Characterization of the Inclusion Complexes of Isothiocyanates with γ-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
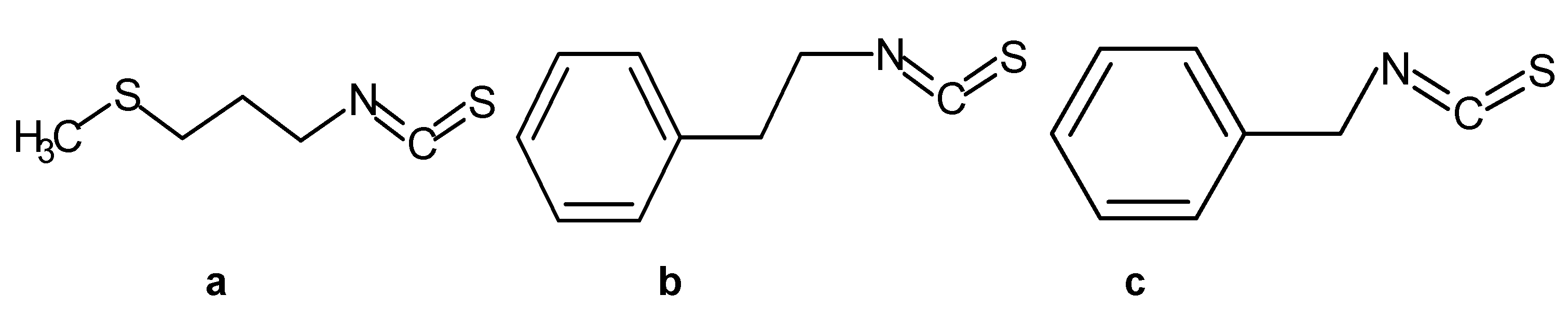
2.1. Chemicals and Bacterial Strains
2.2. Preparation of the Inclusion Complexes
2.3. Characterization of the Inclusion Complexes
2.4. In Vitro Release Study
2.5. Antibacterial Assays
2.6. Biofilm Analysis
2.6.1. Crystal Violet Quantitative Assay
2.6.2. SEM Analysis
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Primary Modeling
2.9. Secondary Modeling
2.10. Validation of Predictive Models
2.11. Statistical Analysis
3. Results and Discussion
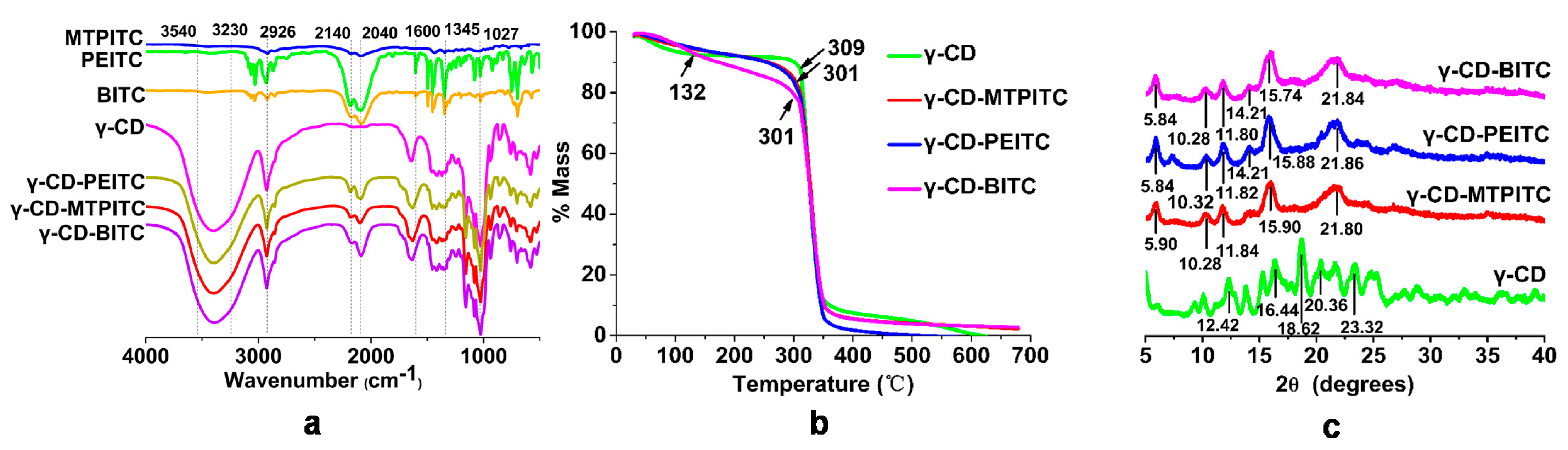
3.1. Characterization of the ITCs Inclusion Complexes with γ-CD
3.2. Antibacterial Activities of ITCs and γ-CD-ITCs against S. aureus
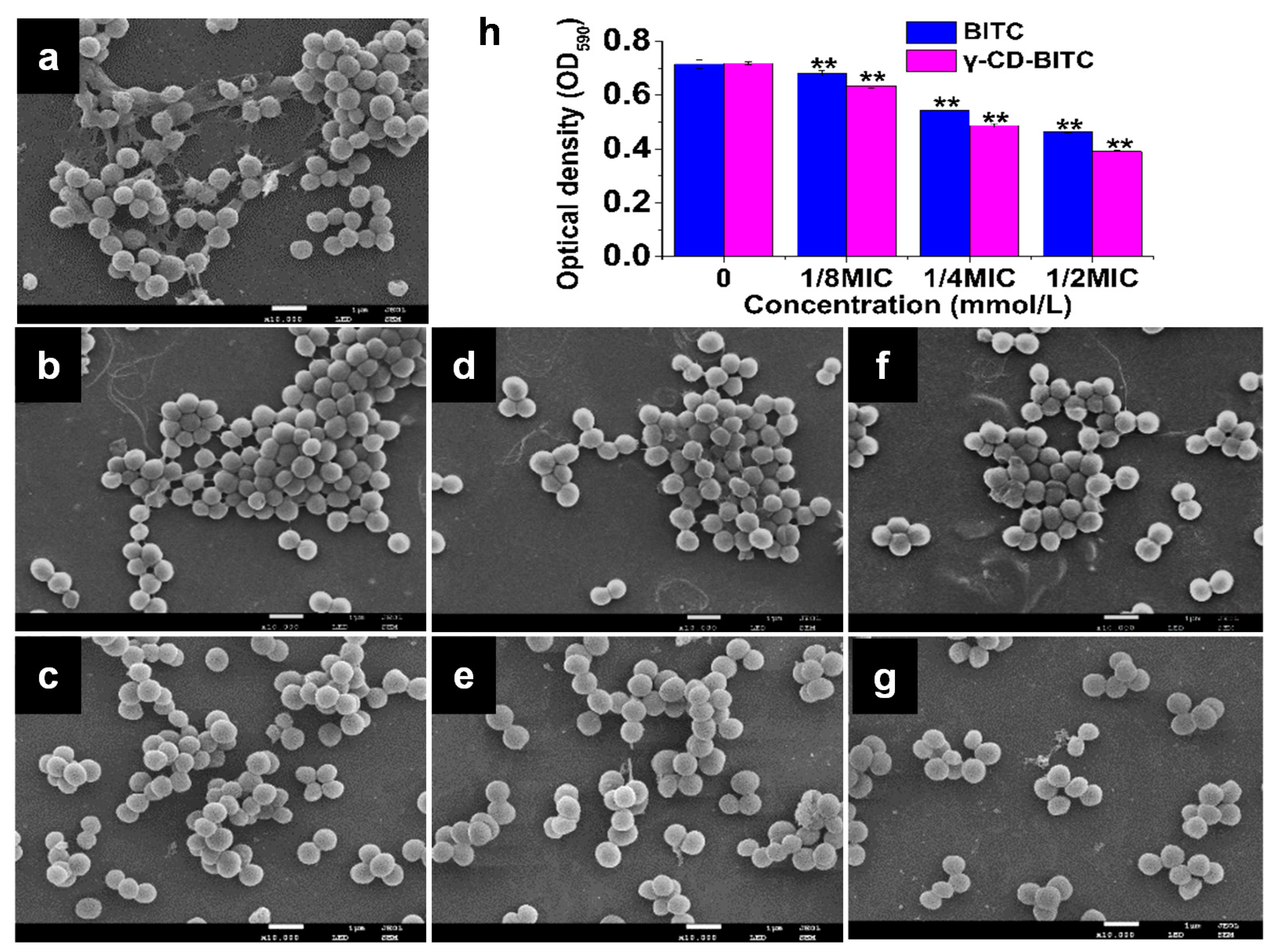
3.3. Effects of BITC and γ-CD-BITC against S. aureus Biofilm Formation
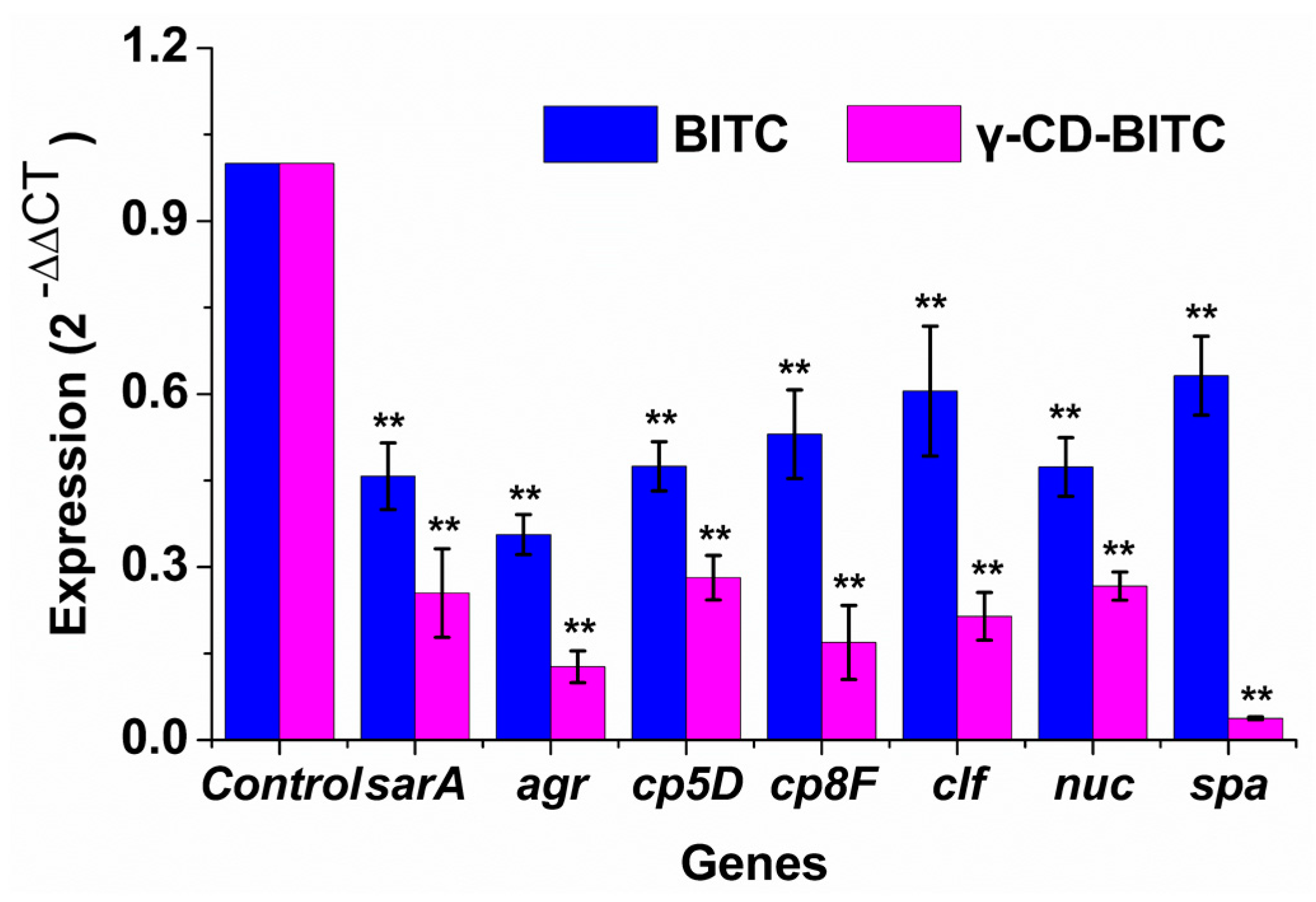
3.4. Effects of BITC and γ-CD-BITC on Virulence Gene Expression in S. aureus
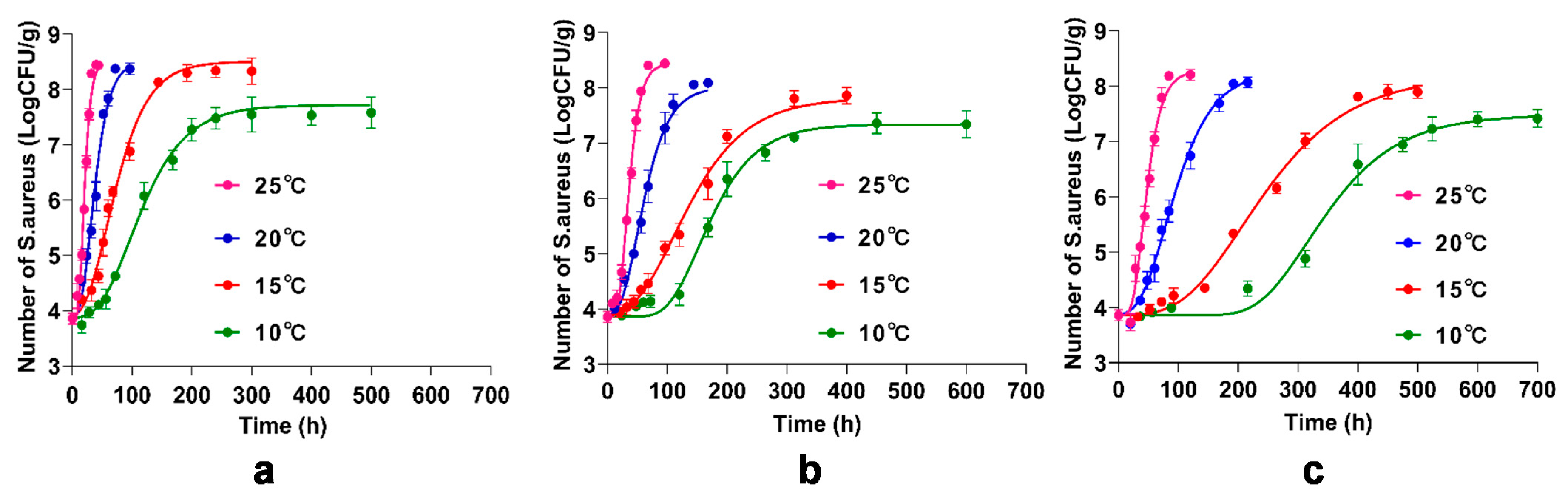
3.5. Primary Model for the Inhibitory of BITC and γ-CD-BITC on S. aureus Growth in Cooked Chicken Breasts
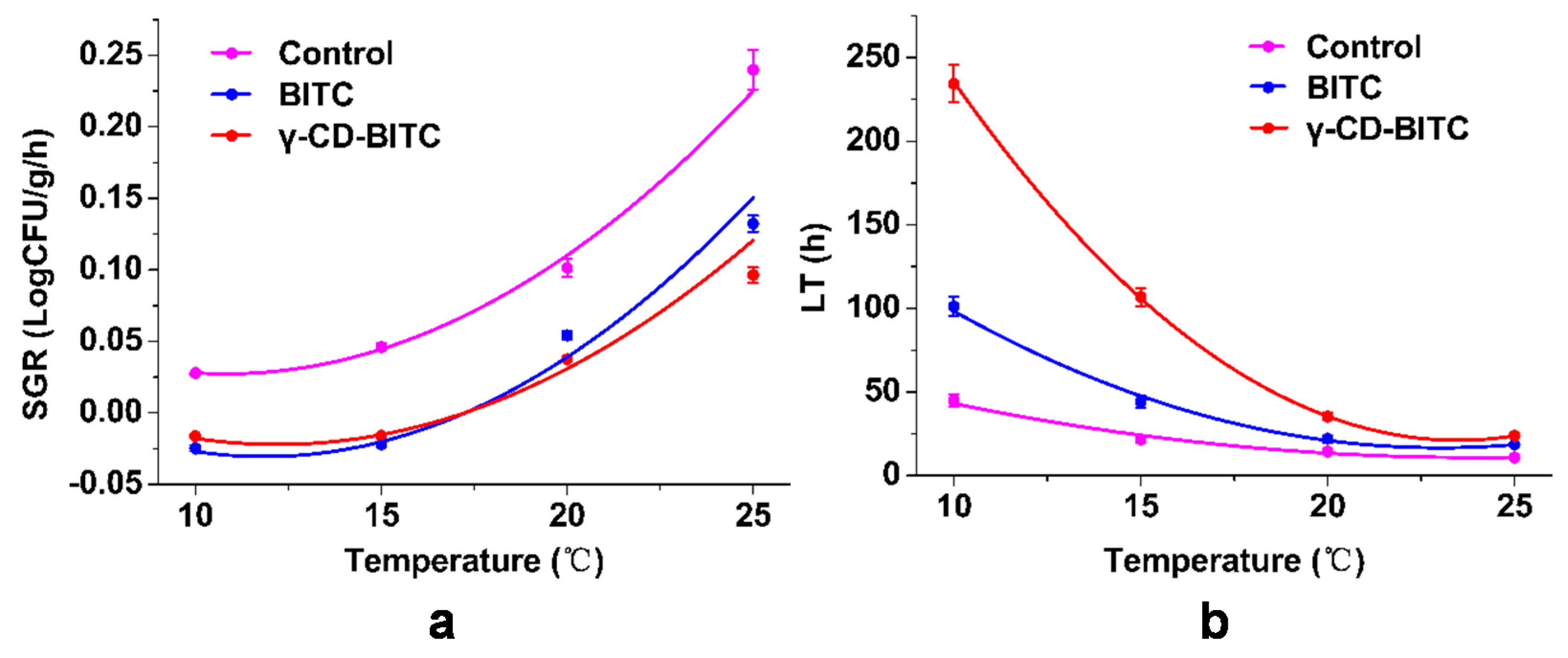
3.6. Secondary Model for Growth Factors of the Inhibitory of BITC and γ-CD-BITC on S. aureus in Cooked Chicken Breasts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostoglou, D.; Protopappas, I.; Giaouris, E. Common Plant-Derived Terpenoids Present Increased Anti-Biofilm Potential against Staphylococcus Bacteria Compared to a Quaternary Ammonium Biocide. Foods 2020, 9, 697. [Google Scholar] [CrossRef]
- Büttner, H.; Mack, D.; Rohde, H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell. Infect. Microbiol. 2015, 5, 14. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Figueiredo, A.; Ferreira, F.A.; Beltrame, C.O.; Côrtes, M. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit. Rev. Microbiol. 2017, 43, 602–620. [Google Scholar] [CrossRef]
