Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Extraction with Supercritical CO2
2.3. Solvent Extraction
2.4. Determination of Dry Matter Content
2.5. Determination of the Total Carotenoid Content
2.6. Determination of the Individual Carotenoids
2.7. Determination of Individual Phenolic Compounds
2.8. Determination of Antioxidant Activity
2.9. Calculations
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction as Function of Time
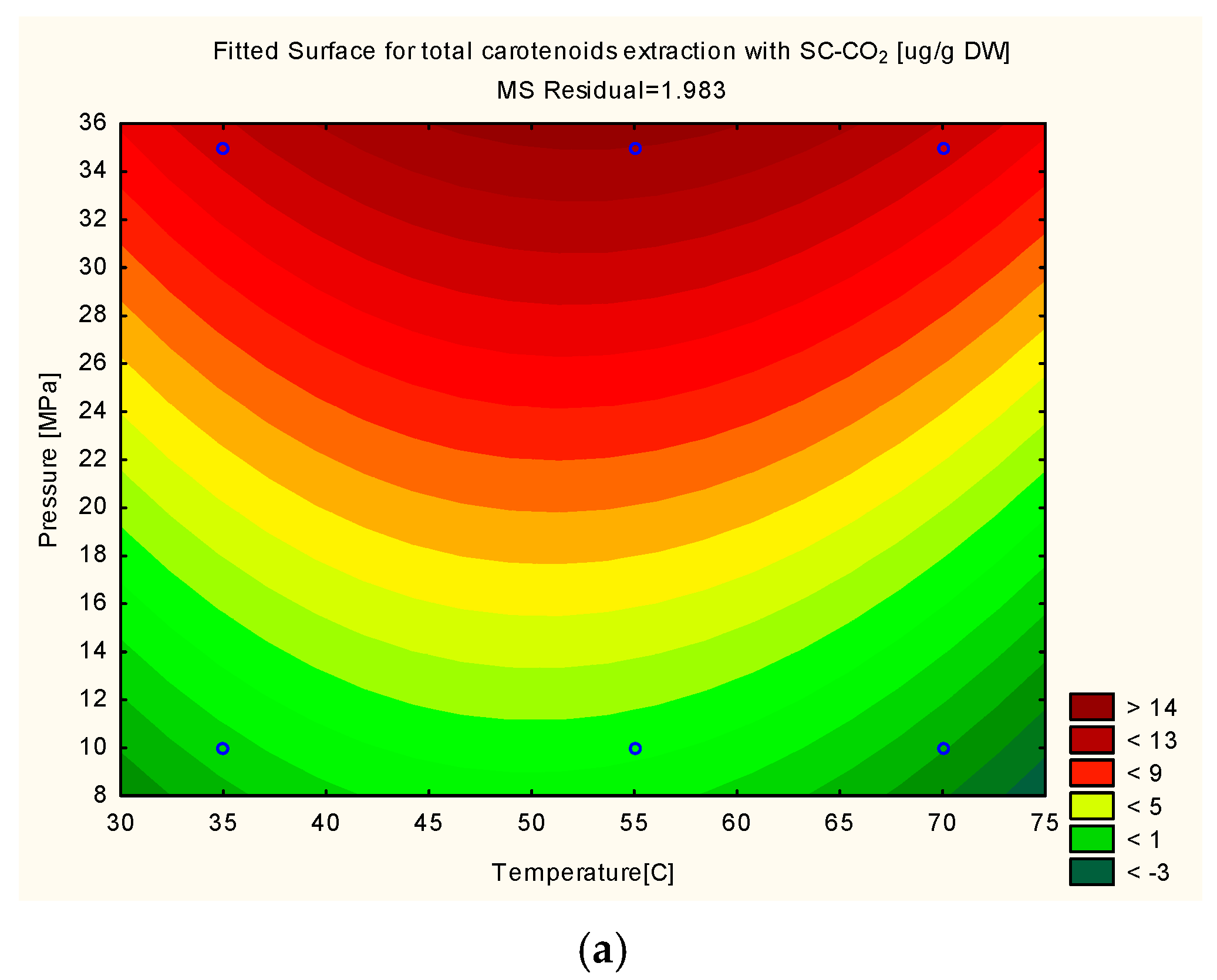
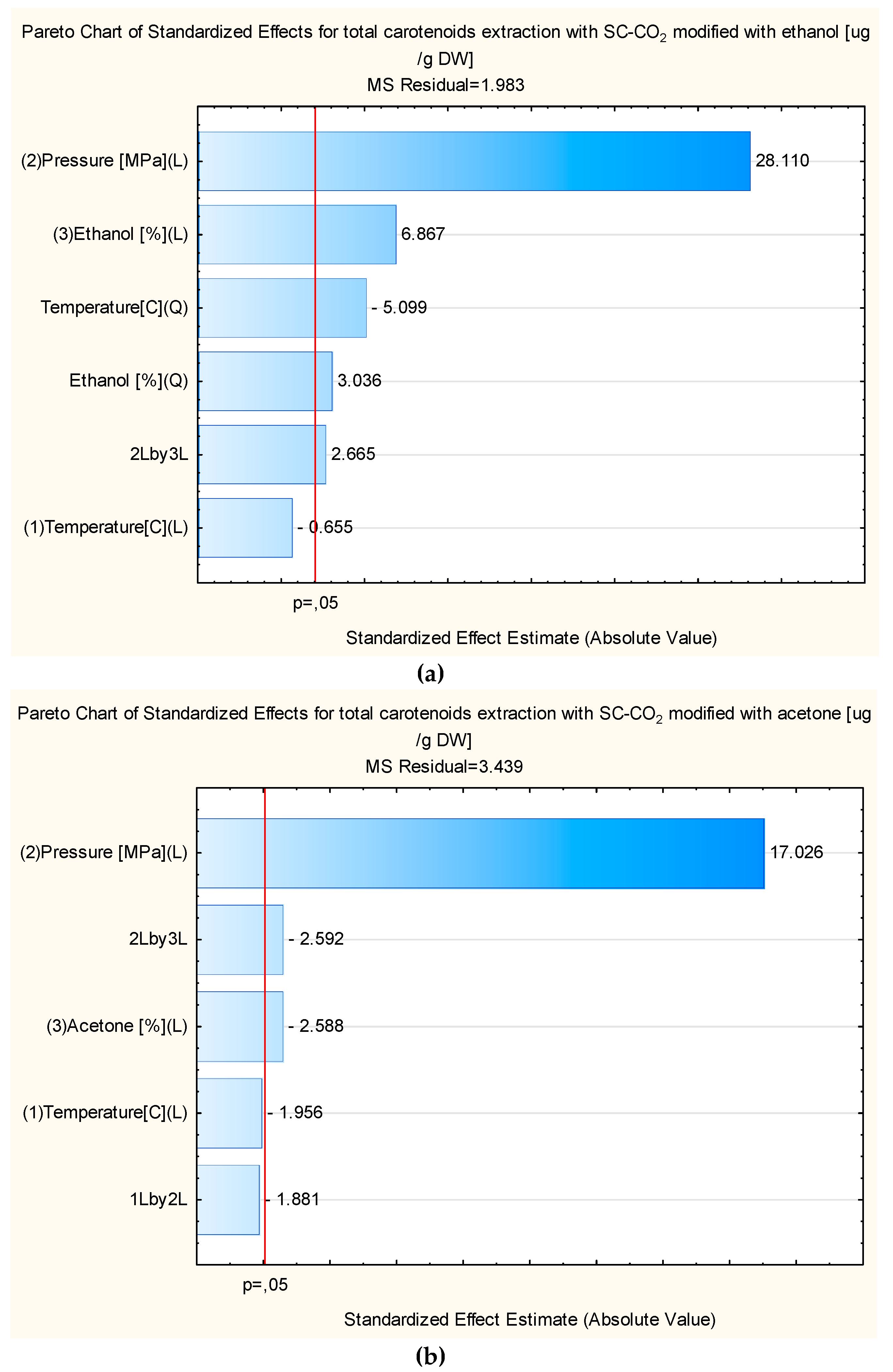
3.2. Optimization of the Total Carotenoids Extraction via RSM Modelling
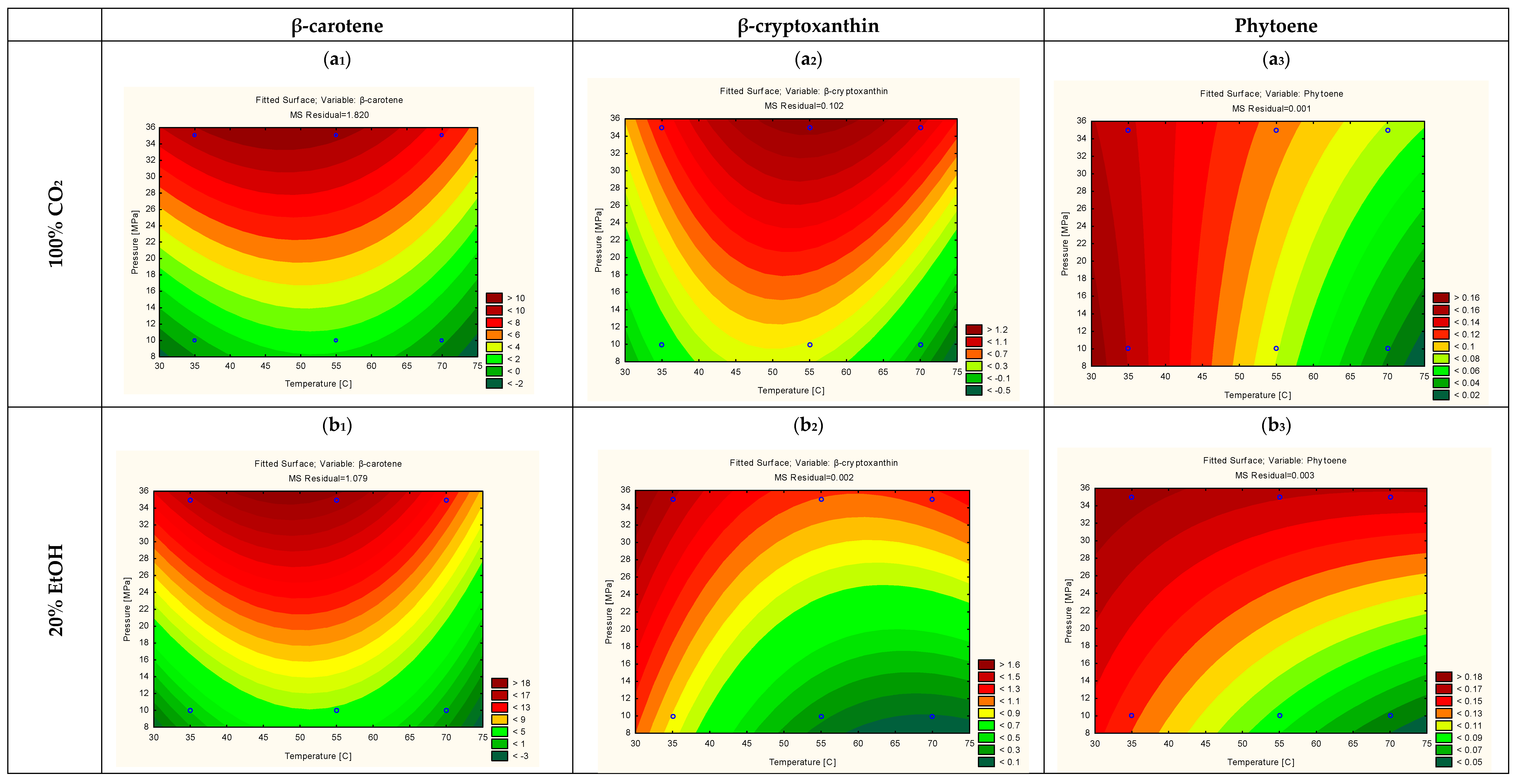
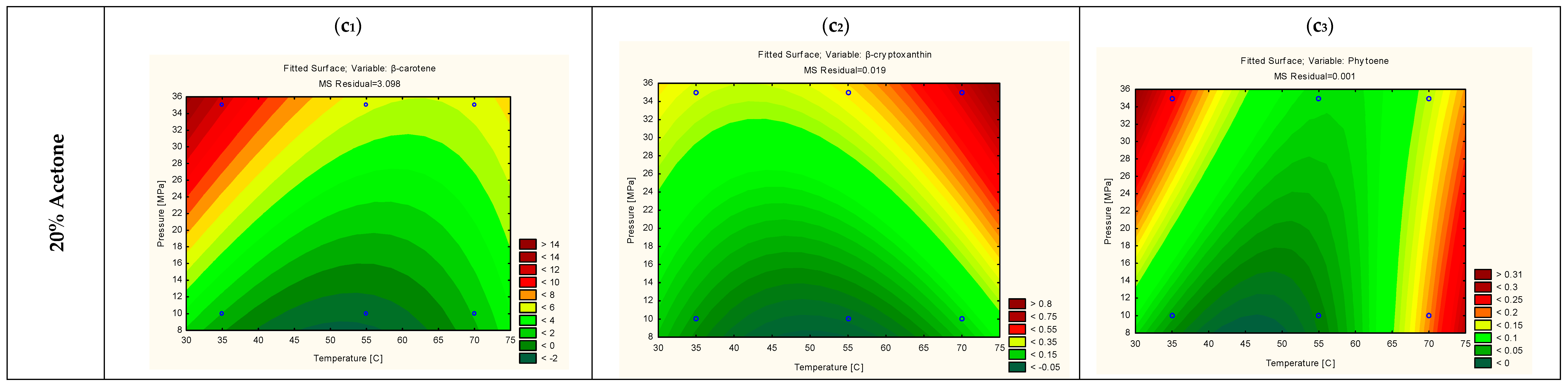
3.3. Optimization of the Extraction Yield of the Individual Carotenoids via RSM Modelling
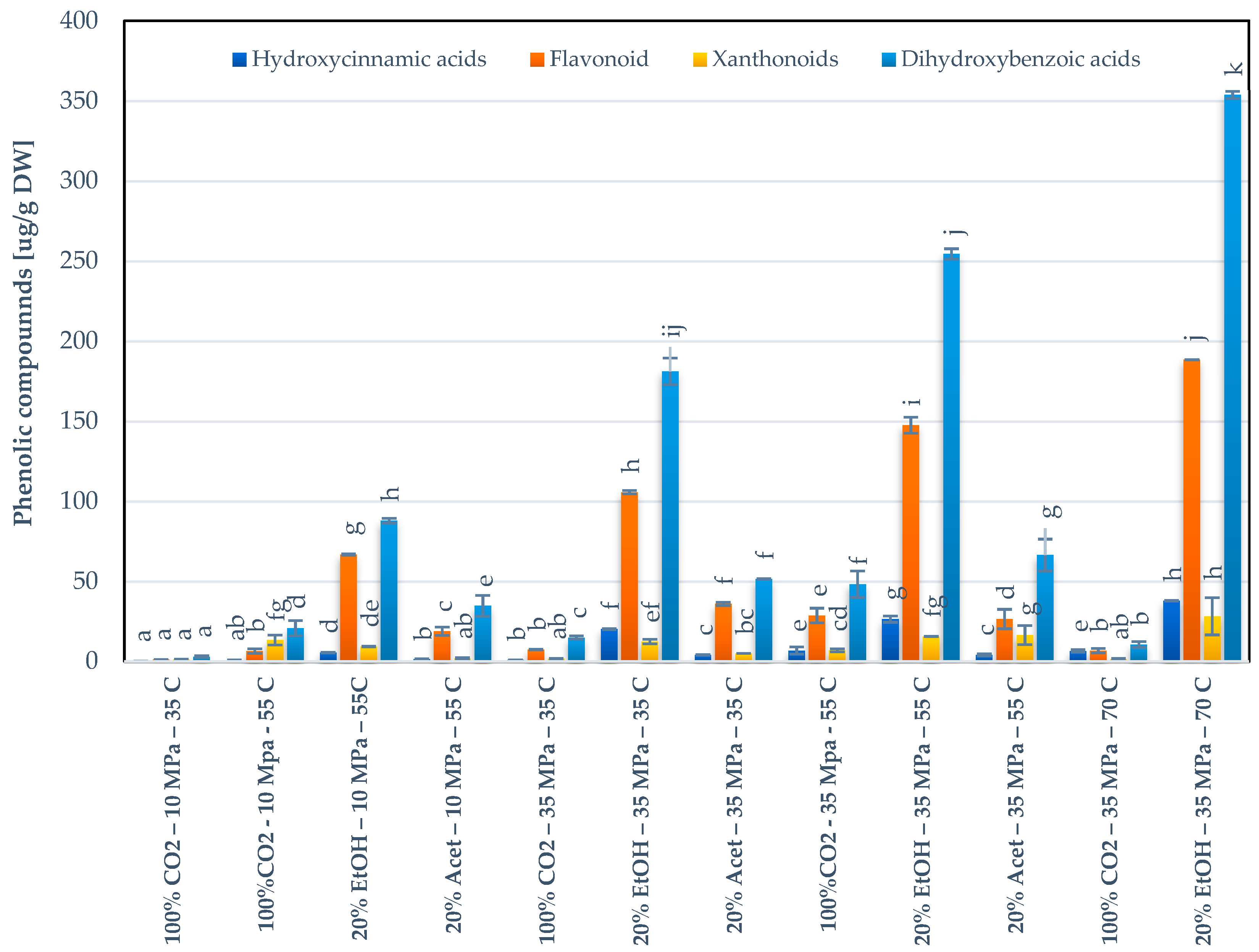
3.4. Validation of the Model for Carotenoids Extraction and Behavior of the Phenolic Compounds
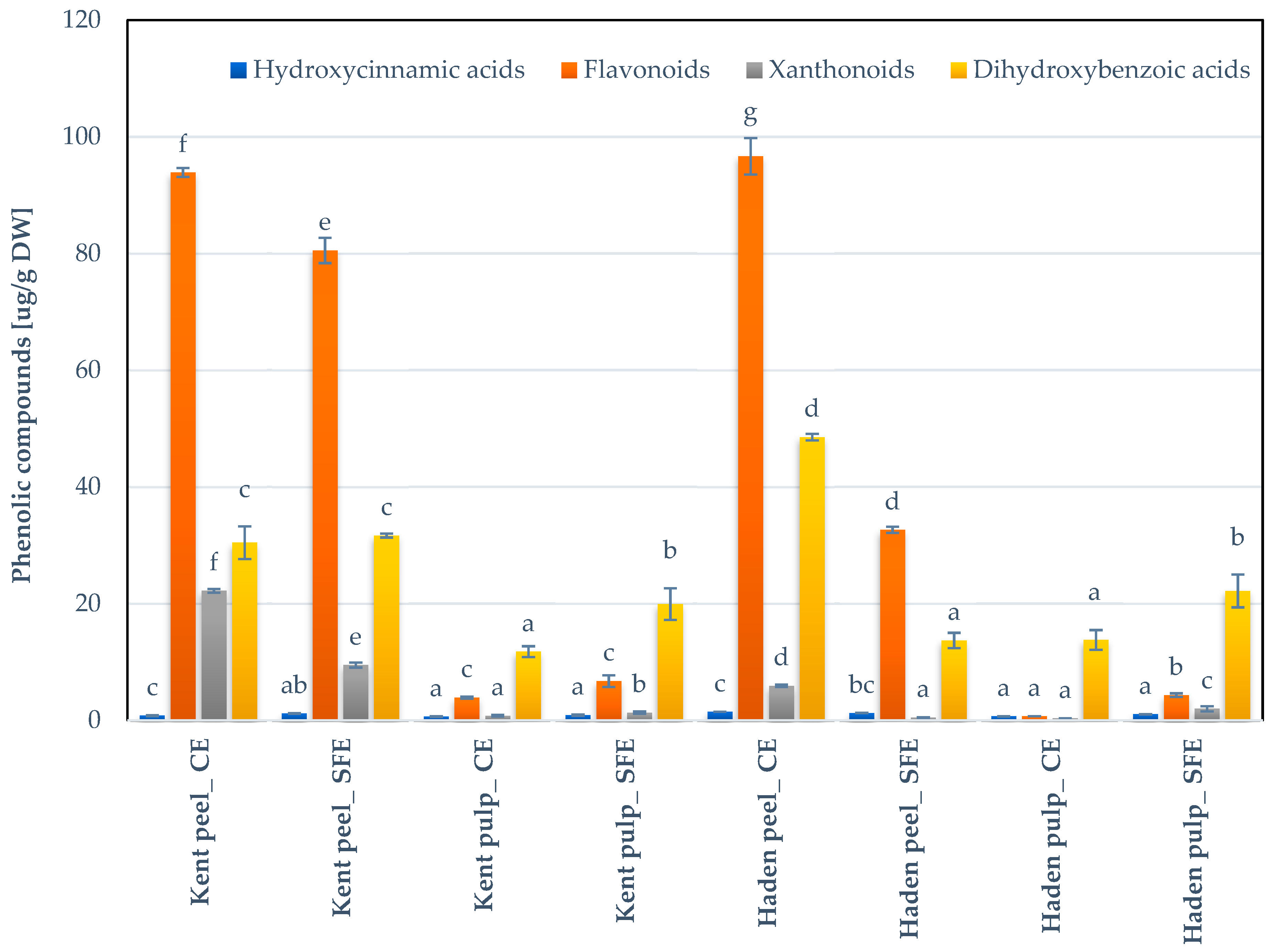
3.5. Extraction of Carotenoids and Phenolic Compounds at Optimized Conditions on By-Products Obtained from Different Ecuadorian Mango Varieties and Assessment of the Antioxidant Activities
3.5.1. Carotenoids
3.5.2. Phenolic Compounds
3.5.3. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villacís-Chiriboga, J.; Elst, K.; Van Camp, J.; Vera, E.; Ruales, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, R.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Arbizu-Berrocal, S.H.; Kim, H.; Fang, C.; Krenek, K.A.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenols from mango (Mangifera indica L.) modulate PI3K/AKT/mTOR-associated micro-RNAs and reduce inflammation in non-cancer and induce cell death in breast cancer cells. J. Funct. Foods 2019, 55, 9–16. [Google Scholar] [CrossRef]
- Servaes, K.; Maesen, M.; Prandi, B.; Sforza, S.; Elst, K. Polar Lipid Profile of Nannochloropsis oculata Determined Using a Variety of Lipid Extraction Procedures. J. Agric. Food Chem. 2015, 63, 3931–3941. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mendoza, M.P.; Paula, J.T.; Paviani, L.C.; Cabral, F.A.; Martinez-Correa, H.A. