | ||||||
| None | 48.2 ± 4.9 a | 64.9 ± 0.8 a | 3.3 ± 0.2 i | 19.0 ± 0.6 efgh | 37.8 ± 1.1 h | 10.0 ± 1.4 fgh |
| LV1 | 31.6 ± 3.6 bcd | 64.5 ± 0.5 a | 3.5 ± 0.2 i | 19.3 ± 0.3 fghi | 39.0 ± 1.0 gh | 9.7 ± 0.6 gh |
| BY | 29.2 ± 4.5 cde | 64.4 ± 1.5 a | 5.0 ± 0.3 h | 20.2 ± 0.4 i | 42.7 ± 1.0 def | 10.1 ± 0.9 fgh |
| BY + L. fermentum | 30.3 ± 4.8 cde | 63.8 ± 0.7 a | 3.4 ± 0.3 i | 18.6 ± 0.8 def | 37.8 ± 2.0 h | 9.1 ± 0.7 h |
| BY + K. marxianus | 15.1 ± 3.1 ij | 60.9 ± 0.8 b | 5.3 ± 0.2 gh | 20.0 ± 0.6 ghi | 45.1 ± 1.3 bcd | 9.6 ± 1.3 gh |
| BY + inulinase | 25.2 ± 2.5 efgh | 64.3 ± 2.0 a | 5.3 ± 0.3 gh | 20.1 ± 1.1 hi | 42.7 ± 1.5 def | 9.6 ± 1.0 gh |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habuš, M.; Mykolenko, S.; Iveković, S.; Pastor, K.; Kojić, J.; Drakula, S.; Ćurić, D.; Novotni, D. Bioprocessing of Wheat and Amaranth Bran for the Reduction of Fructan Levels and Application in 3D-Printed Snacks. Foods 2022, 11, 1649. https://doi.org/10.3390/foods11111649
Habuš M, Mykolenko S, Iveković S, Pastor K, Kojić J, Drakula S, Ćurić D, Novotni D. Bioprocessing of Wheat and Amaranth Bran for the Reduction of Fructan Levels and Application in 3D-Printed Snacks. Foods. 2022; 11(11):1649. https://doi.org/10.3390/foods11111649
Chicago/Turabian StyleHabuš, Matea, Svitlana Mykolenko, Sofija Iveković, Kristian Pastor, Jovana Kojić, Saša Drakula, Duška Ćurić, and Dubravka Novotni. 2022. "Bioprocessing of Wheat and Amaranth Bran for the Reduction of Fructan Levels and Application in 3D-Printed Snacks" Foods 11, no. 11: 1649. https://doi.org/10.3390/foods11111649