- Rosenstein, R.; Götz, F. What distinguishes highly pathogenic staphylococci from medium- and non-pathogenic? Curr. Top Microbiol. Immunol. 2013, 358, 33–89. [Google Scholar]
- Guo, L.; Wang, Y.; Bi, X.; Duo, K.; Sun, Q.; Yun, X.; Zhang, Y.; Fei, P.; Han, J. Antimicrobial activity and mechanism of action of the Amaranthus tricolor crude extract against Staphylococcus aureus and potential application in cooked meat. Foods 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhang, L.F.; Hu, Q.P.; Hao, D.L.; Xu, J.G. Chemical composition, antibacterial activity of Cyperus rotundus rhizomes essential oil against Staphylococcus aureus via membrane disruption and apoptosis pathway. Food Control 2017, 80, 290–296. [Google Scholar] [CrossRef]
- Pate, J.; Yin, H.B.; Bauchan, G.; Mowery, J. Inhibition of Escherichia coli O157:H7 and Salmonella enterica virulence factors by benzyl isothiocyanate. Food Microbiol. 2020, 86, 103303. [Google Scholar] [CrossRef]
- Rampal, G.; Thind, T.S.; Arora, R.; Vig, A.P.; Arora, S. Synergistic antimutagenic effect of isothiocyanates against varied mutagens. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Bergaentzlé, M.; Bindler, F.O.; Marchioni, E.; Lintz, A.; Ennahar, S.D. Invitro efficacies of various isothiocyanates from cruciferous vegetables as antimicrobial agents against foodborne pathogens and spoilage bacteria. Food Control 2013, 30, 318–324. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, H.S. Growth-inhibiting activities of phenethyl isothiocyanate and its derivatives against intestinal bacteria. J. Food Sci. 2010, 74, M467–M471. [Google Scholar] [CrossRef]
- Huang, L.H. IPMP 2013—A comprehensive data analysis tool for predictive microbiology. Int. J. Food Microbiol. 2014, 171, 100–107. [Google Scholar] [CrossRef]
- Yang, C.X.; Wu, H.T.; Li, X.X.; Wu, H.Y.; Hou, H.M. Comparison of the inhibitory potential of benzyl isothiocyanate and phenethyl isothiocyanate on Shiga toxin-producing and enterotoxigenic Escherichia Coli. LWT 2020, 118, 108806. [Google Scholar] [CrossRef]