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT Food Sci. Technol. 2015, 62, 131–137. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Sforza, S.; Elst, K. Enzymatic pectic oligosaccharides (POS) production from sugar beet pulp using response surface methodology. J. Food Sci. Technol. 2017, 54, 3707–3715. [Google Scholar] [CrossRef]
- Elst, K.; Maesen, M.; Jacobs, G.; Bastiaens, L.; Voorspoels, S.; Servaes, K. Supercritical CO2 Extraction of Nannochloropsis sp.: A Lipidomic Study on the Influence of Pretreatment on Yield and Composition. Molecules 2018, 23, 1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bijttebier, S.K.A.; D’Hondt, E.; Hermans, N.; Apers, S.; Voorspoels, S. Unravelling ionization and fragmentation pathways of carotenoids using orbitrap technology: A first step towards identification of unknowns. J. Mass Spectrom. 2013, 48, 740–754. [Google Scholar] [CrossRef]
- De Paepe, D.; Servaes, K.; Noten, B.; Diels, L.; De Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. An improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 2013, 136, 368–375. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machmudah, S.; Wahyudiono; Goto, M. Supercritical Fluid Extraction of Carotenoids. In High Pressure Fluid Technology for Green Food Processing; Fornari, T., Stateva, R., Eds.; Springer: Cham, Switzerland, 2015; pp. 397–426. [Google Scholar]
- Tirado, D.F.; Calvo, L. The Hansen theory to choose the best cosolvent for supercritical CO2 extraction of Β-carotene from Dunaliella salina. J. Supercrit. Fluids 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Calvo, M.M.; Dado, D.; Santa-María, G. Influence of extraction with ethanol or ethyl acetate on the yield of lycopene, β-carotene, phytoene and phytofluene from tomato peel powder. Eur. Food Res. Technol. 2007, 224, 567–571. [Google Scholar] [CrossRef]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Urrego, F.A. Free solute content and solute-matrix interactions affect apparent solubility and apparent solute content in supercritical CO2 extractions. A hypothesis paper. J. Supercrit. Fluids 2012, 66, 157–175. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Sato, T.; Ikeya, Y.; Adachi, S.I.; Yagasaki, K.; Nihei, K.I.; Itoh, N. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod. Process. 2019, 117, 160–169. [Google Scholar] [CrossRef]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Phenolic acids contribution to antioxidant activities and comparative assessment of phenolic content in mango pulp and peel. S. Afr. J. Bot. 2018, 116, 158–163. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B. Effects of Ripening, Cultivar Differences, and Processing on the Carotenoid Composition of Mango. J. Agric. Food Chem. 1998, 46, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Pott, I.; Marx, M.; Neidhart, S.; Mühlbauer, W.; Carle, R. Quantitative determination of β-carotene stereoisomers in fresh, dried, and solar-dried mangoes (Mangifera indica L.). J. Agric. Food Chem. 2003, 51, 4527–4531. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Su, X.; Yang, Z.; Deng, H.; Yang, Z.; Liang, R.; Huang, J. Carotenoid composition and expression of carotenogenic genes in the peel and pulp of commercial mango fruit cultivars. Sci. Hortic. 2020, 263, 109072. [Google Scholar] [CrossRef]
- Cuttriss, A.J.; Cazzonelli, C.I.; Wurtzel, E.T.; Pogson, B.J. Carotenoids. In Advances in Botanical Research; Rébeillé, F., Douce, R., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 58, pp. 1–36. [Google Scholar]
- Haque, S.; Begum, P.; Khatun, M.; Islam, S.N. Total Carotenoid Content in Some Mango (Mangifera indica) Varieties of Bangladesh. Int. J. Pharm. Sci. Res. 2015, 6, 4875. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Ultrasound-assisted extraction of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on in vitro bioaccessibility. Food Biosci. 2018, 21, 125–131. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Vithana, M.D.K.; Singh, Z.; Johnson, S.K. Harvest maturity stage affects the concentrations of health-promoting compounds: Lupeol, mangiferin and phenolic acids in the pulp and peel of ripe ‘Kensington Pride’ mango fruit. Sci. Hortic. 2019, 243, 125–130. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef]