- Uppal, S.; Sharma, P.; Kumar, R.; Kaur, K.; Bhatia, A.; Mehta, S.K. Effect of benzyl isothiocyanate encapsulated biocom-patible nanoemulsion prepared via ultrasonication on microbial strains and breast cancer cell line MDA MB 231. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124732. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kaur, N.D.; Shukla, G.; Mehta, S.K. Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int. J. Biol. Macromol. 2018, 115, 18–28. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Y.; Ye, J.; Jia, J.F.; Ma, J.F.; Ge, F.H. Preparation of walnut oil microcapsules employing soybean protein isolate and maltodextrin with enhanced oxidation stability of walnut oil. LWT Food Sci. Technol. 2017, 83, 292–297. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhou, R.; Zhu, Y. Study of production and pyrolysis characteristics of sweet orange flavor-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2014, 105, 75–80. [Google Scholar] [CrossRef]
- Li, X.H.; Jin, Z.Y.; Wang, J. Complexation of allyl isothiocyanate by α- and β-cyclodextrin and its controlled release characteristics. Food Chem. 2007, 103, 461–466. [Google Scholar] [CrossRef]
- Valle, E. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH: Weinheim, Germany, 2006; Volume 9, p. 162. [Google Scholar]
- Costa, M.; Rocha, J.E.; Campina, F.F.; Silva, A.R.P.; Da Cruz, R.P.; Pereira, R.L.S.; Quintans-Júnior, L.J.; De Menezes, I.R.A.; Araújo, A.D.S.; De Freitas, T.S. Comparative analysis of the antibacterial and drug-modulatory effect of D-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 2019, 128, 158–161. [Google Scholar] [CrossRef]
- Andrade, T.A.; Freitas, T.S.; Araújo, F.; Menezes, P.P.; Dória, G.; Rabelo, A.S.; Quintans-Júnior, L.; Santos, M.; Bezerra, D.P.; Serafini, M.R. Physico-chemical characterization and antibacterial activity of inclusion complexes of Hyptis martiusii Benth essential oil in β-cyclodextrin. Biomed. Pharmacother. 2017, 89, 201–207. [Google Scholar] [CrossRef]
- De Sousa Oliveira, F.; de Freitas, T.S.; da Cruz, R.P.; do Socorro Costa, M.; Pereira, R.L.S.; Quintans-Júnior, L.J.; de Araújo Andrade, T.; dos Passos Menezes, P.; de Sousa, B.M.H.; Nunes, P.S. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed. Pharmacother. 2017, 92, 1111–1118. [Google Scholar] [CrossRef]
- Nguyen, T.V.A.; Yoshii, H. Release behavior of allyl sulfide from cyclodextrin inclusion complex of allyl sulfide under different storage conditions. Biosci. Biotechnol. Biochem. 2018, 82, 848–855. [Google Scholar] [CrossRef]
- Phunpee, S.; Ruktanonchai, U.R.; Yoshii, H.; Assabumrungrat, S.; Soottitantawat, A. Encapsulation of lemongrass oil with cyclodextrins by spray drying and its controlled release characteristics. J. Agric. Chem. Soc. Jpn. 2017, 81, 718–723. [Google Scholar] [CrossRef]
- Moradi, S.; Barati, A.; Tonelli, A.E.; Hamedi, H. Chitosan-based hydrogels loading with thyme oil cyclodextrin inclusion compounds: From preparation to characterization. Eur. Polym. J. 2020, 122, 109303. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef]
- Goni, P.; Lopez, P.; Sanchez, C.; Gomez-Lus, R.; Becerril, R.; Nerin, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Ferment. Ind. 2010, 116, 982–989. [Google Scholar]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Chaabouni, Y.; Mahdouani, K.; Bakhrouf, A.; Chaieb, K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017, 104, 56–63. [Google Scholar] [CrossRef]
- Kang, J.; Li, Q.; Liu, L.; Jin, W.; Sun, Y. The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Appl. Microbiol. Biotechnol. 2018, 102, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Ivak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar]