- Rumainum, I.M.; Worarad, K.; Srilaong, V.; Yamane, K. Fruit quality and antioxidant capacity of six Thai mango cultivars. Agric. Nat. Resour. 2018, 52, 208–214. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Ofelia Vila Buso, M.A.; Ruiz Sauri, A.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.d.O.; Ranquine, L.G.; Monteiro, M.; Torres, A.G. Pomegranate (Punica granatum L.) seed oil enriched with conjugated linolenic acid (cLnA), phenolic compounds and tocopherols: Improved extraction of a specialty oil by supercritical CO2. J. Supercrit. Fluids 2019, 147, 126–137. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.L.; Xie, L.X.; Hu, Y.; Fang, H.; Sun, X.D.; Wang, T.; Xiao, R.; Yu, R.Q. Direct and interference-free determination of thirteen phenolic compounds in red wines using a chemometrics-assisted HPLC-DAD strategy for authentication of vintage year. Anal. Methods 2017, 9, 3361–3374. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; García-Ruiz, A.; Baenas, N.; Moreno, D.A.; Meléndez-Martínez, A.J.; Stinco, C.M.; Jerves-Andrade, L.; León-Tamariz, F.; Ortiz-Ulloa, J.; Ruales, J. Changes in phytochemical composition, bioactivity and in vitro digestibility of guayusa leaves (Ilex guayusa Loes.) in different ripening stages. J. Sci. Food Agric. 2018, 98, 1927–1934. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Wu, C.H.; Kuan, A.W.; Guo, H.R.; Lin, Y.H.; Wang, P.K. Free Radical-Scavenging, Anti-Inflammatory, and Antibacterial Activities of Water and Ethanol Extracts Prepared from Compressional-Puffing Pretreated Mango (Mangifera indica L.) Peels. J. Food Qual. 2018, 2018, 1025387. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Fratianni, A.; Adiletta, G.; Di Matteo, M.; Panfili, G.; Niro, S.; Gentile, C.; Farina, V.; Cinquanta, L.; Corona, O. Evolution of carotenoid content, antioxidant activity and volatiles compounds in dried mango fruits (Mangifera indica L.). Foods 2020, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]

| 10 MPa | 35 MPa | |||||||
|---|---|---|---|---|---|---|---|---|
| α-Carotene [µg·g−1 DW] | β-Cryptoxantin [µg·g−1 DW] | β-Carotene [µg·g−1 DW] | Phytoene [µg·g−1 DW] | α-Carotene [µg·g−1 DW] | β-Cryptoxantin [µg·g−1 DW] | β-Carotene [µg·g−1 DW] | Phytoene [µg·g−1 DW] | |
| 35 °C; 100% CO2 | <LOQ | 0.24 ± 0.01 d | 0.31 ± 0.02 ab | 0.16 ± 0.04 ef | 1.03 ± 0.07 ef | 0.65 ± 0.01 abc | 8.96 ± 0.23 d | 0.13 ± 0.00 abc |
| 35 °C; 10% EtOH | <LOQ | 0.14 ± 0.07 cd | 0.17 ± 0.02 a | <LOD | 1.24 ± 0.11 g | 1.52 ± 0.27 ef | 6.81 ± 0.19 c | 0.19 ± 0.03 cde |
| 35 °C; 20% EtOH | <LOQ | 0.91 ± 0.08 e | 1.52 ± 0.08 c | 0.11 ± 0.02 d | 1.51± 0.09 h | 1.49 ± 0.17 ef | 15.28 ± 0.92 h | 0.19 ± 0.01 de |
| 35 °C; 10% Ac | <LOQ | 0.03 ± 0.00 a | 0.25 ± 0.08 ab | 0.08 ± 0.02 cd | 0.26 ± 0.03 b | 0.33 ± 0.07 a | 11.36 ± 0.37 e | 0.26 ± 0.03 e |
| 35 °C; 20% Ac | <LOQ | 0.02 ± 0.00 a | 0.13 ± 0.00 a | 0.04 ± 0.02 bc | 0.39 ± 0.01 c | 0.39 ± 0.01 ab | 12.16 ± 0.38 f | 0.23 ± 0.01 de |
| 55 °C; 100% CO2 | <LOQ | 0.04 ± 0.01 a | 0.11 ± 0.02 a | 0.05 ± 0.01 bc | 1.32 ± 0.02 g | 1.68 ± 0.03 f | 11.63 ± 1.14 ef | 0.14 ± 0.04 bcd |
| 55 °C; 10% EtOH | <LOQ | 0.07 ± 0.00 ab | 1.44 ± 0.17 c | 0.06 ± 0.02 bc | 1.01 ± 0.12 e | 2.23 ± 0.14 g | 13.41 ± 0.42 g | 0.07 ± 0.00 ab |
| 55 °C; 20% EtOH | <LOQ | 0.19 ± 0.01 de | 3.70 ± 0.87 d | 0.15 ± 0.02 e | 1.13 ± 0.14 f | 1.13 ± 0.04 de | 18.74 ± 0.21 i | 0.10 ± 0.07 ab |
| 55 °C; 10% Ac | <LOQ | 0.10 ± 0.02 bc | 0.11 ± 0.02 a | 0.09 ± 0.02 d | 0.39 ± 0.08 c | 1.52 ± 0.04 ef | 5.81 ± 0.23 b | 0.09 ± 0.02 ab |
| 55 °C; 20% Ac | <LOQ | 0.13 ± 0.00 c | 0.22 ± 0.00 ab | 0.03 ± 0.00 ab | 0.27 ± 0.02 b | 0.24 ± 0.18 a | 3.35 ± 0.34 a | 0.07 ± 0.06 ab |
| 70 °C; 100% CO2 | <LOQ | 0.07 ± 0.04 ab | <LOD | 0.05 ± 0.04 bc | 0.41 ± 0.04 cd | 0.81 ± 0.09 cd | 6.23 ± 0.52 bc | 0.06 ± 0.02 a |
| 70 °C; 10% EtOH | <LOQ | 0.07 ± 0.01 ab | 0.01 ± 0.00 a | 0.05 ± 0.00 bc | 0.52 ± 0.00 d | 1.29 ± 0.10 ef | 5.80 ± 0.57 b | 0.08 ± 0.01 ab |
| 70 °C; 20% EtOH | <LOQ | 0.11 ± 0.00 bc | 0.61 ± 0.05 b | 0.02 ± 0.00 ab | 0.38 ± 0.05 c | 1.13 ± 0.00 de | 11.08 ± 0.20 e | 0.19 ± 0.04 cde |