- Yu, H.H.; Song, Y.J.; Kim, Y.J.; Lee, H.Y.; Choi, Y.S.; Lee, N.K.; Paik, H.D. Predictive model of growth kinetics for Staphylococcus aureus in raw beef under various packaging systems. Meat Sci. 2020, 165, 108108. [Google Scholar] [CrossRef]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Microbiol. 1996, 81, 501–508. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Yang, H.P.; Liu, Q.F.; Zhang, N.; Du, Y.D.; Zhu, H.P. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015, 184, 99–104. [Google Scholar]
- Yuan, H.N.; Yao, S.J.; Shen, L.Q.; Mao, J.W. Preparation and Characterization of Inclusion Complexes of β-Cyclodextrin BITC and β-Cyclodextrin PEITC. Ind. Eng. Chem. Res. 2009, 48, 5070–5078. [Google Scholar] [CrossRef]
- Braga, S.S.; El-Saleh, F.; Lysenko, K.; Paz, F.A.A. Inclusion Compound of Efavirenz and γ-Cyclodextrin: Solid State Studies and Effect on Solubility. Molecules 2021, 26, 519. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, M.A.; Alves, R.; Menezes, P.; Serafini, M.R.; Antunes, D.; Bezerra, D.P.; Júnior, L.Q. Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β-cyclodextrin, improves the pharmacological response on cancer pain experimental protocols. Chem. Biol. Interact. 2015, 227, 69–76. [Google Scholar] [CrossRef]
- Silva, A.; Monteiro, M.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.; Cardoso, S.M. Inclusion Complex of Resveratrol with γ-Cyclodextrin as a Functional Ingredient for Lemon Juices. Foods 2020, 10, 16. [Google Scholar] [CrossRef]
- Pinheiro, P.G.; Santiago, G.; Silva, F.; Araújo, A.; Oliveira, C.; Freitas, P.R.; Rocha, J.E.; Neto, J.; Silva, M.; Tintino, S.R. Antibacterial activity and inhibition against Staphylococcus aureus NorA efflux pump by ferulic acid and its esterified derivatives. Asian Pac. J. Trop. Biomed. 2021, 11, 405–413. [Google Scholar]