| 70 °C; 10% Ac | <LOQ | 0.11 ± 0.01 bc | 0.02 ± 0.01 a | 0.04 ± 0.00 b | 0.05 ± 0.00 a | 0.83 ± 0.49 cd | 8.65 ± 0.03 d | 0.07 ± 0.00 ab |
| 70 °C; 20% Ac | <LOQ | 0.07 ± 0.01 ab | 0.03 ± 0.00 a | 0.19 ± 0.02 g | 0.04 ± 0.00 a | 0.74 ± 0.07 bcd | 6.46 ± 0.25 bc | 0.13 ± 0.08 bcd |
| Total Carotenoids [µg·g−1 DW] | α-Carotene [µg·g−1 DW] | β-Kryptoxanthin [µg·g−1 DW] | β-Carotene [µg·g−1 DW] | Lutein [µg·g−1 DW] | Violaxanthin [µg·g−1 DW] | Phytoene [µg·g−1 DW] | Phytofluene [µg·g−1 DW] | Zeaxanthin [µg·g−1 DW] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Kent peel | CE * | 44.71 ± 0.02 a | 0.99 ± 0.01 a | 0.28 ± 0.06 a | 34.38 ± 1.89 a | 1.26 ± 0.03 a | 0.08 ± 0.01 b | 0.43 ± 0.02 a | 0.76 ± 0.01 b | 0.26 ± 0.06 a |
| SFE ** | 43.12 ± 6.53 b | 1.45 ± 0.59 b | 0.26 ± 0.01 a | 35.52 ± 4.09 a | 1.69 ± 0.12 b | 0.17 ± 0.02 c | 0.57 ± 0.04 b | 0.92 ± 0.02 c | 0.33 ± 0.02 b | |
| Kent pulp | CE | 32.23 ± 0.99 a | 1.36 ± 0.34 a | 0.76 ± 0.06 a | 24.02 ± 0.49 a | 0.94 ± 0.14 a | <LOQ | 0.01 ± 0.02 a | <LOQ | 0.24 ± 0.03 a |
| SFE | 22.00 ± 0.12 b | 0.38 ± 0.19 b | 0.33 ± 0.01 b | 17.87 ± 1.88 b | <LOQ | <LOQ | 0.14 ± 0.02 b | <LOQ | <LOQ | |
| Haden peel | CE | 65.66 ± 4.45 a | 7.09 ± 0.59 a | 1.50 ± 0.21 a | 46.47 ± 1.13 a | 1.29 ± 0.00 a | 0.45 ± 0.13 b | 0.69 ± 0.14 b | 0.78 ± 0.01 b | 0.14 ± 0.01 a |
| SFE | 53.87 ± 0.87 b | 2.52 ± 0.04 b | 1.12 ± 0.09 b | 33.30 ± 4.16 b | 1.05 ± 0.07 b | 0.14 ± 0.03 b | 0.20 ± 0.06 a | 0.19 ± 0.00 c | 0.12 ± 0.02 b | |
| Haden pulp | CE | 17.35 ± 0.33 a | 0.16 ± 0.05 a | 0.24 ± 0.03 a | 11.33 ± 8.45 a | 0.07 ± 0.00 a | 0.03 ± 0.00 b | 0.28 ± 0.01 a | 0.23 ± 0.01 b | 0.03 ± 0.01 a |
| SFE | 13.09 ± 0.91 b | 0.17 ± 0.06 a | 0.26 ± 0.08 a | 8.72 ± 0.22 a | 0.04 ± 0.00 a | 0.01 ± 0.00 c | 0.19 ± 0.02 a | 0.24 ± 0.00 b | 0.01 ± 0.01 b | |
| Kent Peel | Kent Pulp | Haden Peel | Haden Pulp | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CE * | SFE ** | CE | SFE | CE | SFE | CE | SFE | ||
| Hydroxycinnamic acids | Caffeic acid | 0.19 ± 1.03 b | 0.27 ± 4.82 c | 0.16 ± 6.90 a | 0.22 ± 13.26 b | 0.39 ± 0.22 e | 0.24 ± 0.05 b | 0.30 ± 1.46 d | 0.41 ± 3.53 e |
| Chlorogenic acid | <LOQ | <LOQ | <LOQ | <LOQ | 0.17 ± 1.36 b | 0.02 ± 8.97 a | <LOQ | <LOQ | |
| Ferulic acid | 0.14 ± 16.09 c | 0.18 ± 3.77 d | 0.05 ± 0.33 a | 0.08 ± 9.50 b | 0.25 ± 0.95 f | 0.21 ± 1.73 e | 0.15 ± 5.99 c | 0.27 ± 8.88 g | |
| p-Coumaric acid | 0.46 ± 8.79 c | 0.71 ± 3.63 f | 0.43 ± 6.79 c | 0.57 ± 9.39 d | 0.66 ± 1.59 e | 0.76 ± 2.65 g | 0.22 ± 5.08 a | 0.35 ± 1.87 b | |
| Flavonoids | Cymaroside | 0.06 ± 4.63 b | 0.02 ± 4.00 a | <LOQ | <LOQ | 0.10 ± 3.04 c | <LOQ | <LOQ | <LOQ |
| Aromadendrin | 0.02 ± 4.04 e | 0.03 ± 4.08 f | 0.01 ± 13.20 d | 0.01 ± 10.95 c | 0.01 ± 0.67 a | 0.02 ± 1.82 b | <LOQ | 0.01 ± 6.28 a | |
| Avicularin | 10.53 ± 3.18 c | 10.41 ± 1.26 c | 0.02 ± 65.27 a | 0.27 ± 18.11 a | 10.97 ± 4.16 d | 4.49 ± 4.05 b | 0.01 ± 1.51 a | 0.26 ± 40.31 a | |
| Astragalin | 0.86 ± 2.50 d | 1.94 ± 4.04 e | <LOQ | 0.11 ± 18.29 a | 0.56 ± 4.29 b | 0.64 ± 3.96 c | <LOQ | 0.11 ± 24.90 a | |
| Hyperin | 26.57 ± 4.89 e | 23.97 ± 4.19 d | <LOQ | 1.40 ± 17.65 b | 27.98 ± 4.31 e | 10.73 ± 2.05 c | 0.07 ± 3.22 a | 1.58 ± 2.31 b | |
| Phlorizin | 0.18 ± 3.88 d | 0.16 ± 2.82 c | <LOQ | 0.01 ± 12.79 a | 0.18 ± 0.05 d | 0.06 ± 3.75 b | <LOQ | <LOQ | |
| Taxifolin | 0.37 ± 1.64 e | 0.77 ± 2.68 f | 0.03 ± 7.59 a | 0.06 ± 16.93 b | 0.15 ± 1.33 c | 0.27 ± 0.75 d | 0.02 ± 3.66 a | 0.03 ± 5.55 a | |
| Naringenin | 0.15 ± 12.59 e | 0.15 ± 4.21 e | 0.02 ± 7.15 a | 0.03 ± 14.16 c | 0.16 ± 2.79 d | 0.14 ± 3.79 b | <LOQ | 0.01 ± 7.85 a | |
| Epicathecin | 0.14 ± 1.49 d | 0.06 ± 4.69 c | 0.03 ± 4.99 b | 0.03 ± 11.95 b | 0.14 ± 3.86 d | 0.01 ± 1.16 a | 0.01 ± 15.69 a | <LOQ | |
| Kaempferol | 0.04 ± 78.95 a | 0.07 ± 18.32 b | <LOQ | <LOQ | 0.07 ± 1.24 b | 0.09 ± 1.56 c | <LOQ | 0.07 ± 28.42 b | |
| Isoquercitrin | 27.66 ± 1.50 g | 26.81 ± 1.95 f | 0.11 ± 31.36 b | 1.11 ± 17.34 c | 27.47 ± 2.01 f | 11.11 ± 0.60 e | 0.08 ± 6.35 a | 1.26 ± 2.51 d | |
| Quercetin | 0.99 ± 13.54 c | 1.67 ± 5.37 d | <LOQ | 0.29 ± 38.82 a | 2.78 ± 0.71 f | 1.92 ± 5.28 e | <LOQ | 0.53 ± 9.01 b | |
| Quercitrin | 5.03 ± 5.75 e | 4.34 ± 3.12 d | 0.01 ± 26.32 a | 0.08 ± 21.53 b | 5.01 ± 1.27 e | 1.70 ± 0.68 c | <LOQ | 0.08 ± 25.13 b | |