- Uppal, S.; Kaur, K.; Kumar, R.; Kahlon, N.K.; Singh, R.; Mehta, S.K. Encompassment of Benzyl Isothiocyanate in cyclodextrin using ultrasonication methodology to enhance its stability for biological applications. Ultrason. Sonochem. 2017, 39, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.M.; Patel, K.K.; Pandey, N.; Tilak, R.; Singh, S. Development of Anacardic Acid/hydroxypropyl-β-cyclodextrin inclusion complex with enhanced solubility and antimicrobial activity. J. Mol. Liq. 2019, 296, 112085. [Google Scholar] [CrossRef]
- Iordache, F.; Grumezescu, V.; Grumezescu, A.M.; Curutu, C.; Ditu, L.M.; Socol, G.; Ficai, A.; Trusca, R.; Holban, A.M. Gamma-cyclodextrin/usnic acid thin film fabricated by MAPLE for improving the resistance of medical surfaces to Staphylococcus aureus colonization. Appl. Surf. Sci. 2015, 336, 407–412. [Google Scholar] [CrossRef]
- Ythier, M.; Resch, G.; Waridel, P.; Panchaud, A.; Gfeller, A.; Majcherczyk, P.; Quadroni, M.; Moreillon, P. Proteomic and transcriptomic profiling of Staphylococcus aureus surface LPXTG-proteins: Correlation with agr genotypes and adherence phenotypes. Mol. Cell. Proteom. 2012, 11, 1123–1139. [Google Scholar] [CrossRef]
- Liu, L.; Shen, X.; Yu, J.; Cao, X.; Yu, F. Subinhibitory Concentrations of Fusidic Acid May Reduce the Virulence of S. aureus by Down-Regulating sarA and saeRS to Reduce Biofilm Formation and α-Toxin Expression. Front. Microbiol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Liang, Z.; Qi, Y.; Guo, S.; Hao, K.; Zhao, M.; Guo, N. Effect of AgWPA nanoparticles on the inhibition of Staphylococcus aureus growth in biofilms. Food Control 2019, 100, 240–246. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Schlievert, P.M. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 2003, 112, 1620–1625. [Google Scholar] [CrossRef]
- Hentzer, M.; Givskov, M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Cirioni, V.; Giacometti, A.; Ghiselli, R.; Braunstein, G.B.; Silvestri, C.; Mocchegiani, F.; Saba, V.; Scalise, G. Treatment of Staphylococcus aureus biofilm infection by the quorum-sensing inhibitor RIP. Antimicrob. Agents Chemother. 2007, 51, 2226–2229. [Google Scholar] [CrossRef]