| Catechin | 21.28 ± 0.99 e | 10.13 ± 1.43 d | 3.67 ± 3.75 c | 3.31 ± 8.64 c | 21.06 ± 3.95 e | 1.45 ± 4.50 b | <LOQ | 0.35 ± 1.20 a | |
| Xanthonoids | Mangiferin | 22.19 ± 1.44 h | 9.47 ± 4.54 g | 0.78 ± 16.39 c | 1.30 ± 15.86 d | 5.90 ± 3.45 f | 0.48 ± 10.95 b | 0.34 ± 0.87 a | 1.96 ± 23.30 e |
| Dihydroxybenzoic acids | Gallic acid | 29.70 ± 8.11 e | 30.45 ± 0.99 e | 11.55 ± 7.74 a | 18.98 ± 13.52 c | 48.21 ± 1.15 f | 13.13 ± 7.87 b | 13.21 ± 8.33 b | 21.15 ± 11.89 d |
| Protocatechuic acid | 0.72 ± 70.58 d | 1.18 ± 2.53 f | 0.22 ± 14.14 a | 0.94 ± 16.33 e | 0.29 ± 5.50 b | 0.55 ± 60.96 c | 0.56 ± 18.65 c | 1.01 ± 27.49 f | |
| DPPH [µg·mL−1] | FRAP [µmol Trolox g−1] | ||
|---|---|---|---|
| Kent peel | CE * | 0.63 ± 0.00 a | 10.89 ± 0.27 a |
| SFE ** | 5.68 ± 0.08 b | 2.46 ± 0.15 b | |
| Kent pulp | CE | 7.97 ± 0.24 a | 2.60 ± 0.15 a |
| SFE | 3.68 ± 0.08 b | 2.06 ± 0.02 b | |
| Haden peel | CE | 0.64 ± 0.02 a | 17.45 ± 0.13 a |
| SFE | 1.34 ± 0.03 b | 4.25 ± 0.16 b | |
| Haden pulp | CE | 6.11 ± 0.27 a | 2.09 ± 0.03 a |
| SFE | 17.36 ± 0.63 b | 0.91 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villacís-Chiriboga, J.; Voorspoels, S.; Uyttebroek, M.; Ruales, J.; Van Camp, J.; Vera, E.; Elst, K. Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp. Foods 2021, 10, 2201. https://doi.org/10.3390/foods10092201
Villacís-Chiriboga J, Voorspoels S, Uyttebroek M, Ruales J, Van Camp J, Vera E, Elst K. Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp. Foods. 2021; 10(9):2201. https://doi.org/10.3390/foods10092201
Chicago/Turabian StyleVillacís-Chiriboga, José, Stefan Voorspoels, Maarten Uyttebroek, Jenny Ruales, John Van Camp, Edwin Vera, and Kathy Elst. 2021. "Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp" Foods 10, no. 9: 2201. https://doi.org/10.3390/foods10092201
APA StyleVillacís-Chiriboga, J., Voorspoels, S., Uyttebroek, M., Ruales, J., Van Camp, J., Vera, E., & Elst, K. (2021). Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp. Foods, 10(9), 2201. https://doi.org/10.3390/foods10092201