- Murphy, E.; Lin, S.L.; Nunez, L.; Andrew, L.; Fink, P.S.; Dilts, D.A.; Hoiseth, S.K.; Jansen, K.U.; Anderson, A.S. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: Monitoring antigenic diversity. Hum. Vaccines 2014, 7, 51–59. [Google Scholar] [CrossRef][Green Version]
- Nanra, J.S.; Buitrago, S.M.; Crawford, S.; Ng, J.; Fink, P.S.; Hawkins, J.; Scully, I.L.; Mcneil, L.K.; Aste-Amézaga, J.; Cooper, D. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum. Vaccines 2013, 9, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Thakker, M.; Park, J.S.; Carey, V.; Lee, J.C. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 1998, 66, 5183–5189. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, B.; Vicedo, B.; Aznar, R. PCR-based procedures for detection and quantification of Staphylococcus aureus and their application in food. J. Appl. Microbiol. 2010, 100, 352–364. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, J.; Shi, C.; Hervin, K.; Fratamico, P.M.; Shi, X. Characterization and comparative analysis of a second thermonuclease from Staphylococcus aureus. Microbiol. Res. 2013, 168, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.V.P.; Fortaleza, C.M.C.B.; Riboli, D.F.M.; Rocha, R.S.; Rocha, C.; de Souza, M.d.R. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in a burn unit from Brazil. Burns 2013, 39, 1242–1249. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nam, G.W.; Yoon, K.S. Growth and survival of Staphylococcus aureus on beef jerky as a function of temperature. J. Food Saf. 2018, 38, e12495. [Google Scholar] [CrossRef]
- Yong, J.L.; Jung, B.S.; Kim, K.T.; Paik, H.D. Predictive model for the growth kinetics of Staphylococcus aureus in raw pork developed using Integrated Pathogen Modeling Program (IPMP) 2013. Meat Sci. 2015, 107, 20–25. [Google Scholar]
- Ross, T. Predictive microbiology for the meat industry. Meat Livest. Aust. 1999, 196. [Google Scholar]
- Baranyi, J.; Ross, T.; Mcmeekin, T.A.; Roberts, T.A. Effects of parameterization on the performance of empirical models used in ‘predictive microbiology’. Food Microbiol. 1996, 13, 83–91. [Google Scholar] [CrossRef]

| Times (Day) | Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Control | MTPITC | PEITC | BITC | γ-CD-MTPITC | γ-CD-PEITC | γ-CD-BITC | |
| 1 | 0.00 ± 0.00 Aa | 7.9 ± 0.4 Bb | 10.8 ± 0.4 Bc | 14.9 ± 0.7 Cd | 8.1 ± 0.5 Cb | 10.8 ± 0.6 Dc | 14.7 ± 0.6 Dd |
| 3 | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 9.1 ± 0.25 Bc | 3.1 ± 0.42 Bb | 9.1 ± 0.6 Cc | 11.1 ± 0.7 Cd |
| 5 | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 6.3 ± 0.6 Ba | 9.1 ± 0.9 Bb |
| 10 | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 7.2 ± 0.9 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wu, H.; Ao, X.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Characterization of the Inclusion Complexes of Isothiocyanates with γ-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus. Foods 2022, 11, 60. https://doi.org/10.3390/foods11010060
Liu J, Wu H, Ao X, Hao H, Bi J, Hou H, Zhang G. Characterization of the Inclusion Complexes of Isothiocyanates with γ-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus. Foods. 2022; 11(1):60. https://doi.org/10.3390/foods11010060
Chicago/Turabian StyleLiu, Jianan, Hongyan Wu, Xinying Ao, Hongshun Hao, Jingran Bi, Hongman Hou, and Gongliang Zhang. 2022. "Characterization of the Inclusion Complexes of Isothiocyanates with γ-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus" Foods 11, no. 1: 60. https://doi.org/10.3390/foods11010060
APA StyleLiu, J., Wu, H., Ao, X., Hao, H., Bi, J., Hou, H., & Zhang, G. (2022). Characterization of the Inclusion Complexes of Isothiocyanates with γ-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus. Foods, 11(1), 60. https://doi.org/10.3390/foods11010060







